Abstract
Purposes/Aim: Glucocorticoid steroids can induce expression of PPARγ gene and enhance adipogenesis by bone marrow mesenchymal stem cells (BMSCs), which may result in osteonecrosis of the femoral head. Currently, there are no medications available to prevent steroid-induced osteonecrosis. We hypothesized that siRNA targeting PPARγ gene may prevent steroid-induced adipogenesis and osteonecrosis in rabbit. The purpose of this study was to evaluate the preventive effects of siRNA targeting PPARγ gene on steroid-induced adipogenesis and osteonecrosis.
Methods: Forty-eight healthy New Zealand rabbits were randomized into four groups with Group M treated with dexamethasone only, Group S with dexamethasone and a recombinant adenovirus shuttle vector carrying siRNA targeting PPARγ gene, Group Con with dexamethasone and a vector carrying irrelative sequence, and Group N with no treatment serving as control. Expressions of the PPARγ, osteocalcin and Runx2 genes, as well as histopathologic changes were evaluated.
Results: The levels of PPARγ gene expression were decreased while the levels of osteocalcin and Runx2 gene expression were increased in rabbits treated with dexamethasone and recombinant adenovirus shuttle vector carrying siRNA targeting PPARγ gene (Group S), compared to rabbits treated either with dexamethasone alone (Group M) or with both dexamethasone and a vector carrying irrelative sequence (Group Con). The marrow necrosis, adipocyte hypertrophy and proliferation, diminished hematopoiesis, thinner and sparse trabeculae, and increased empty osteocyte lacunae in the femoral head were observed in Group M and Group Con rabbits. However, no such changes were seen in Group S rabbits that were treated with dexamethasone and a recombinant adenovirus shuttle vector carrying siRNA targeting PPARγ gene.
Conclusion: siRNA targeting PPARγ gene can inhibit adipogenic differentiation of BMSCs and prevent steroid-induced osteonecrosis in rabbit. The inhibition of bone-marrow adipogenesis and concomitant enhancement of osteogenesis with RNAi may provide a novel approach to the prevention of steroid-induced osteonecrosis.
Introduction
Osteonecrosis of the femoral head [ONFH] is a common orthopaedic disease (Citation1–3). Non-traumatic ONFH is associated with approximately 40 etiologic factors, but steroid administration and alcohol abuse are the most common causes (Citation1,Citation2,Citation4–9). Approximately 80% of patients suffering from ONFH will progress to a collapse of the femoral head, resulting in the loss of joint function if they do not receive appropriate treatment. ONFH is a significantly disabling disease that currently has no effective preventative or treatment options. Most of the patients with late stage ONFH will eventually require a total hip replacement, which is not ideal, especially in younger patients (Citation6). Therefore, steroid-induced ONFH continues to be a challenging problem to both scientists and surgeons in the orthopaedic field (Citation2,Citation3,Citation5,Citation7,Citation9).
Typically implicated in the pathology of ONFH is the peroxisome proliferator-activated receptor-γ [PPARγ], which is a specific transcription factor to induce adipogenic differentiation. In the literature, in vivo and in vitro studies have demonstrated that steroids stimulated the expression of the PPARγ gene in both rabbits and human bone marrow mesenchymal stem cells [BMSCs]. Upregulation of the expression of the PPARγ gene led to increased amounts of adipogenic differentiation, decreased amounts of osteogenic differentiation (Citation5,Citation9), and subsequently increased lipid generation and decreased bone formation. The accumulation of fat in the femoral head can block blood circulation, and finally result in ONFH (Citation2,Citation3,Citation7,Citation8). Downregulation of PPARγ expression can inhibit adipogenic differentiation of BMSCs (Citation5,Citation9). This evidence demonstrates that expression of the PPARγ gene is an important key in the development of steroid-induced ONFH.
As a tool of gene therapy, RNA interference [RNAi] is a rapidly developing technology with promising applications (Citation10). Small interfering RNA [siRNA]-mediated RNAi can induce specific gene silencing and provide a novel approaches to the treatment of human diseases. The adenovirus genome is highly homologous with the human genome. Currently, the delivery of siRNA into target tissues for the interference of expression of disease-related specific genes using an adenoviral vector has achieved satisfactory results (Citation11–14). However, to our knowledge, there is no report of siRNA targeting the PPARγ gene aimed at preventing steroid-induced ONFH.
Our previous in vitro study showed that three recombinant adenovirus shuttle vectors, 1.0-CMV-971, 1.0-CMV-1253 and 1.0-CMV-1367, carrying siRNA targeted at the PPARγ genes, were successfully constructed allowing high titers of the adenovirus to be obtained (Citation2). Among those the shuttle vector 1.0-CMV-1253 was found to be the most effective suppressor of the PPARγ gene by BMSCs (Citation15). In this in vivo study, we used the same vector to infect femoral head cells of rabbits treated with steroids, and subsequently investigated the preventive and therapeutic effects of siRNA targeting on the PPARγ gene in steroid-induced ONFH. Our results indicated that siRNA recombinant adenovirus shuttle vectors targeting the PPARγ gene can effectively suppress steroid-induced PPARγ gene expression and its concomitant adipogenic differentiation of BMSCs resulting in diminished bone marrow changes within the femoral head of rabbits, in vivo. These findings provide a scientific foundation for the potential clinical applications of siRNA to prevent steroid-induced ONFH.
Materials and methods
Reagents and instruments
The ultraviolet spectrophotometer used was from Sartorius (Dourdan, France). The CO2 constant-temperature incubator and ultra-low temperature freezer were from Sanyo (Tokyo, Japan). The ultrapure water system was from Millipore (Temecula, CA). The Mouse anti-rabbit PPARγ, osteocalcin, Runx2 monoclonal antibody, mouse anti-rabbit GAPDH polyclonal antibody, and goat anti-mouse IgG/enzyme were from Santa Cruz Biotechnology (Santa-Cruz, CA). Antibody diluent was purchased from DAKO (Troy, MI). RNA distilling kit was from QIAGEN (Hilden, Germany). Reverse transcriptase AMV, Trizol and RNasin were purchased from Promega (Madison, WI). DEPC was from Fluka (Newport News, VA). FBS and high glucose DMEM were purchased from Gibco (Grand Island, NY). The gel imaging analysis system was from GeneGenius, Syngene (Los Altos, CA). Experimental animals were bought from Experimental Animal Center of Henan province, with an animal license: SYXK [HENAN] 2009-0001.
siRNA adenovirus shuttle vector
The recombinant adenovirus shuttle vectors carrying siRNA targeting PPARγ genes were already synthesized and successfully constructed in our earlier study (Citation2). We selected the recombinant adenovirus shuttle vector 1.0-CMV-1253, which is the most effective vector to suppress PPARγ gene expression (Citation15). The shuttle vector 1.0-CMV–Con carrying irrelative sequence that was ineffective at targeting PPARγ served as a control. The virus titer was diluted to 1.2 × 1011 IU/ml, and was preserved at low temperature.
Animals and experiment grouping
Forty-eight healthy New Zealand rabbits [Experimental Animal Center, Henan Province, China], 7-month-old [weight, 4 ± 0.5 kg], were randomized into four groups. Group M: rabbits were treated with dexamethasone only. Group S: rabbits were treated with dexamethasone and a [n] recombinant adenovirus shuttle vector 1.0-CMV-1253 that was effective at targeting the PPARγ gene. 25 µl [3 × 109 IU] of the virus was injected into one side of the femoral heads [depth 5 mm] randomly with special bone marrow puncture needle and microsyringe, and block off with bone wax. Group Con: the rabbits were treated with dexamethasone and a vector carrying irrelative sequence that was ineffective at targeting the PPARγ gene with the same dose and method to group S. Group N: rabbits with no treatment served as controls. The rabbits were sacrificed in batches at 4 and 8 weeks after treatment. The study protocol was approved by the Animal Review Board of the University.
Histopathologic examinations
Femoral head specimens were cut into two symmetrical parts along a coronal plane. One half of the specimen was fixed in 10% formalin for 24 hours and then decalcified in 10% ethylenediaminetetraacetic acid with a Tris-HCl buffer. The tissue was then embedded in paraffin and Five-µm sections were cut and stained using hematoxylin and eosin. The remaining specimen was sent for frozen section and stained with Sudan III. We examined five sections from each animal using a modified method described in the paper by Warner et al. (Citation16). Briefly, five fields within the subchondral area of the femoral head of each section were chosen. The first field was located at the estimated center of the subchondral bone of weight-bearing areas, and the remaining four fields were located on either sides of the first field with two fields on each side. The mean of the five fields from each section was representative of that section. Next, the mean of the five sections from each animal was used as the value for that rabbit. The following parameters were assessed: [1] percentage of empty osteocyte lacunae: 200 osteocyte lacunae in each established field were counted under a light microscope at × 200 magnification, and then the percentage of the empty osteocyte lacunae was determined. [2] Fraction of trabecular bone area: the fraction was surveyed in each field with computer image analysis software. [3] Largest adipocyte diameter: the average diameter of the biggest adipocyte was measured in each field using an ocular micrometer under a light microscope at × 200 magnification (Citation3,Citation7,Citation17) and the average diameter for fat cells in each animal was calculated.
Determination of the expression of the PPARγ, osteocalcin and Runx2 mRNA
Total RNA was extracted using a Trizol method when bone tissue homogenate of femoral head was collected at the 4th and 8th weekends and used as templates for cDNA synthesized through reverse transcription under the catalysis of M-MuLV. The expression of the PPARγ, osteocalcin and Runx2 mRNA were determined by TaqMan real time-PCR [TaqMan probe fluorescence detection], and the PCR primer sequences and probes used in this study were as follows:
PPARγ:
forward [F], 5′-GCATCCCCACCCTACTATTCTG-3′
reverse [R], 5′-GAGGGAGTTGGAAGGCTCTTC-3′
fluorescence probe sequence [probe], FAM5′-CTCAGCTCTACAATAAGACT-3′
Osteocalcin:
F, 5′-TGCTTTGGGATGGCAGAAG-3′
R, 5′-TCTGTTCCCTCCCTGCTGTCT-3′
probe, FAM5′-AAGGGACAAACTACCAGAGAG-3′TAMR
Runx2:
F, 5′-AACCCACGAATGCACTATCCA-3′
R, 5′-GGGACATGCCTGAGGTGACT-3′
probe, FAM5′-CCACCTTTACTTACACCCCG-3′
GAPDH:
F, 5′-CGCCTGGAGAAAGCTGCTAA-3′
R, 5′-CCTCGGATGCCTGCTTCA-3′
probe, FAM5′-TATGACGACATCAAGAAGGT-3′
Each specimen was amplified in five parallel tubes, including gene amplification of the PPARγ, osteocalcin, Runx2 and an internal reference GAPDH gene was used as control. The DNA standard of the PPARγ, osteocalcin, Runx2 and an internal reference GAPDH with a 5-gradient series dilution was used at every amplification event. The standard curve was plotted, and PCR products were quantified according to the standard curve.
Determination of the expression of PPARγ, osteocalcin and Runx2 proteins through Western blotting
The PPARγ, osteocalcin and Runx2 protein was extracted using a cell protein extraction kit from femoral head tissue homogenate that were collected at the 4th and 8th weekends. A bicinchoninic acid method was used to determine the total protein concentration. Protein samples [50 μg] were separated by 12% SDS-polyacrylamide gel electrophoresis, and GAPDH was used as a control. The protein was transferred to polyvinylidene difluoride membranes. The membranes were subsequently blocked and incubated with a 1:200 diluted mouse anti-rabbit PPARγ, osteocalcin, Runx2 monoclonal antibody and mouse anti-rabbit GAPDH polyclonal antibody at 4 °C overnight and, again, incubated with a 1:500 diluted goat anti-mouse IgG polyclonal antibody. After washing, the blots were developed using BCIP/NBT color detection system. The relative levels of PPARγ, osteocalcin, and Runx2 protein expression were presented as a ratio of the gray value of the specific protein compared to that of GAPDH.
Statistical analysis
Data are presented as mean ± standard deviation [SD]. Means of biggest adipocytes size, and gene expression were compared using one-way ANOVA, followed by the SNK multiple comparison procedure. Rates of empty osteocyte lacunae and bone trabecular area fraction were compared using the multisample Kruskal–Wallis test.
Results
General state of experimental animals
Throughout the experimental period, the rabbits in group S, treated with both dexamethasone and adenovirus shuttle vector, and group N, without treatment, appeared unaffected and the weights of the animals remained stable or gradually increased. The rabbits in group M showed a poorer mental state, evidence of anorexia, and less activity, with a decrease in weight [0.5 ± 0.11 kg] when compared to the other groups. The Con group, treated with both dexamethasone and a vector carrying an irrelative sequence, showed a decrease in weight [0.3 ± 0.21 kg] when compared to the other groups.
Histopathologic changes in the femoral head
Eight weeks after treatment, gross examination revealed that the femoral heads of rabbits in the group M and group Con became whitish, soft, and easily cut. Under microscopy, we found evidence of bone marrow necrosis, diminished hematopoiesis, increased fat, thinning of trabecular bone, with evidence of trabecular breakage. The fraction of trabecula decreased, and the percentage of empty osteocyte lacunae occurring in the subchondral region of the femoral head increased in both Group M and group Con. However, no such pathologic change was observed in either Group N or Group S []. Four and 8 weeks after treatment, the percentage of empty osteocyte lacunae was increased in animals of group M and group Con, and was significantly higher compared with both group N and group S [p < 0.05], and the percentage of empty osteocyte lacunae in group S did not increase compared with the animals in group N [p > 0.05] []. The average diameter of the largest adipocyte increased in both the M group and Con group when compared to animals in group N and group S [p < 0.05] []. The fractional area of trabecular bone decreased in both the M group and Con group when compared to the N and S groups [p < 0.05]. No differences in the fractional area of trabecular bone were seen between group N and group S [p > 0.05] [].
Figure 1. A subchondral area of the femoral head is shown. At 8 weeks, significantly increased number of empty osteocyte lacunae was found in group M (A) treated with steroid only and group Con (B) treated with both steroid and the vector carrying irrelative sequence, while less empty osteocyte lacunae were found in group S (C) treated with both steroid and adenovirus shuttle vectors carrying siRNA targeting the PPARγ gene and group N (D) with no treatment (Stain, hematoxylin and eosin; original magnification, ×400).
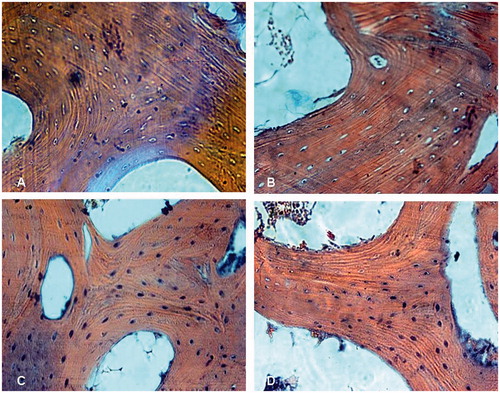
Figure 2. The thinner, sparse and broken bone trabeculae, bone and marrow necrosis in the subchondral area of the femoral head were found in group M (A) treated with steroid only (Stain, hematoxylin and eosin; original magnification, ×40) and group Con (B) treated with both steroid and the vector carrying irrelative sequence (Stain, hematoxylin and eosin; original magnification, ×100), compared with normal in siRNA group S (C) treated with both steroid and adenovirus shuttle vectors carrying siRNA targeting the PPARγ gene and group N (D) with no treatment (Stain, hematoxylin and eosin; original magnification, ×100).
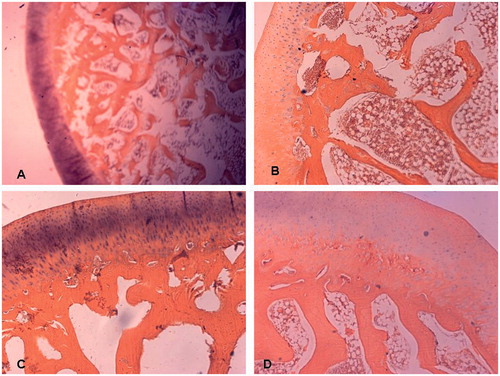
Figure 3. Hypertrophy and proliferation of adipocyte, increased fatty tissue in subchondral area of the femoral head noticed in animals in group M (A) treated with steroid only and group Con (B) treated with both steroid and the vector carrying irrelative sequence, while less adipocytes were found in group S (C) treated with both steroid and adenovirus shuttle vectors carrying siRNA targeting the PPARγ gene and group N (D) with no treatment (Stain, Sudan III; original magnification, ×100).
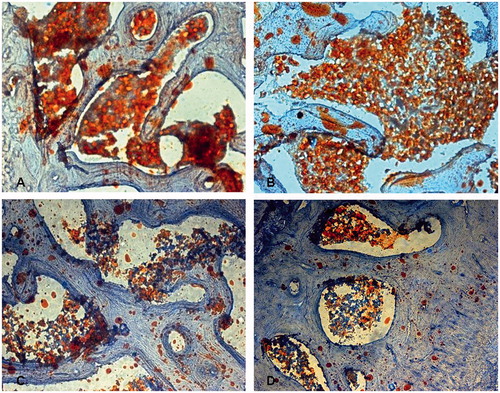
Table 1. Histopathologic change of the femoral heads of rabbits (, n = 6).
Expression of PPARγ, osteocalcin and Runx2 mRNA
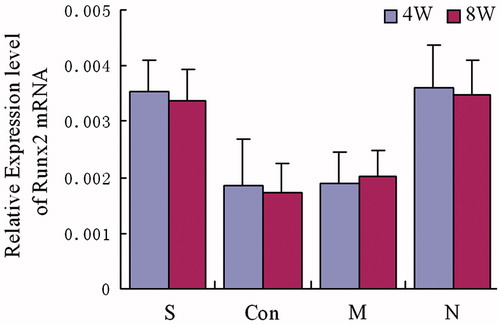
Four and 8 weeks after treatment, the expression of PPARγ mRNA in animals of group S and group N were significantly lower than animals in groups M and Con [p < 0.05]. The PPARγ mRNA expression was also found to not be statistically significant between group S and group N [p > 0.05]. Furthermore, there was no significant difference between group M and group Con [p > 0.05] []. The expression level of osteocalcin and Runx2 mRNA in animals of both group S and group N were significantly higher than those in groups M and Con [p < 0.05]. The osteocalcin and Runx2 mRNA expression levels were also found to not be statistically different between groups S and N [p > 0.05]. Finally, there were no significant differences between groups M and the Con Group [p > 0.05] [ and ].
Figure 4. Expression of PPARγ mRNA. The group S was treated with both steroid and adenovirus shuttle vectors, carrying siRNA targeting the PPARγ gene; Group Con was treated with both steroid and a vector carrying irrelative sequence; Group M was treated with steroid only, group N received no treatment serving as control. Statistical difference among 4 groups was treated by analysis of variance, in week 4: F = 38.08, p = 0.00; in week 8: F = 29.46, p = 0.00. In week 4 and week 8, group S: compare to group Con and M, p < 0.05, compare to group N, p > 0.05; group Con: compared to group M, p > 0.05, compared to group N, p < 0.05; group M: compared to group N p < 0.05.
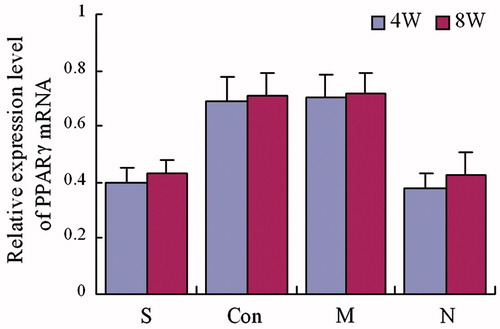
Figure 5. Expression of osteocalcin mRNA. The group S was treated with both steroid and adenovirus shuttle vectors carrying siRNA targeting the PPARγ gene, group Con was treated with both steroid and a vector carrying irrelative sequence, group M was treated with steroid only, group N received no treatment serving as control. Statistical difference among 4 groups was assessed by analysis of variance, in week 4: F = 21.89, p = 0.00; in week 8: F = 16.72, p = 0.00. In week 4 and week 8, group S: compare to group Con and M, p < 0.05, compare to group N, p > 0.05; group Con: compared to group M, p > 0.05, compared to group N, p < 0.05; group M: compared to group N p < 0.05.
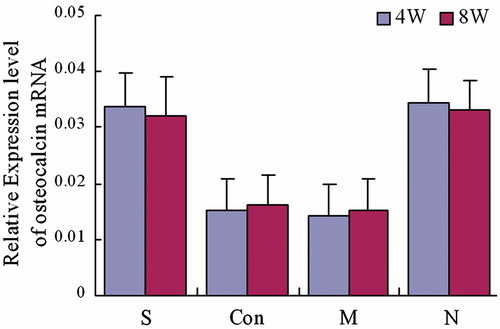
Figure 6. Expression of Runx2 mRNA. The group S was treated with both steroid and adenovirus shuttle vectors carrying siRNA targeting the PPARγ gene, group Con was treated with both steroid and a vector carrying irrelative sequence, group M was treated with steroid only, group N was with no treatment serving as control. Statistical difference among 4 groups was assessed by analysis of variance, in week 4: F = 12.31, p = 0.00; in week 8: F = 16.66, p = 0.00. In week 4 and week 8, group S: compare to group Con and M, p < 0.05, compare to Group N, p > 0.05; group Con: compared to group M, p > 0.05, compared to group N, p < 0.05; group M: compared to group N p < 0.05.
Expression levels of the PPARγ, osteocalcin and Runx2 protein
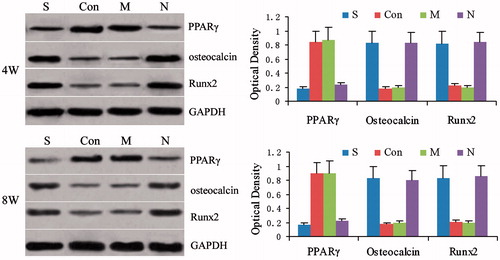
Four and 8 weeks after treatment, the expression of PPARγ protein in animals of group S and group N was significantly lower than that in animals in either group M or group Con [p < 0.05]. The level of expression of the PPARγ protein was also not statistically different between groups S and N [p > 0.05], and was also not significantly different between the M and Con groups [p > 0.05] []. The expression of osteocalcin and Runx2 protein in animals of both group S and group N was found to be significantly higher than the levels of animals in either group M or group Con [p < 0.05]. The osteocalcin and Runx2 protein expression were not significantly different between the S and N group [p > 0.05], as well as between group M and group Con [p > 0.05] [].
Figure 7. Expression of PPARγ protein, osteocalcin protein and Runx2 protein at 4 and 8 weeks after treatment. The group S was treated with both steroid and adenovirus shuttle vectors carrying siRNA targeting the PPARγ gene, group Con was treated with both steroid and a vector carrying irrelative sequence, group M was treated with steroid only, group N received no treatment serving as control. Optical density among 4 groups was assessed by analysis of variance at week 4: PPARγ F = 32.17, p = 0.00, osteocalcin F = 28.31, p = 0.00 and Runx2 F = 23.19, p = 0.00; group S: compared to group Con and M, p < 0.05, compared to group N, p > 0.05; group Con: compared to group M p > 0.05, compared to group N, p < 0.05; group M: compared to group N, p < 0.05. Statistical difference Optical density among 4 groups was assessed by analysis of variance at week 8: PPARγ F = 35.98, p = 0.00, osteocalcin F = 31.59, p = 0.00 and Runx2 F = 28.44, p = 0.00; group S: compared to group Con and M, p < 0.05, compared to group N, p > 0.05; group Con: compared to group M, p > 0.05, compared to group N, p < 0.05; group M: compared to group N p < 0.05.
Discussion
One of the most common risk factors for ONFH is the use of glucocorticoid steroids (Citation2,Citation3,Citation9,Citation18). Excessive use of exogenous steroids can cause many side effects on the skeleton through either direct or indirect effects (Citation19,Citation20). Although a number of studies have tried to protect the femoral head against ON, the mechanisms of ONFH remain unknown and presently there are no effective treatments for the condition (Citation21,Citation22).
Several possible hypotheses of ON have been proposed in the literature, including an increase in the size and number of fatty cells, increased intraosseous pressure, fatty degeneration of osteocytes, fatty embolisms, and extraosseous arterial occlusions due to abnormal changes in histologic features, hemodynamics, metabolism, and biochemical features within the femoral head (Citation3,Citation4,Citation23–28). Hypertrophy and proliferation of fat cells, diminished hematopoiesis, lipid deposition within osteocytes, fatty degeneration of osteocytes, marrow necrosis, and thinner or sparse trabeculae are histopathologic changes occurring throughout the early stages of steroid-induced ONFH (Citation3,Citation7,Citation8,Citation26,Citation29–35). The development of ONFH may be associated with the adipogenic differentiation of BMSCs induced by steroids (Citation2–5,Citation8,Citation9,Citation30,Citation36,Citation37). In this study, classic histopathologic changes of osteonecrosis were observed in the femoral heads of animals treated with steroids. At 4 weeks after treatment, the percentage of empty osteocyte lacunae increased in animals treated with steroids. Furthermore, at 8 weeks after treatment, diminished hematopoiesis, hypertrophy and proliferation of adipocytes, bone marrow necrosis, thinning and paucity of trabeculae, as well as an increase in the percentage of empty osterocytes were observed in the subchondral region of the femoral head of animals in both the group M treated with steroid and group Con treated with both steroid and the vector carrying irrelative sequence. However, no pathologic changes were observed in either the control group [group N, treated with no steroid] or the siRNA group [group S] treated with both steroid and adenovirus shuttle vectors targeting PPARγ.
It is well known that BMSCs can differentiate into osteoblasts, adipocytes, fibroblasts, chondrocytes, and even myoblasts (Citation38,Citation39). Recent Studies have shown that most BMSCs could differentiate into osteoblasts and osteocytes, and a few differentiate into adipocytes, under normal in vitro culture conditions (Citation4,Citation5,Citation40,Citation41). BMSCs can be induced to differentiate into a large number of adipocytes and decreased number of osteoblasts if the cells are treated with longer durations and higher concentrations of steroids, and the levels of ALP activity and osteocalcin decrease (Citation4,Citation5,Citation35,Citation40,Citation41). These findings indicate that steroids can increase adipogenesis and decrease osteogenesis in bone marrow stroma, and induce intracellular lipid deposition causing fat degeneration and the death of osteocytes, which may be associated with the development of osteonecrosis (Citation3,Citation7,Citation8,Citation26,Citation28).
Adipogenic differentiation is a complex process regulated by several factors. PPARγ is closely involved in the induction of adipogenic differentiation (Citation3,Citation5,Citation9,Citation42) and PPARγ is a specific adipogenic transcription factor that belongs to the nucleus-hormone receptor subgroup. Like most members of Nuclear-receptor families, its activity is regulated by ligans (Citation43). PPARγ mRNA appears prior to the activation of many other adipocyte genes in adipogenic differentiation of 3T3-L1 and 3T3-F442A cells (Citation44). Several in vivo and in vitro studies have shown that steroids can also stimulate the expression of PPARγ mRNA in both rabbit and human bone marrow stroma cells (Citation3,Citation42,Citation43). Upregulation of PPARγ expression can promote adipogenic differentiation, reduce osteogenic differentiation (Citation5,Citation9), increase fatty accumulation, heighten intraosseous pressure, block blood circulation in the femoral head, and eventually lead to ONFH (Citation2,Citation3,Citation7,Citation8). Downregulation of PPARγ expression can effectively inhibit adipogenesis and maintain osteogenesis of cells, which may decrease the incidence of steroid-induced ONFH (Citation7,Citation42). This evidence indicates that PPARγ is an important target gene in the development of steroid-induced ONFH.
As a tool of gene therapy, RNA interference [RNAi] is a rapidly developing technology with promising applications (Citation10). Small interfering RNA [siRNA]-mediated RNAi can induce specific gene silencing and provide a novel approach to the treatment of human diseases. How to effectively deliver siRNA into target tissues is an essential obstacle of this technology, which is the subject of many basic and clinical science projects. Currently, viral and non-viral methods are used for siRNA delivery. Compared to non-viral vectors, viral vectors are more effective at targeting cells and causing stable expression of siRNA in host cells. Thus, viral vectors, such as retrovirus, lentivirus and adenovirus, are used for siRNA delivery. The adenovirus genome is a linear, non-segmented double-stranded [ds] DNA, which is highly homologous with the human genome. Adenovirus can be produced at a high virus titer, and has been used extensively to deliver siRNA or other genes into a variety of mammalian cells, with the effective working time last for about two weeks (Citation15,Citation45,Citation46). Furthermore, adenoviral integration events and integration mutations are rare (Citation47,Citation48). Currently, the delivery of siRNA into target tissues for the interference in expression of disease-related specific genes by using adenoviral vectors has yielded satisfactory results (Citation12–15).
Our previous in vitro studies have demonstrated that siRNA targeting of PPARγ could suppress the expression of the PPARγ gene (Citation9,Citation49), inhibit steroid-induced adipogenic differentiation and maintain osteogenic differentiation of the bone marrow stromal cells treated with 10−7 mol/L dexamethasone and siRNA adenovirous 1 × 106 IU. Similarly, our previous in vivo study (Citation7) showed that siRNA adenoviral vectors targeting PPARγ efficaciously suppressed the expression of the PPARγ gene and adipogenesis in the femoral head of animals induced by alcohol, indicating that the silencing of PPARγ played a role in the prevention of alcohol-induced ONFH. However, to our knowledge, there are no reports utilizing the siRNAs to target the PPARγ gene in order to prevent steroid-induced ONFH in vivo.
In summary, the current study using the most effective shuttle vector 1.0-CMV-1253 showed that the expression of PPARγ mRNA and protein was significantly lower in animals treated either with siRNA and steroids or without treatment [control] [p < 0.05] compared with animals treated with steroids. The expression of osteocalcin and Runx2 mRNA and their proteins were significantly higher in animals treated with siRNA and steroids [p < 0.05] compared with animals treated with steroids alone. Also, there were no histological changes indicative of osteonecrosis of the femoral head in animals treated with siRNA and steroids. These results indicate that siRNA targeting the PPARγ gene can inhibit adipogenic differentiation of BMSCs and prevent steroid-induced osteonecrosis of the femoral head in rabbits.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Each author certifies that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangements) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This study was supported by a grant from the National Natural Science Foundation of China (No. 81071520).
Investigation was performed at Orthopaedic Institute of Zhengzhou University & Open Laboratory of Unode Science of Clinical Medicine of Henan Province, Zhengzhou, Henan, China.
References
- Jones JP Jr. Etiology and pathogenesis of osteonecrosis. Semin Arthroplasty 1991;2:160–8
- Liu M, Wang YS, Li YB, Zhao GQ. Construction and identification of the recombinant adenovirus vector carrying a small interfering RNA targeting the peroxisome proliferator-activated receptor-γ. Chin Med J (Engl) 2012;125:671–5
- Wang YS, Yin L, Li YB, Liu PL, Cui QJ. Preventive effects of puerarin on alcohol-induced osteonecrosis. Clin Orthop Relat Res 2008;466:1059–67
- Cui QJ, Wang YS, Saleh KJ, Wang GJ, Balian G. Alcohol-induced adipogenesis in a cloned bone-marrow stem cell. J Bone Joint Surg Am 2006;88:148–54
- Li YB, Cao YW, Wu XJ, Wang YS. Steroid-induced regulation of gene involved in the osteogenesis and adipogenesis of human bone marrow mesenhymal stem cells. Chin J Exp Surg (Chin) 2006;23:1522–3
- Li ZR. Establishment of osteonecrosis and joint reconstruction center in Sino-Japan friendship Hospital. Chin J Bone Tumors Bone Dis 2004;3:M002
- Wang YS, Liu M, Zhang LF, Shen ZL, Wang SH, Yin L. The animal study of prevention of the alcohol-induced osteonecrosis of the femoral head in rabbit by the small interfering RNA for the target on specific gene. Chin J Exp Surg (Chin) 2011;28:611–14
- Wang YS, Li YB, Mao KY, Li J, Cui QJ, Wang GJ. Alcohol-induced adipogenesis in bone and marrow: a possible mechanism for osteonecrosis. Clin Orthop Relat Res 2003;410:213–24
- Wang YS, Wang XG, Li YB, Wang SH. Effects of RNAi for target on PPARγ to maintain osteogenic differentiation of BMSCs of rabbit inhibited by steroid. J Zhengzhou Univ (Med Sci) (Chin) 2009;44:275–9
- Mello CC, Conte D Jr. Revealing the world of RNA interference Nature 2004;431:338–42
- Gomes-da-Silva LC, Fonseca NA, Moura V, Pedroso de Lima MC, Simões S, Moreira JN. Lipid-based nanoparticles for siRNA delivery in cancer therapy: paradigms and challenges. Acc Chem Res 2012;45:1163–71
- Lambeth LS, Smith CA. Short hairpin RNA-mediated gene silencing. Methods Mol Biol 2013;942:205–32
- Sonwane AA, Dahiya SS, Saini M, Chaturvedi VK, Singh RP, Gupta PK. Inhibition of rabies virus multiplication by siRNA delivered through adenoviral vector in vitro in BHK-21 cells and in vivo in mice. Res Vet Sci 2012;93:498–503
- Wang L, Feng J, Da L, Li Y, Li Z, Zhao M. Adenovirus-mediated delivery of siRNA targeting TM4SF4 attenuated liver cancer cell growth in vitro and in vivo. Acta Biochim Biophys Sin 2013;45:213–19
- Wang YS, Li JF, Liu M, Zhao GQ, Hao LY, Li YB. Inhibition of peroxisome proliferator-activated receptor-γ in steroid-induced adipogenic differentiation of the bone marrow mesenchymal stem cells of rabbit using small interference RNA. Chin Med J (Engl) 2014;127:130–6
- Warner JJ, Philip JH, Brodsky GL, Thornhill TS. Studies of nontraumatic osteonecrosis. Manometric and histologic studies of the femoral head after chronic steroid treatment: an siRNA study in rabbits. Clin Orthop Relat Res 1987;225:128–40
- Wang GJ, Sweet DE, Reger SI, Thompson RC. Fat-cell changes as a mechanism of avascular necrosis of the femoral head in cortisone-treated rabbits. J Bone Joint Surg Am 1977;59:729–35
- Mont MA, Jones LC, Hungerford DS. Nontraumatic osteonecrosis of the femoral head: ten years later. J Bone Joint Surg Am 2006;88:1117–32
- Tan G, Kang PD, Pei FX. Glucocorticoids affect the metabolism of bone marrow stromal cells and lead to osteonecrosis of the femoral head: a review. Chin Med J (Engl) 2012;125:134–9
- Weinstein RS. Glucocorticoids, osteocytes, and skeletal fragility: the role of bone vascularity. Bone 2010;46:564–70
- Lee MS, Hsieh PH, Shih CH, Wang CJ. Non-traumatic osteonecrosis of the femoral head – from clinical to bench. Chang Gung Med J 2010;33:351–60
- Masada T, Iwakiri K, Oda Y, Kaneshiro Y, Iwaki H, Ohashi H, Takaoka K. Increased hepatic cytochrome P4503A activity decreases the risk of developing steroid-induced osteonecrosis in a rabbit model. J Orthop Res 2008;26:91–5
- Arlet J. Nontraumatic avascular necrosis of the femoral head: past, present, and future. Clin Orthop Relat Res 1992;277:12–21
- Hungerford DS, Lennox DW. The importance of increased intraosseous pressure in the development of osteonecrosis of the femoral head: implications for treatment. Orthop Clin North Am 1985;16:635–54
- Jones JP Jr. Alcoholism, hypercortisonism, fat embolism and osseous avascular necrosis. Clin Orthop Relat Res 2001;393:4–12
- Kawai K, Tamaki A, Hirohata K. Sreroid-induced accumulation of lipid in the osteocytes of the rabbit femoral head: a histochemical and electron microscopic study. J Bone Joint Surg Am 1985;67:755–63
- Mankin HJ. Nontraumatic necrosis of bone (osteonerosis). New Eng J Med 1992;326:1473–9
- Wang GJ, Dughman SS, Reger SI, Stamp WG. The effects of core decompression on femoral head blood flow in steroid-induced avascular necrosis of the femoral head. J Bone Joint Surg Am 1985;67:121–4
- Cheng T, Li YB, Wang YS. To establish the model of steroid-induced osteonecrosis of femoral head of chicken treated with large dose steroid during short-term. J Zhengzhou Univ (Med Sci) (Chin) 2009;44:285–7
- Cui Q, Wang GJ, Balian G. Steroid-induced adipogensis in a pluripotential cell from bone marrow. J Bone Joint Surg Am 1997;79:1054–63
- Jacobs B. Epidemiology of traumatic and nontraumatic osteonecrosis. Clin Orthop Relat Res 1978;130:51–67
- Jones JP Jr. Intravascular coagulation and osteonecrosis. Clin Orthop Relat Res 1992;277:41–53
- Turner RT, Evans GL, Zhang M, Sibonga JD. Effects of parathyroid hormone on bone formation in a rat model for chronic alcohol abuse. Alcohol Clin Exp Res 2001;25:667–71
- Wang Y, Mao K, Li Y, Xu Z. The development and observation of animal model of the femoral head necrosis induced by alcohol. Chin J Exper Surg (Chin) 1998;15:182–3
- Wang YS, Dou XF Tie WB. Make an animal model of alcohol-induced osteonecrosis of femoral head of cock on the sensitized condition with horse-serum. Chin J Exper Surg (Chin) 2009;26:1077–9
- Suh KT, Kim SW, Roh HL, Youn MS, Jung JS. Decreased osteogenic differentiation of mesenchymal stem cells in alcohol-induced osteonecrosis. Clin Orthop Relat Res 2005;431:220–5
- Wu XJ, Yin WL, Li YB, Wang YS. Effects of alcohol on mRNA expression of PPARγ and osteocalcin in human bone marrow mesenchymal stem cells. J Zhengzhou Univ (Med Sci) (Chin) 2006;41:1098–100
- Bianco P, Robey PG. Marrow stromal stem cells. J Clin Invest 2000;105:1663–8
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 1997;276:71–4
- Li J, Wang YS, Li YB, Xu JZ, Xiong TB. Alcohol-induced regulation of adipogenic and osteogenic gene expression of marrow stromal cells. Chin Orthop J (Chin) 2003;23:493–5
- Yin L, Li YB, Wang YS. Dexamethasone-induced adipogenesis in primary marrow stromal cell cultures: mechanism of steroid-induced osteonecrosis. Chin Med J (Engl) 2006;119:581–8
- Li X, Cui Q, Kao C, Wang GJ, Balian G. Lovastatin inhibits adi-pogenic and stimulates osteogenic differentiation by suppressing PPARgamma2 and increasing Cbfa1/Runx2 expression in bone marrow mesenchymal cell cultures. Bone 2003;33:652–9
- Lowell BB. PPARgamma: an essential regulator of adipogenesis and modulator of fat cell function. Cell 1999;99:239–42
- Smas CM, Sul HS. Control of adipocyte differentiation. Biochem J 1995;309:697–710
- Nie B, Shen Z, Wen JB, Wong OG, Hsueh WD, Huo LF, Kung HF, Jiang B, Lin MC. AAV-HGFK1 and Ad-p53 cocktail therapy prolongs survival of mice with colon cancer. Mol Cancer Ther 2008;7:2855–65
- Huang Q, Zhang H, Pei FX, Chen ZY, Wang GL, Shen B, Yang J, Zhou ZK, Kong QQ. Use of small interfering ribonucleic acids to inhibit the adipogenic effect of alcohol on human bone marrow-derived mesenchymal cells. Int Orthop 2010;34:1059–68
- Stephan AV, Kelly KH. Adenoviral gene therapy. Oncologist 2002;7:46–59
- Wang YS, Wu XJ, Zhao GQ. Progression of RNA interference application. Henan Med Res (Chin) 2007;16:268–77
- Wang YS, Wang SH, Li YB, Zhao GQ, Liu M, Wang XG. Interfere effect of siRNA adenovirus vector to adipogenic differentiation in marrow stromal cells of rabbit. Chin J Exper Surg (Chin) 2009;26:519–21
