Abstract
Increasing evidence points to extracellular matrix (ECM) components playing integral roles in regulating the muscle satellite cell (SC) niche. Even small alterations to the niche ECM can have profound effects on SC localization, activation, self-renewal, proliferation and differentiation. This review will focus on the ECM components that comprise the niche, how they are modulated in health and disease and how these changes are thought to affect SC function. Particular emphasis will be placed on the pathological niche and interventions that aim to restore healthy structure and function, as a better understanding of the interplay between the SC and its environment will drive more targeted and effective therapies.
Introduction
Stem cells live in a specialized microenvironment in tissues, commonly referred to as the “niche”. However, the niche is much more than an anatomical location; it is a dynamic circuit board transmitting mechanical and chemical signals that continuously relay the status and requirements of the tissue to its regenerative cell source. It protects the inactive, quiescent stem cell population from depletion and conveys signals for activation, proliferation and differentiation in response to tissue damage. In fact, the interplay between the stem cell and its niche is so important that alterations to components of the niche have been shown to result in defective regeneration in nearly every stem cell compartment in the body (Citation1).
A growing body of evidence supports extracellular matrix (ECM) components as essential signal mediators in the niche, both for maintaining stem cell identity and regulating activation. One of the major roles of this ECM is to provide structural integrity to the niche, physically separating the stem cell pool from other tissue resident cells and interstitial matrix (Citation2). However, it also plays a role in localizing molecules such as growth factors and glycoproteins that regulate the balance between activation and quiescence. Stem cells are also able to sense and respond to the composition, porosity and stiffness of the ECM in their niche as they directly interact with it through focal adhesions (Citation3–5). Though the multifaceted 3D nature of the in vivo niche makes it difficult to isolate individual regulators, experiments using engineered niches have demonstrated that variations in the ECM, in absence of compounding factors, are capable of influencing the proliferation (Citation6), migration (Citation7,Citation8) and differentiation of stem cells (Citation4,Citation9). More recently, matrix cues have been examined in combination, in 3D and with growth factors, all of which complicate the niche and do not necessarily have additive effects (Citation3,Citation10).
Understanding how environmental factors modulate tissue regeneration is critical for successful strategies in regenerative medicine, and this is particularly evident in skeletal muscle in conditions that cause high tissue turnover and poor regeneration, e.g. muscular dystrophy. Healthy skeletal muscle is one of the most adaptive and regenerative tissues in the adult body. Its regeneration capacity is so robust that, following widespread destruction of its myofibers by experimental myotoxin injection (Citation11), mechanical crush (Citation12), prolonged freeze injury (Citation13) or ex vivo mincing and replacement (Citation14), a muscle is able to regain near normal morphology and force production in a matter of weeks. The primary source for regeneration in skeletal muscle is the satellite cell. These cells reside in a distinct niche and express a unique panel of surface markers and transcription factors, making them relatively easy to identify, target and isolate. This fact, combined with the spectrum of muscle-specific transgenic animals, the variety of muscle injury models and access to patient biopsies make the satellite cell niche a unique model system to study ECM regulation and cell–matrix interactions. A better understanding of the role of the ECM in regulating satellite cell function will provide new directions and targets for therapies aimed at improving muscle regeneration and tissue regeneration as a whole; this forms the focus of our review below, specifically introducing the satellite cell, its niche, how it is activated and how it responds in disease models.
The satellite cell niche
The satellite cell (SC) is an undifferentiated, unipotent muscle progenitor that resides within the basal lamina adjacent to the plasma membrane of a muscle fiber (). The majority of SCs are quiescent, but in response to increases in loading or tissue damage, SCs become activated and begin to divide. A fraction of these activated SCs will continue to proliferate and migrate as myoblasts before terminally differentiating and fusing into muscle fibers (Citation15). Though they share many behavioral characteristics, quiescent SCs, activated SCs and myoblasts are transcriptionally and functionally distinct. Much of our understanding of SC behavior has come about from animal models, but considerable inter-species variability in tissue composition and cell behavior may lead to species-to-species differences. Therefore, care will be taken in this discussion to differentiate in vitro from in vivo studies and those in animal models from those in humans.
Figure 1. Schematic diagram of the satellite cell (SC) niche. SCs reside between the basal lamina (BL) and the muscle fiber sarcolemma where they interact with matrix components of the niche. Through integrins, SCs bind to collagen type IV and laminin. The ECM protein nidogen helps cross-link these two components into a matrix. They in turn bind to collagen type VI and several proteoglycans including perlecan and decorin. Collagen type VI integrates the BL with the reticular lamina composed primarily of collagen types I and III and fibronectin. On the other side of the SC niche, the muscle fiber sarcolemma links to the BL through the dystroglycan complex, which binds to the actin cytoskeleton thorough dystrophin and to laminin in the BL.
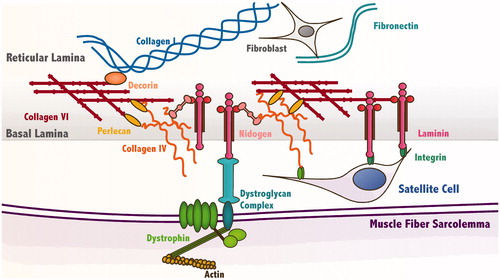
The ECM surrounding muscle fibers is composed of collagens, laminins, fibronectin and glycosaminoglycans (GAGs), short polysaccharide chains which bind to a protein core to form proteoglycans (Citation16). This matrix is known as the basement membrane (BM), and has two layers, the basal lamina (BL) and the reticular lamina. SCs reside in the BL, whose two primary constituents, collagen type IV and laminin-2 (α2, β1 and γ1 chains), assemble into two cross-linked networks, which are then linked by the glycoprotein nidogen. The concentration of these two components of the BL varies as a function of muscle fiber type. The predominately slow Soleus muscle of adult rats has twice the concentration of collagen IV and less than half the concentration of laminin-2 in the BL compared with the predominately fast rectus femoris (Citation17). Interestingly, the concentration of SCs in slow rat muscle fibers is also double what is measured in fast muscle fibers (Citation18). Whether or how this is related to the differences in the ECM of the niche is unknown.
In addition to collagen IV and laminin-2, there are several other critical components of BM worth noting. Perlecan, a heparin sulfate proteoglycan, as well as decorin and biglycan, SLRP proteoglycans, are distributed throughout the BM (Citation19). These negatively charged proteoglycans bind and sequester a variety of growth factors, giving them both structural and signaling roles in the BM. Perlecan binds to collagen type IV and laminin-2 in the BL while decorin binds to collagen type I in the reticular lamina. In addition to collagen type IV, the BL also contains collagen type VI, which connects the BL to the reticular lamina. Fibronectin is primarily localized to the reticular lamina, with which SCs are typically not in contact, but may be transiently expressed and localized to the BL during regeneration (Citation20). The BL is also linked to the cytoskeleton of the muscle fiber at repeating assemblies of proteins called dystroglycan complexes (DGC). Within the muscle fiber, dystrophin links actin to the DGC, and in the basal lamina β-dystroglycan then binds to laminin. This integration further stabilizes the structure of the SC niche.
Cells adhere to the ECM not only for structural stability but also for signaling, beginning with integrins, a family of cell surface receptors which bind to ECM proteins and from which focal adhesions form. Assembled within these structures are proteins that both serve structural and force-sensitive signaling roles, e.g. vinculin (Citation21); these proteins also allow for so called “outside-in” signaling to relay outside conditions to inside the cell (Citation22). Integrins α7 and β1 are the major isoforms expressed by SCs and together form a receptor complex that binds to laminin-2 in the BL (Citation23). However, integrin expression varies as a function of the activation state of SCs. Activated, but not quiescent, mouse satellite cells express integrin β3 which likely complexes with integrin αv to form a receptor for proteins bearing an exposed Arg–Gly–Asp (RGD) tripeptide including fibronectin, osteopontin and some degraded laminins and collagens (Citation24). Activated human myoblasts also express integrin α5 in vitro which, in combination with β1, is a receptor for fibronectin (Citation25). The temporal variation of integrin expression in SCs suggests that they may have unique regulatory roles in muscle, some promoting the initiation of myogenesis and some maintaining homeostasis.
Remodeling of the niche
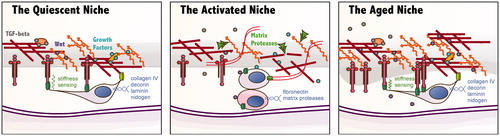
Muscle BM substantially remodels during regeneration post-injury (transition from left to center panels in ). Experimentally controlled ECM remodeling has demonstrated a critical role for modifications in restoring strength and morphology following injury (Citation26). Focal muscle damage frequently involves an initial insult to the BM followed by further degradation by proteases. ECM fragments and growth factors that are released during the process of matrix degradation play a critical role in the migration and homing of inflammatory, endothelial and myogenic cell types. Furthermore, the reconstruction of the damaged matrix can scavenge these same factors, signaling the end of cellular infiltration and differentiation. Transcriptional profiles conducted using a cardiotoxin injury rodent model show a consistent temporal pattern of gene expression marked by a peak in expression of matrix degrading enzymes followed by upregulation of numerous ECM components of the BL (Citation27,Citation28). This is a dynamic process involving the interplay between a variety of cell types in and around the SC niche.
Figure 2. Schematic diagrams illustrating some differences between the quiescent, activated and aged satellite cell (SC) niche. (Far left) The quiescent SC senses the stiffness of its niche through integrins and expresses various matrix proteins to maintain its extracellular matrix (ECM). Within this matrix, growth factors and signaling molecules such as Wnts and TGF-β are sequestered, maintaining the “quiet” state. (Center) In response to injury, components of the basal lamina are degraded by matrix proteases which results in the release of signaling molecules that play a role in activation and proliferation of the SC. The activated SC divides and some daughter cells begin to differentiate. (Far right) In the aged niche, matrix components accumulate to form a denser and thicker basal lamina. The stiffness sensing and sequestration of signaling molecules may be affected by this change.
The major enzymes responsible for the physiological breakdown of ECM are matrix metalloproteinases (MMPs), which work in tandem with the urokinase plasminogen activator. Two isoforms, MMP2 and MMP9, target collagen type IV and laminin in the BL and are significantly upregulated during muscle regeneration (Citation29,Citation30). Cultured human myoblasts constitutively synthesize and secrete MMP2 and the urokinase plasminogen activator and can be induced to secrete MMP9 (Citation31,Citation32). This is consistent with in vivo data from a regenerating mouse model, localizing expression of MMP2 and MMP9 to activated SCs (Citation33,Citation34). This data suggests that SCs are involved in the breakdown of their own niche, allowing them to leave the niche and migrate to the site of injury.
Resident muscle fibroblasts are considered to be the main contributor to the ECM of skeletal muscle. In addition to secreting the primary fibrous collagens found in the interstitial connective tissue (types I and III), fibroblasts have also been shown to secrete the major basal lamina collagens (types IV and VI) (Citation33,Citation34). Zou et al. (Citation34) took advantage of a mutation to the collagen type VI gene which caused the protein to be synthesized, but not secreted by cells. They observed large significant collagen VI staining in interstitial fibroblasts and an absence of positive staining in myogenic cells suggesting that fibroblasts are the major, if not the only, contributor of collagen VI in human skeletal muscle.
However, fibroblasts are not the only contributor to the SC niche ECM. In addition to participating in the degradation of their surrounding ECM, SCs also secrete a variety of BL components in addition to MMPs. Cross-species co-cultures of mouse-derived fibroblasts with quail-derived myoblasts show collagen IV incorporation into the BL of myotubes of both mouse and quail origin (Citation33), suggesting that both myogenic cells and fibroblasts contribute collagen IV to the SC niche. Studies in vitro and in vivo have also demonstrated expression and secretion of laminin and fibronectin by mouse myoblasts (Citation35,Citation36). In fact, Bentzinger et al. (Citation36) demonstrated that not only do proliferating SCs express fibronectin, but expression by SCs is important for efficient regeneration. In this study, fibronectin knockdown in SCs decreased engraftment efficiency upon injection into recipient mice, demonstrating that SC-derived fibronectin specifically is important for SC engraftment and function. ECM proteins collagen IV, decorin, perlecan, laminin chains α2 and γ1, and nidogen are more highly expressed in quiescent mouse SCs compared with activated SCs (Citation36,Citation37). These data provide evidence that quiescent SCs may reside in a different niche than either activated or proliferating SCs and that they may require a complex combination of ECM proteins for optimal function and survival. It should also be noted that the time scale for ECM assembly is >24–48 h (Citation38) and thus most of the in vitro data examining adhesion may not observe the same differential effects as longer term or in vivo assays. Regardless of amount, assembly state and composition, the field is emerging around the concept that matrix does indeed establish and control a niche and the stem cells within it.
ECM regulation of SC quiescence and activation
One of the most important functions of the progenitor/stem cell niche is maintaining the balance between quiescence and activation. When proliferation is inhibited, tissue regeneration is severely blunted. On the other hand, overexuberant proliferation of SCs would lead to overpopulation of the niche and potential tumorigenesis. Interactions with the ECM have been suggested to regulate the switch between symmetric and asymmetric division in a variety of stem cell niches (Citation39). However, BL ECM is an intricate composite with a variety of matrix components and complex geometry. Determining the component or combination of components that control cellular behavior is difficult.
In order to study cell–matrix interactions in a controlled environment, many researchers have turned to in vitro cell culture models where specific components or attributes of the ECM can be easily manipulated. Unfortunately, one of the strongest pieces of evidence for the SC niche being involved in the maintenance of quiescence is that when SCs are removed from their niche, they quickly withdraw from quiescence, enter the cell cycle and lose their myogenic properties (Citation6). However, several studies have identified matrix cues that promote the maintenance of quiescence in vitro. When cultured on various ECM coatings, a larger fraction of freshly isolated murine and porcine SCs were shown to express two transcriptional markers of quiescence, Pax7 and Pax3, in response to matrigel (a purified BM secreted by murine Engelbreth–Holm–Swarm tumor cells) or laminin compared with collagen I, gelatin or fibronectin (Citation40,Citation41). Also, when grown on matrigel with the addition of native collagen VI, a greater percentage of murine SCs expressed Pax7 than in cultures without collagen VI (Citation42). Furthermore, when collagen VI knockout fibers were cultured on collagen VI plus matrigel, SCs were better able to maintain Pax7 expression compared with matrigel alone. Although Matrigel is a heterogeneous mixture of ECM proteins and growth factors, several of its major constituents, including laminin, are primary components of the skeletal muscle BL. Taken together, these data suggest that the ECM composition of the BL may play a role in maintaining SC quiescence in vivo.
In addition to ligand cues, the stiffness of the culture substrate influences SC quiescence. Gilbert et al. (Citation6) cultured freshly isolated mouse SCs on tunable polyethylene glycol (PEG) hydrogels of different elastic moduli, and found greater SC survival and expression of Pax7 on hydrogels that approximate the physiological stiffness of muscle. Furthermore, when these cells were injected back into regenerating mouse muscle, the SCs grown on the hydrogels of muscle-like stiffness were able to repopulate the native niche at rates similar to freshly isolated SCs. This ability is rapidly lost in SCs cultured on stiff tissue culture plastic. Furthermore, culturing aged mouse SCs on soft hydrogel substrates was shown to improve their functional capacity when transplanted into recipient muscle (Citation43). These data underscore the importance of understanding and mimicking the niche in moving forward with tissue engineered and cell-based therapies that rely on extensive culture periods.
Symmetric division, in which a SC divides into two identical daughter cells, and asymmetric division, in which one of the progeny remains a SC and the other daughter cell differentiates, are critical for balancing maintenance of the SC pool with the need for muscle repair (Citation44). Emerging evidence has pointed to the importance of the SC niche as a regulator of symmetric and asymmetric division. Polarity achieved through exposure to the basal lamina versus the apical side of the SC niche has been established as a driver of asymmetric division (Citation45). The apical side of the SC expresses m-cadherin receptors, which allow the cell to interact with the muscle fiber, whereas the basal side expresses the laminin receptor integrin α7β1. Studies in mice have demonstrated apical-basal oriented SC divisions where the daughter cell that remains in contact with the BL remains to repopulate the niche while the daughter cell closest to the fiber differentiates (Citation46).
Fibronectin as an individual ECM protein component of the SC niche is critical for maintenance of the SC pool. Knockdown of fibronectin in mouse SCs leads to a drop in symmetric cell division in SCs, reducing the fraction of Pax 7 positive cells in vitro (Citation36). The regulation of symmetric cell division by fibronectin is achieved through the interaction of the fibronectin receptor syndecan-4 and the Wnt7a receptor frizzled-7. Collagen VI is another ECM component shown to be important in maintenance of the SC pool. In mouse muscle lacking collagen VI the SC pool is depleted, as SCs fail to sufficiently self-renew (Citation42). Furthermore, this defect was able to be rescued by injecting collagen VI expressing fibroblasts into the affected muscle indicating that the absence of this ECM protein, as opposed to a SC intrinsic defect, was driving the aberrant SC behavior. In addition to the fibrous matrix components, proteoglycans in the muscle BL play a role in regulating SC behavior. Heparan sulfate proteoglycans syndecan-3 and syndecan-4 have been shown to regulate SC activation and proliferation as SC self-renewal is impaired in mouse knockout muscle (Citation47). Furthermore, matrix resident proteoglycans such as perlecan and decorin can bind signaling molecules such as Notch and Wnts, known to influence SC asymmetric/symmetric division (Citation48). Thus, it is evident that the interactions between ECM proteins and cell surface receptors are necessary for striking the balance between differentiation and self-renewal, and that further research is needed to examine the synergistic effects of multiple ECM proteins present in the SC niche.
ECM regulation of differentiation
The ability of SCs to repair damaged muscle hinges on their capacity to differentiate and fuse into myofibers. Thus, there is great interest in determining the factors that promote SC differentiation both in vitro and in vivo. ECM factors regulating myogenic differentiation include specific ECM ligands, soluble growth factors sequestered within the matrix and the physical properties of the matrix itself.
Differentiation of SCs involves a temporal sequence of transcription factor expression, notably myf-5, myoD, desmin and myogenin, followed by fusion with neighboring myoblasts or with existing myotubes. Each of these steps is affected by the ECM in some fashion. Primary mouse myoblasts cultured on substrates of gelatin, Matrigel, laminin, fibronectin, collagen I or collagen IV fused most robustly on Matrigel (Citation40,Citation49). Similarly, porcine myoblasts cultured on substrates of collagen type I, gelatin, fibronectin, Matrigel and laminin (Citation41) expressed the late myogenic marker, myogenin, at lowest levels on collagen type I and at the highest level on Matrigel. Thus, it is evident that some constituents of Matrigel possess myogenic properties, and that single substrates are insufficient to promote the levels of myogenic differentiation seen with Matrigel; however, as the exact composition of Matrigel is undefined, the factors involved in SC proliferation and differentiation remain unclear.
To further elucidate the in vivo ECM components ideal for SC culture in vitro, murine SCs were cultured on an enactin–laminin–collagen (ECL) substrate, collagen IV, poly-d-lysine and laminin (Citation50,Citation51). Higher myotube fusion rates were observed on poly-d-lysine or laminin than on collagen IV or ECL. However, ECL substrates in combination with glycosaminoglycans (GAGs) promoted myotube fusion better than ECL, GAGs, collagen type I or laminin single substrates (Citation33,Citation52). Thus, GAGs, already known to be important components of the ECM, are also an important component of the SC niche. However, as ECL substrate and GAGs are both heterogeneous mixtures of numerous proteins, they are still undefined substrates in these culture systems, making it difficult to distinguish the contributions of individual proteins to the SC niche. Further research is needed to disentangle the combinatorial effects of multiple ECM proteins on SC culture and to determine which components of Matrigel and ECL are instrumental in SC differentiation.
In addition to playing a role in regulating SC proliferation, proteoglycans participate in SC differentiation. Heparan sulfate proteoglycans (HSPGs) interact with a large number of growth factors in the muscle BL including insulin-like growth factor (IGF), fibrobast growth factor (FGF), hepatocyte growth factor (HGF) and transforming growth factor beta (TGF-β), all known to influence SC proliferation and differentiation (Citation53,Citation54). HSPGs can increase the local concentration of growth factors, or sequester them away from cells and even participate in their function by complexing with them, dramatically affecting the local environment and driving cell behavior.
The mechanical properties of the in vitro culture environment have also been shown to affect myoblast differentiation and fusion. Engler et al. (Citation55) induced fusion in immortalized mouse myoblasts on polyacrylamide gels of varying stiffness and found maximal myosin heavy chain striation, a marker of myotube maturity, on gels of muscle-like stiffness (∼11 kPa). Similarly, primary mouse SCs cultured on tunable PEG hydrogels of muscle-like stiffness (∼12 kPa) exhibited significantly greater engraftment efficiency upon injection into recipient mice than SCs cultured on tissue culture plastic (Citation6). These data not only underscore the need to mimic the mechanics of the in vivo environment in culture models, but also suggest that the progressive stiffening of the ECM that is a feature of many muscle diseases may have a significant negative impact on the SC population.
Pathological alterations to the SC niche
Under ideal conditions, damaged muscle would be completely replaced with fresh, healthy contractile fibers, resulting in a full recovery of force production and function. This is usually the case. Bouts of high intensity or unaccustomed exercise cause microtears, or disruptions to the sarcomeric structure, followed by an inflammatory response and ultimate reconstruction of the damaged area, frequently with gains in muscle mass and strength. However, the ability of the muscle to respond to such cues for regeneration and growth can be dramatically affected by aging and disease. Both of these processes are typically characterized by pathological changes to muscle ECM, including increased deposition, density and stiffness (Citation26,Citation56). Though alterations to the BL have received less focus than those to the interstitial matrix, significant changes to BL components have also been noted as a function of age and disease ( far right).
Aging
The regenerative potential of skeletal muscle declines significantly with age. Studies in human muscle have come to conflicting conclusions about whether this decline is associated with intrinsic changes to the SC pool such as a reduction in SC numbers or proliferative capacity (Citation57,Citation58). However, studies that expose aged SCs to a young environment, either in vitro or by heterochronic parabiosis in vivo suggest that at least some of the deficit is environmental (Citation59,Citation60). Studies examining the basal lamina of aged muscle and other tissues have demonstrated a pronounced thickening with a loss of laminated structure, becoming irregular and amorphous (Citation61,Citation62). Collagen IV concentration increases preferentially in slow muscles with age, while laminin increases preferentially in fast muscles (Citation17). This could affect the ability of the BL to store and release growth factors and other signaling molecules involved in maintaining the niche. Increased concentration of the glycoprotein osteopontin has been documented in the BL of mouse muscle with age (Citation63). This cytokine negatively regulates myoblast differentiation in vitro and muscle regeneration in vivo. Increased levels of other matrix associated negative regulators of myogenesis such as TGF-β and Wnt have also been documented in the aged SC niche (Citation64,Citation65).
Disease
A striking number of primary myopathies originate in mutations to components or to proteins that bind to components of the BL (Citation66). The most common and well studied of these are the muscular dystrophies, a heterogeneous group of inherited progressive disorders characterized by pronounced muscle weakness, fibrosis and fatty infiltration. The most prevalent dystrophy is Duchenne Muscular Dystrophy (DMD), arising from a mutation in the gene encoding dystrophin, which participates in the linkage between the muscle fiber cytoskeleton and the BL. Loss of this integration is thought to cause destabilization of the fiber membrane resulting in repeated cycles of degeneration and regeneration, which eventually exhaust the regenerative potential of the SC pool.
Pathological alterations are seen in the BL of muscle from DMD patients including decreased accumulation of laminin α2 and β1 and increased accumulation of collagen IV (Citation67). This is consistent with gene expression studies showing lower expression of laminin α2 (Citation68), higher expression of collagen IV (Citation69) and higher expression of integrin α7 (Citation70) in primary DMD myoblast cultures. In addition to changes in laminin and collagen IV, gene expression studies also show increased expression of TGF-β and osteopontin, pro-fibrotic cytokines that inhibit myogenesis (Citation71). Interestingly, osteopontin was recently identified as determining factor of disease severity in DMD patients, with lower levels of osteopontin correlated with greater weakness and earlier loss of ambulation (Citation72).
In addition to DMD, there are a variety of less well-known dystrophies resulting from mutations to laminin (congenital muscular dystrophy type 1A), collagen IV (Walker–Warburg syndrome), collagen VI (Ulrich congenital muscular dystrophy and Bethlem myopathy) and components of the dystroglycan complex (dystroglycanopathies). These disorders are all characterized by progressive muscle weakness, from mild to debilitating depending on severity, and frequently exhibit disruptions to the BM. Studies in mice lacking collagen VI, the mouse model for Bethlem myopathy, demonstrate a significant reduction in muscle stiffness, impaired muscle regeneration and a progressive depletion of the SC pool (Citation42). Interestingly, this effect could be rescued by the transplantation of collagen VI expressing wild-type fibroblasts, which, within 12 days resulted in significant increases in collagen VI deposition, muscle stiffness and SC concentration. This suggests that modulation of the SC niche could have a positive effect on SCs even after long-term exposure to a diseased environment. Understanding the remodeling that occurs in the SC niche with the progression of muscle disease is critically important for therapies targeting the SC niche – both those that seek to repopulate it with exogenous cells and those that seek to modify the behavior of the resident SCs.
Therapeutic implications
A large quantity of data has now been collected pointing to the critical role the BM plays in the maintenance of muscle integrity. In all of the dystrophies described above, muscle develops normally, but then progressively degenerates as a result of the loss of some reticular lamina – BL – cytoskeleton linkage. This makes these disorders excellent candidates for interventional regenerative therapies. A variety of therapeutic approaches are currently under investigation to either target or supplement the SC population, and some of the most promising involve the creation of tissue engineered niches mimicking those in healthy muscles.
As discussed above, the ECM composition and stiffness of the substrate on which SCs are grown in culture can have a dramatic effect on their ability to fuse into myotubes in vitro as outlined below. A natural next step for this finding is to use these model culture systems to “condition” cells for engraftment prior to injection into muscle, a strategy that has already shown promise in mice (Citation6). Taking this one step further, the engineered niche environment could be injected or implanted with the SCs to further promote survival and engraftment.
Extracellular matrix scaffolds have shown considerable promise in the repair or replacement of a variety of diseased tissues, including muscle. When cross-sections of the rat abdominal wall were reconstructed with porcine-derived ECM constructs, force production and fatigue resistance was returned to native tissue levels, compared with polypropylene mesh reconstruction which was unable to improve muscle function (Citation73). Even though these constructs were acellular at the time of implantation, they promoted cellular infiltration of the injured area, resulting in the formation of new muscle fibers within the scaffold. Differences in regenerative efficiency have been noted for scaffolds of different materials highlighting the need for a thorough understanding of the signaling effects of different ECM proteins on SC activation, migration and differentiation (Citation73,Citation74).
Decellularized matrix may also be milled and lyophilized into a powder, which can then be reconstituted and injected for minimally invasive applications. One such material is matrigel, which will form a solid gel in response to physiological temperatures. As discussed above, matrigel promotes maintenance of the SC pool and myoblast fusion in vitro making it an excellent candidate for this type of therapy. Other ECM components have also shown therapeutic benefit when injected into or overexpressed in dystrophic mouse muscle. Rooney et al. (Citation75) showed that injection of purified laminin α1β1γ1 into the tibialis anterior muscles of α7 integrin knockout mice improves regeneration and ameliorates satellite cell proliferation and differentiation deficits. Similarly, systematically delivered human recombinant biglycan reduces the pathology in the mouse model for Duchenne Muscular Dystrophy (Citation76). Furthermore, injectable hydrogels derived from skeletal muscle matrices have been shown to promote infiltration of muscle progenitors and tissue repair in a rat hindlimb ischemia model over those composed only of collagen, highlighting the importance of recapitulating the native ECM properties in artificial constructs (Citation77).
Extracellular matrix-mimicking hydrogels have the advantage that their shape and material properties can be precisely controlled. As discussed above, in addition to responding to ligand cues, SCs are sensitive to the stiffness of their environment. Hydrogels have been developed with precisely tunable mechanical properties (Citation8,Citation78) even some with stiffness that changes temporally to mimic the changes seen in developing or regenerating tissue (Citation79). Furthermore, hydrogels can be designed to mimic complex 3D environments using 3D printing, enabling precise arrangement of cells and growth factors (Citation80).
Conclusions
The field of tissue engineering and regenerative medicine is expanding fast. Though our understanding of native tissue regeneration sometimes lags behind our ability to engineer novel and intricate tools, it is becoming increasingly clear that the success of the latter will depend heavily on the former. Therapeutic applications for muscle disease will require an understanding of the biology of the SC, the composition of its niche, the interactions between the two and how all of these things change as a function of age and disease. This will enable the application of optimal environmental cues to generate a precisely targeted and efficient regenerative response.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Jones DL, Wagers AJ. No place like home: anatomy and function of the stem cell niche. Nat Rev Mol Cell Biol 2008;9:11–21
- Hay ED. Cell biology of the extracellular matrix. 2nd ed. New York: Plenum Press; 1991
- Flaim CJ, Chien S, Bhatia SN. An extracellular matrix microarray for probing cellular differentiation. Nat Methods 2005;2:119–25
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell 2006;126:677–89
- Khetan S, Burdick JA. Patterning network structure to spatially control cellular remodeling and stem cell fate within 3-dimensional hydrogels. Biomaterials 2010;31:8228–34
- Gilbert PM, Havenstrite KL, Magnusson KEG, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 2010;329:1078–81
- Raab M, Swift J, Dingal PCDP, Shah P, Shin J-W, Discher DE. Crawling from soft to stiff matrix polarizes the cytoskeleton and phosphoregulates myosin-II heavy chain. J Cell Biol 2012;199:669–83
- Vincent LG, Choi YS, Alonso-Latorre B, Del Alamo JC, Engler AJ. Mesenchymal stem cell durotaxis depends on substrate stiffness gradient strength. Biotechnol J 2013;8:472–84
- Saha K, Keung AJ, Irwin EF, Li Y, Little L, Schaffer DV, Healy KE. Substrate modulus directs neural stem cell behavior. Biophys J 2008;95:4426–38
- Rowlands AS, George PA, Cooper-White JJ. Directing osteogenic and myogenic differentiation of MSCs: interplay of stiffness and adhesive ligand presentation. Am J Physiol Cell Physiol 2008;295:C1037–44
- Vignaud A, Hourdé C, Butler-Browne G, Ferry A. Differential recovery of neuromuscular function after nerve/muscle injury induced by crude venom fromNotechis scutatus, cardiotoxin fromNaja atraand bupivacaine treatments in mice. Neurosci Res 2007;58:317–23
- Fink E, Fortin D, Serrurier B, Ventura-Clapier R, Bigard AX. Recovery of contractile and metabolic phenotypes in regenerating slow muscle after notexin-induced or crush injury. J Muscle Res Cell Motil 2003;24:421–9
- Warren GL, Hulderman T, Jensen N, McKinstry M, Mishra M, Luster MI, Simeonova PP. Physiological role of tumor necrosis factor alpha in traumatic muscle injury. FASEB J 2002;16:1630–2
- Carlson BM, Gutmann E. Development of contractile properties of minced muscle regenerates in the rat. Exp Neurol 1972;36:239–49
- Seale P, Rudnicki MA. A new look at the origin, function, and “stem-cell” status of muscle satellite cells. Dev Biol 2000;218:115–24
- Sanes JR. The basement membrane/basal lamina of skeletal muscle. J Biol Chem 2003;278:12601–4
- Kovanen V, Suominen H, Risteli J, Risteli L. Type IV collagen and laminin in slow and fast skeletal muscle in rats – effects of age and life-time endurance training. Coll Relat Res 1988;8:145–53
- Schultz E. A quantitative study of satellite cells in regenerated soleus and extensor digitorum longus muscles. Anat Rec 1984;208:501–6
- Thorsteinsdóttir S, Deries M, Cachaço AS, Bajanca F. The extracellular matrix dimension of skeletal muscle development. Dev Biol 2011;354:191–207
- Sanes JR. Laminin, fibronectin, and collagen in synaptic and extrasynaptic portions of muscle fiber basement membrane. J Cell Biol 1982;93:442–51
- Holle AW, Engler AJ. More than a feeling: discovering, understanding, and influencing mechanosensing pathways. Curr Opin Biotechnol 2011;22:648–54
- Harburger DS, Calderwood DA. Integrin signalling at a glance. J Cell Sci 2009;122:159–63
- Blanco-Bose WE, Yao CC, Kramer RH. Purification of mouse primary myoblasts based on α7 integrin expression. Exp Cell Res 2001;265:212–20
- Liu H, Niu A, Chen S-E, Li Y-P. Beta3-integrin mediates satellite cell differentiation in regenerating mouse muscle. FASEB J 2011;25:1914–21
- Blaschuk KL, Holland PC. The regulation of α 5 β 1 integrin expression in human muscle cells. Dev Biol 1994;164:475–83
- Serrano AL, Muñoz-Cánoves P. Regulation and dysregulation of fibrosis in skeletal muscle. Exp Cell Res 2010;316:3050–8
- Goetsch SC, Hawke TJ, Gallardo TD, Richardson JA, Garry DJ. Transcriptional profiling and regulation of the extracellular matrix during muscle regeneration. Physiol Genomics 2003;14:261–71
- Kherif S, Lafuma C, Dehaupas M, Lachkar S, Fournier JG, Verdière-Sahuqué M, Fardeau M, Alameddine HS. Expression of matrix metalloproteinases 2 and 9 in regenerating skeletal muscle: a study in experimentally injured and mdx muscles. Dev Biol 1999;205:158–70
- Koskinen SO, Wang W, Ahtikoski AM, Kjaer M, Han XY, Komulainen J, Kovanen V, Takala TE. Acute exercise induced changes in rat skeletal muscle mRNAs and proteins regulating type IV collagen content. Am J Physiol Regul Integr Comp Physiol 2001;280:R1292–300
- Lewis MP, Tippett HL, Sinanan AC, Morgan MJ, Hunt NP. Gelatinase-B (matrix metalloproteinase-9; MMP-9) secretion is involved in the migratory phase of human and murine muscle cell cultures. J Muscle Res Cell Motil 2000;21:223–33
- Guérin CW, Holland PC. Synthesis and secretion of matrix-degrading metalloproteases by human skeletal muscle satellite cells. Dev Dyn 1995;202:91–9
- Fibbi G, Barletta E, Dini G, Del Rosso A, Pucci M, Cerletti M, Del Rosso M. Cell invasion is affected by differential expression of the urokinase plasminogen activator/urokinase plasminogen activator receptor system in muscle satellite cells from normal and dystrophic patients. Lab Invest 2001;81:27–39
- Kühl U, Ocalan M, Timpl R, Mayne R, Hay E, Mark, von der K. Role of muscle fibroblasts in the deposition of type-IV collagen in the basal lamina of myotubes. Differentiation 1984;28:164–72
- Zou Y, Zhang R-Z, Sabatelli P, Chu M-L, Bönnemann CG. Muscle interstitial fibroblasts are the main source of collagen VI synthesis in skeletal muscle: implications for congenital muscular dystrophy types Ullrich and Bethlem. J Neuropathol Exp Neurol 2008;67:144–54
- Schuler F, Sorokin LM. Expression of laminin isoforms in mouse myogenic cells in vitro and in vivo. J Cell Sci 1995;108:3795–805
- Bentzinger CF, Wang YX, Maltzahn, von J, Soleimani VD, Yin H, Rudnicki MA. Fibronectin regulates Wnt7a signaling and satellite cell expansion. Cell Stem Cell 2013;12:75–87
- Fukada S-I, Uezumi A, Ikemoto M, Masuda S, Segawa M, Tanimura N, Yamamoto H, Miyagoe-Suzuki Y, Takeda S. Molecular signature of quiescent satellite cells in adult skeletal muscle. Stem Cells 2007;25:2448–59
- Sechler JL, Takada Y, Schwarzbauer JE. Altered rate of fibronectin matrix assembly by deletion of the first type III repeats. J Cell Biol 1996;134:573–83
- Yamashita YM. Cell adhesion in regulation of asymmetric stem cell division. Curr Opin Cell Biol 2010;22:605–10
- Grefte S, Vullinghs S, Kuijpers-Jagtman AM, Torensma R, Von den Hoff JW. Matrigel, but not collagen I, maintains the differentiation capacity of muscle derived cells in vitro. Biomed Mater 2012;7:055004 (11 pp)
- Wilschut KJ, Haagsman HP, Roelen BAJ. Extracellular matrix components direct porcine muscle stem cell behavior. Exp Cell Res 2010;316:341–52
- Urciuolo A, Quarta M, Morbidoni V, Gattazzo F, Molon S, Grumati P, Montemurro F, Tedesco FS, Blaauw B, Cossu G, Vozzi G, Rando TA, Bonaldo P. Collagen VI regulates satellite cell self-renewal and muscle regeneration. Nat Commun 2013;4:1964
- Cosgrove BD, Gilbert PM, Porpiglia E, Mourkioti F, Lee SP, Corbel SY, Llewellyn ME, Delp SL, Blau HM. Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nat Med 2014;20:255–64
- Kuang S, Gillespie MA, Rudnicki MA. Niche regulation of muscle satellite cell self-renewal and differentiation. Cell Stem Cell 2008;2:22–31
- Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell 2004;116:769–78
- Kuang S, Kuroda K, Le Grand F, Rudnicki MA. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell 2007;129:999–1010
- Cornelison DD, Filla MS, Stanley HM, Rapraeger AC, Olwin BB. Syndecan-3 and syndecan-4 specifically mark skeletal muscle satellite cells and are implicated in satellite cell maintenance and muscle regeneration. Dev Biol 2001;239:79–94
- Brack AS, Conboy IM, Conboy MJ, Shen J, Rando TA. A temporal switch from notch to Wnt signaling in muscle stem cells is necessary for normal adult myogenesis. Cell Stem Cell 2008;2:50–9
- Maley MA, Davies MJ, Grounds MD. Extracellular matrix, growth factors, genetics: their influence on cell proliferation and myotube formation in primary cultures of adult mouse skeletal muscle. Exp Cell Res 1995;219:169–79
- Schultz E. Fine structure of satellite cells in growing skeletal muscle. Am J Anat 1976;147:49–70
- Boonen KJM, Rosaria-Chak KY, Baaijens FPT, van der Schaft DWJ, Post MJ. Essential environmental cues from the satellite cell niche: optimizing proliferation and differentiation. Am J Physiol Cell Physiol 2009;296:C1338–45
- Rønning SB, Pedersen ME, Andersen PV, Hollung K. The combination of glycosaminoglycans and fibrous proteins improves cell proliferation and early differentiation of bovine primary skeletal muscle cells. Differentiation 2013;86:13–22
- Allen RE, Boxhorn LK. Regulation of skeletal muscle satellite cell proliferation and differentiation by transforming growth factor-beta, insulin-like growth factor I, and fibroblast growth factor. J Cell Physiol 1989;138:311–15
- Gutiérrez J, Brandan E. A novel mechanism of sequestering fibroblast growth factor 2 by glypican in lipid rafts, allowing skeletal muscle differentiation. Mol Cell Biol 2010;30:1634–49
- Engler AJ, Griffin MA, Sen S, Bönnemann CG, Sweeney HL, Discher DE. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J Cell Biol 2004;166:877–87
- Gillies AR, Lieber RL. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve 2011;44:318–31
- Roth SM, Martel GF, Ivey FM, Lemmer JT, Metter EJ, Hurley BF, Rogers MA. Skeletal muscle satellite cell populations in healthy young and older men and women. Anat Rec 2000;260:351–8
- Renault V, Thornell L-E, Eriksson P-O, Butler-Browne G, Mouly V, Thorne L-E. Regenerative potential of human skeletal muscle during aging. Aging Cell 2002;1:132–9
- Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 2005;433:760–4
- Collins CA, Zammit PS, Ruiz AP, Morgan JE, Partridge TA. A population of myogenic stem cells that survives skeletal muscle aging. Stem Cells 2007;25:885–94
- Candiello J, Cole GJ, Halfter W. Age-dependent changes in the structure, composition and biophysical properties of a human basement membrane. Matrix Biol 2010;29:402–10
- Snow MH. The effects of aging on satellite cells in skeletal muscles of mice and rats. Cell Tissue Res 1977;185:399–408
- Paliwal P, Pishesha N, Wijaya D, Conboy IM. Age dependent increase in the levels of osteopontin inhibits skeletal muscle regeneration. Aging (Albany NY) 2012;4:553–66
- Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 2007;317:807–10
- Carlson ME, Hsu M, Conboy IM. Imbalance between pSmad3 and Notch induces CDK inhibitors in old muscle stem cells. Nature 2008;454:528–32
- Carmignac V, Durbeej M. Cell–matrix interactions in muscle disease. J Pathol 2012;226:200–18
- Hayashi YK, Engvall E, Arikawa-Hirasawa E, Goto K, Koga R, Nonaka I, Sugita H, Arahata K. Abnormal localization of laminin subunits in muscular dystrophies. J Neurol Sci 1993;119:53–64
- Sterrenburg E, van der Wees CGC, White SJ, Turk R, de Menezes RX, van Ommen G-JB, den Dunnen JT, ‘t Hoen PAC. Gene expression profiling highlights defective myogenesis in DMD patients and a possible role for bone morphogenetic protein 4. Neurobiol Dis 2006;23:228–36
- Zanotti S, Saredi S, Ruggieri A, Fabbri M, Blasevich F, Romaggi S, Morandi L, Mora M. Altered extracellular matrix transcript expression and protein modulation in primary Duchenne muscular dystrophy myotubes. Matrix Biol 2007;26:615–24
- Hodges BL, Hayashi YK, Nonaka I, Wang W, Arahata K, Kaufman SJ. Altered expression of the alpha7beta1 integrin in human and murine muscular dystrophies. J Cell Sci 1997;110(Pt 22):2873–81
- Haslett JN, Sanoudou D, Kho AT, Bennett RR, Greenberg SA, Kohane IS, Beggs AH, Kunkel LM. Gene expression comparison of biopsies from Duchenne muscular dystrophy (DMD) and normal skeletal muscle. Proc Natl Acad Sci USA 2002;99:15000–5
- Kyriakides T, Pegoraro E, Hoffman EP, Piva L, Cagnin S, Lanfranchi G, Griggs RC, Nelson SF. SPP1 genotype is a determinant of disease severity in Duchenne muscular dystrophy: predicting the severity of Duchenne muscular dystrophy: implications for treatment. Neurology 2011;77:1858–author reply 1858–9
- Valentin JE, Turner NJ, Gilbert TW, Badylak SF. Functional skeletal muscle formation with a biologic scaffold. Biomaterials 2010;31:7475–84
- Hinds S, Bian W, Dennis RG, Bursac N. The role of extracellular matrix composition in structure and function of bioengineered skeletal muscle. Biomaterials 2011;32:3575–83
- Rooney JE, Gurpur PB. Yablonka-Reuveni Z, Burkin DJ, Laminin-111 restores regenerative capacity in a mouse model for α7 integrin congenital myopathy. Am J Pathol 2009;174:256–64
- Amenta AR, Yilmaz A, Bogdanovich S, McKechnie BA, Abedi M, Khurana TS, Fallon JR. Biglycan recruits utrophin to the sarcolemma and counters dystrophic pathology in mdx mice. Proc Natl Acad Sci 2011;108:762–7
- DeQuach JA, Lin JE, Cam C, Hu D, Salvatore MA, Sheikh F, Christman KL. Injectable skeletal muscle matrix hydrogel promotes neovascularization and muscle cell infiltration in a hindlimb ischemia model. Eur Cell Mater 2012;23:400–12 discussion 412
- Tse JR, Engler AJ. Preparation of hydrogel substrates with tunable mechanical properties. Curr Protoc Cell Biol 2010;Chapter 10, Unit 10.16
- Young JL, Engler AJ. Hydrogels with time-dependent material properties enhance cardiomyocyte differentiation in vitro. Biomaterials 2011;32:1002–9
- Fedorovich NE, Alblas J, de Wijn JR, Hennink WE, Verbout AJ, Dhert WJA. Hydrogels as extracellular matrices for skeletal tissue engineering: state-of-the-art and novel application in organ printing. Tissue Eng 2007;13:1905–25
