Abstract
In clinically stable asthma the exhaled NO values (FENO) are generally higher than in control subjects. Therefore, reference values are of limited importance in clinical practice. This is demonstrated in this case report, but it is also shown that NO parameters from non-linear modelling do have a clinical value. A subject with asthma was treated with inhaled corticosteroids for 1 week. The non-linear NO model was used to measure the response to treatment. The NO parameters from subjects with atopic rhinitis and asthma were fed into a computer program to generate theoretical FENO0.05 values, i.e. target values. There was a dramatic decrease in FENO0.05 due to treatment, from 82 to 34 ppb, but it remained higher than in healthy controls. This is due to the elevated diffusion rate of NO, unchanged by treatment. When the NO parameters are known, a personal best value of FENO0.05 (fractional concentration of exhaled NO in the gas phase, 0.05 L/s) can be calculated, which can be the target value when only FENO0.05 can be monitored. In conclusion, reference values for NO parameters are shown to be clinically useful. It is essential that every patient receives his/her target value of FENO0.05, when only a single NO measurement is available. In our opinion, this is the reason why there are few successful studies of trying to target the NO value with inhaled corticosteroids.
Key words::
Introduction
Studies have been designed to use exhaled NO to target the treatment of asthma, and recently a Cochrane review has concluded that, at present, defining the dose of inhaled corticosteroids based on exhaled NO cannot be routinely advocated (Citation1). It has also been concluded in an American Thoracic Society/European Respiratory Society (ATS/ERS) document on standardizing end-points for clinical asthma trials and clinical practice that in clinically stable asthma the exhaled NO (FENO) values are generally higher than in healthy control subjects (Citation2). Therefore, reference NO values are of limited use in guiding the clinician in the treatment of patients with asthma. In the present case study it is demonstrated why the FENO0.05 (fractional concentration of exhaled NO in the gas phase, 0.05 L/s) remains high after treatment and that NO parameters from non-linear modelling are clinically useful.
Case study
A male subject, 30 years of age, with the diagnosis of atopic asthma since childhood was investigated. The baseline NO analysis was done without inhaled corticosteroids (ICS). After one week on 800 microgram ICS twice a day, FENO values were obtained again with multiple flow rates using a CLD 88sp NO analyser (ECO Medics AG, Switzerland). The NO production of the respiratory system was computed with the non-linear NO model by Högman and Meriläinen (Citation3).
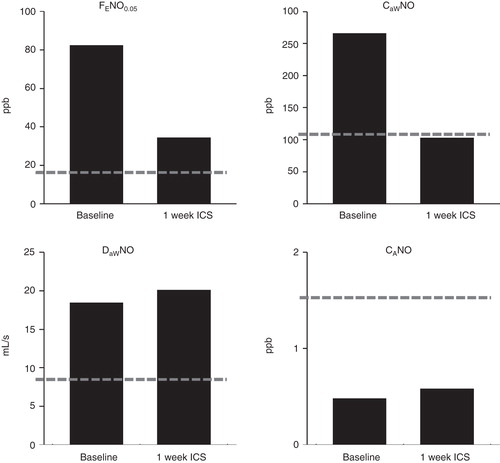
There was a dramatic decrease in FENO0.05 of 58% in 1 week of treatment, but it was still higher than reference values for healthy controls (Citation4). In it can be seen that there was no change in airway diffusion capacity of NO (DawNO) due to ICS. DawNO is known to be increased in atopic rhinitis and atopic asthma (Citation5) and not affected by ICS (Citation6). The alveolar NO levels (CANO) were low. Noteworthily, the airway tissue content (CawNO) was reduced by ICS to levels presented for healthy controls (Citation4).
Figure 1. FENO0.05 and NO parameters, airway tissue concentration of NO (CawNO), airway diffusion capacity for NO (DawNO), and alveolar levels of NO (CANO) in a case of allergic asthma. Values are given before and after 1 week of inhaled corticosteroids. Reference values for healthy controls are marked with a broken line (Högman et al. (Citation4)). Note the decline in CawNO to reference values while FENO0.05 remained high.
Theoretical example
When the NO parameters are known, the non-linear model can be used to calculate the FENO0.05 (Citation3). Different values of CawNO, DawNO, and CANO can be fed into a Microsoft Office Excel spreadsheet, where NO volumes at different expiratory flow rates are visualized and FENO for specific flow rates are given. For the illustration, typical NO values in health, atopy, and asthma are shown in . Since DawNO is not affected by ICS, this value can be used in the calculations together with CawNO and CANO values for healthy controls (Citation3). This calculation will result in a personal best or target value of FENO0.05 to be used during treatment. This target value of FENO0.05 was quite similar to the value after 1 week of treatment in this case study and in a group of asthmatics in a study by Silkoff et al. (Citation6).
Table I. CANO, CawNO, DawNO, and FENO0.05 values in health, atopy, and asthma. During steroid treatment, the target value of FENO0.05 can be calculated when the DawNO is known.
Discussion
One week of ICS reduced the CawNO to reference levels for healthy controls. The FENO0.05 level stayed elevated, which is due to the lack of change in DawNO, known to be high in allergic asthma (Citation5) and not affected by ICS in asthma (Citation6).
In a study by Smith et al. (Citation7) it was concluded that optimum FENO levels were best established by using oral rather than inhaled steroid treatment and that values were higher than reference values even though asthma was well controlled. The finding in this case study gives other solutions to finding the personal best value of FENO0.05. One solution is to use the non-linear NO model and follow the ICS treatment with CawNO. Another solution is to determine the DawNO for the patient and then use the CawNO and CANO for healthy controls to identify a target value of FENO0.05. In our patient it was 33 ppb. The FENO value can then be followed with a simple portable NO device in primary care.
The importance of controlling FENO has been shown in children, where airway hyper-responsiveness improved with lower FENO (Citation8), and in difficult-to-treat asthma, where an increased NO value was a predictor of accelerated decline in lung function (Citation9). Therefore NO values should preferentially be monitored in allergic asthma in both children and adults, and DawNO is useful for targeting FENO0.05. Further studies have to be designed to evaluate the personal best NO value by an approach presented in this case report.
Declaration of interest: The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
References
- Petsky HL, Cates CJ, Li A, Kynaston JA, Turner C, Chang AB. Tailored interventions based on exhaled nitric oxide versus clinical symptoms for asthma in children and adults. Cochrane Database Syst Rev. 2009:CD006340.
- Reddel HK, Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180:59–99.
- Högman M, Meriläinen P. Extended NO analysis in asthma. J Breath Res. 2007;1:024001.
- Högman M, Lafih J, Meriläinen P, Bröms K, Malinovschi A, Janson C. Extended NO analysis in a healthy subgroup of a random sample from a Swedish population. Clin Physiol Funct Imaging. 2009;29:18–23.
- Högman M, Holmkvist T, Wegener T, Emtner M, Andersson M, Hedenström H, Extended NO analysis applied to patients with COPD, allergic asthma and allergic rhinitis. Respir Med. 2002;96:24–30.
- Silkoff PE, Sylvester JT, Zamel N, Permutt S. Airway nitric oxide diffusion in asthma. Role in pulmonary function and bronchial responsiveness. Am J Respir Crit Care Med. 2000;161:1218–28.
- Smith AD, Cowan JO, Taylor DR. Exhaled nitric oxide levels in asthma: personal best versus reference values. J Allergy Clin Immunol. 2009;124:714–18.
- Pijnenburg MW, Bakker EM, Hop WC, de Jongste JC. Titrating steroids on exhaled nitric oxide in children with asthma: a randomized controlled trial. Am J Respir Crit Care Med. 2005;172:831–6.
- van Veen IH, Ten Brinke A, Sterk PJ, Sont JK, Gauw SA, Rabe KF, Exhaled nitric oxide predicts lung function decline in difficult-to-treat asthma. Eur Respir J. 2008;32:344–9.