Abstract
Thoracic ossification of the ligamentum flavum (OLF) has been widely recognized as a main cause of thoracic myelopathy in Asia, particularly in Japan. However, thoracic OLF rarely causes radiculopathy. We report a rare case of thoracic radiculopathy caused by OLF. A 67-year-old male presented with a chief complaint of back pain radiating to the right of the abdomen. Neurological examination revealed mild sensory deficit at the right side of the abdomen at the T9–10 level. Magnetic resonance imaging and computed tomography demonstrated OLF at the right T9–10 level. Thoracic radiculopathy caused by OLF was suspected. Because conservative treatment was not effective to this lesion, surgical intervention was performed, and the pain disappeared immediately after the operation. Thoracic OLF rarely causes radiculopathy, but it should be considered as a differential diagnosis of thoracic radicular pain. When conservative treatment is not effective in this lesion, surgical treatment should be considered.
Introduction
Thoracic radiculopathy is a rare condition with few reports on appropriate management (Citation1). Factors causing thoracic radiculopathy are mainly disc herniation, spondylosis, tumor, cysts, and fracture of vertebral body (Citation2–8). Thoracic ossification of the ligamentum flavum (OLF) has been widely recognized as a main cause of thoracic myelopathy in Asia, especially in Japan (Citation9). However, thoracic OLF rarely causes radiculopathy (Citation10). We present a rare case of thoracic radiculopathy caused by OLF, and an operative intervention improved the symptoms.
Case report
A 67-year-old male visited our clinic with severe back pain radiating to the right abdomen without preceding trauma. The symptom deteriorated while sitting or standing. It was completely relieved by supine position. He has had diabetes mellitus for 5 years treated by medication.
Neurological examination revealed mild sensory deficit at the right side of the abdomen at the T9–10 level. Manual muscle testing showed no muscle weakness of lower extremities. The tendon reflexes were normal.
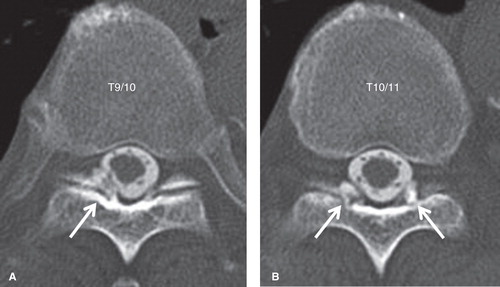
Plain radiographs of the thoracic spine showed mild degenerative changes in the T3 to T11 vertebral bodies and discs (). T2-weighted magnetic resonance imaging (MRI) demonstrated thickening of the ligamentum flavum at the right T9–10 level (). Computed tomography (CT) after conventional myelography demonstrated OLF at the right T9–10 and bilateral T10–11 levels. Though compression of the spinal cord was not clear, compression of the right T9 nerve root at the T9–10 level was suspected (). We performed a selective nerve root block of the right T9 nerve root for diagnostic treatment. The patient's pain decreased just after the procedure; however, the effect was temporary. From these findings, we diagnosed right T9 radiculopathy caused by OLF at the right T9–10 level.
Figure 1. Lateral plain radiograph of the thoracic spine. There were mild degenerative changes in T3 to T11 vertebral bodies and discs.
Figure 2. T2-weighted MR images of the thoracic spine. The ligamentum flavum at the right T9–10 level was thickened (arrows). A: sagittal view. B: axial view.
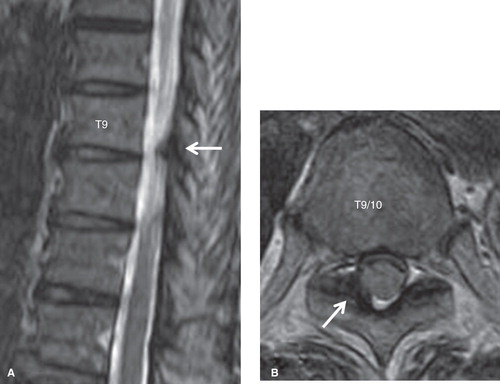
Figure 3. CT images after conventional myelography of the thoracic spine. OLF at the right T9–10 (A) and bilateral T10–11 (B) levels were observed (arrows).
Pain killers were prescribed at first, but the symptoms did not improve. Then an operation was scheduled. Because there remained a possibility that OLF at the right T10–11 level contributed to the symptoms and the patient occasionally complained about left back pain, fenestrations of the right T9–10 and bilateral T10–11 levels were performed under general anesthesia. Epidural fat tissue disappeared at the right T9–10 level, which indicated that OLF compressed the dura matter. OLF at the T10–11 level was smaller than that of the T9–10 level, and there was no apparent compression sign of the T10 nerve roots.
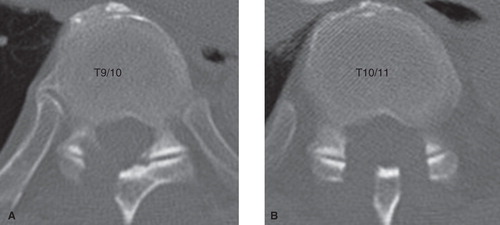
The pain disappeared, and sensory deficit improved just after the operation. CT after the operation demonstrated that decompression was enough at the right T9–10 () and bilateral T10–11 () levels.
Figure 4. CT images of the thoracic spine after the operation. Sufficient decompression was accomplished at the right T9–10 (A) and bilateral T10–11 (B) levels.
The patient was informed that his clinical data would be summarized in a manuscript and be submitted for publication. He gave his consent.
Discussion
Since Polgar first reported a case of OLF in 1920 using plain radiographs (Citation11), most reports of thoracic myelopathy caused by OLF have had their origin in Japan (Citation12). A symptomatic OLF may be a relatively common condition in the elderly population in East Asia (Citation13). Although Kudo reviewed 1744 lateral chest radiographs and revealed OLF in 6.2% of male and 4.8% of female patients, all of the cases were asymptomatic (Citation14). From studying 128 skeletal preparations, Hiraoka concluded that a minimum of 34% of them had at least one OLF (Citation15). The lower thoracic spine (T9–12) is the most frequently affected segments, while the mid-thoracic spine (T5–8) is rarely affected (Citation16).
The underlying etiology and pathogenesis of OLF are still unknown (Citation17). It has been documented that the incidence of thoracic OLF is higher in patients with diffuse idiopathic skeletal hyperostosis, fluorosis, diabetes mellitus, ankylosing spondylitis, and ossification of the posterior longitudinal ligament (OPLL) (Citation9). Recent studies indicate that the development of OPLL is associated with certain genetic factors (Citation18,19). These factors may also play a specific role in the origin of OLF (Citation20). Our patient had no ossification of the other spinal ligaments. However, he had diabetes mellitus, and it has been considered as a risk factor of OLF.
OLF is usually located in the lower thoracic spine (Citation16). This may be explained by the fact that this region is transitioning from the thoracic to the lumbar spine and there is less anatomic protection from the rib cage (Citation21). Ligamentum flavum at this region is repeatedly exposed to mechanical stress due to hypermobility, which might induce OLF (Citation9). In the present case, OLF was localized at the T9–10 and T10–11 levels. It indicated that mechanical stress might have influenced the development of OLF.
Ligamentum flavum has two portions, the interlaminar and the capsular ones (Citation22). Ossification usually begins in the capsular portion and spreads to the laminar portion (Citation20). OLF compresses the spinal cord or root from the posterolateral side and causes symptoms (Citation23).
There are only a few reports of thoracic OLF causing radiculopathy (Citation8,12,24,25). Liao reported 24 cases of thoracic OLF, and only one case presented radiculopathy (Citation8). Most of the reported cases were presenting radiculopathy together with myelopathy. Thoracic OLF causing radiculopathy without myelopathy like our case is extremely rare.
Ikata suggested that thoracic OLF rarely caused radiculopathy because ossification usually begins at the attachment to the capsular portion of the ligamentum flavum and spreads to the posteromedial side, which indicates that the foramen might be intact (Citation10). From studying 49 skeletal preparations, Sakou reported that the ossification was located at the inferior side of the nerve root and hardly compressed the root (Citation26).
Iihara reported a case of thoracic OLF causing radiculopathy. The symptom deteriorated while sitting or standing and was completely relieved by supine position, as in our patient (Citation27). This symptom might be characteristic to thoracic OLF causing radiculopathy.
When conservative treatment is not effective, surgical treatment should be considered. Several posterior decompressive methods (open-door laminectomy, en-bloc laminectomy, fenestration, hemilaminectomy, and key-hole foraminotomy) for the ossified lesions are advocated (Citation28). In the present case, ossifications of the bilateral ligamentum flavum did not fuse in the spinal canal, and we selected fenestrations. We could remove the ossified lesions safely, and the patient's symptoms disappeared completely.
Conclusion
We present a rare case of thoracic OLF causing radiculopathy. In case of patients with thoracic radicular pain, thoracic OLF should be considered as a differential diagnosis. When conservative treatment is not effective, surgical treatment should be considered.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Haufe SM, Baker RA, Pyne ML. Endoscopic thoracic laminoforaminoplasty for the treatment of thoracic radiculopathy: report of 12 cases. Int J Med Sci. 2009;6:224–6.
- Whitmore RG, Williams BJ, Lega BC, Sanborn MR, Marcotte P. A patient with thoracic intradural disc herniation. J Clin Neurosci. 2011;18:1730–2.
- Dickman CA, Rosenthal D, Regan JJ. Reoperation for herniated thoracic discs. J Neurosurg. 1999;91:157–62.
- Zenmyo M, Yamamoto T, Ishidou Y, Komiya S, Ijiri K. Osteoid osteoma near the intervertebral foramen may induce radiculopathy through tumorous inflammation. Diagn Pathol. 2011;6:10.
- Mitra SR, Gurjar SG, Mitra KR. Degenerative disease of the thoracic spine in central India. Spinal Cord. 1996;34:333–7.
- Perrini P, Benedetto N, Buccoliero AM, Di Lorenzo N. Thoracic radiculopathy from a paravertebral mesothelial cyst. Acta Neurochir (Wien). 2006;148:989–91.
- Liveson JA. Thoracic radiculopathy related to collapsed thoracic vertebral bodies. J Neurol Neurosurg Psychiatry. 1984;47:404–6.
- Liao CC, Chen TY, Jung SM, Chen LR. Surgical experience with symptomatic thoracic ossification of the ligamentum flavum. J Neurosurg Spine. 2005;2:34–9.
- Li F, Chen Q, Xu K. Surgical treatment of 40 patients with thoracic ossification of the ligamentum flavum. J Neurosurg Spine. 2006;4:191–7.
- Ikata T, Onomura T. General conception of ossification of ligamenta flava. Rinshou Seikeigeka (Clinical Orthopaedic Surgery). 1977;12:322–4; in Japanese.
- Polgar F. Uber interakuelle wirbelverkalkung. Fortschr Geb Rongenstr Nuklearmed Erganzungsband. 1920;40:292–8.
- Park BC, Min WK, Oh CW, Jeon IH, Kim SY, Kyung HS, Surgical outcome of thoracic myelopathy secondary to ossification of ligamentum flavum. Joint Bone Spine. 2007;74:600–5.
- Inamasu J, Guiot BH. A review of factors predictive of surgical outcome for ossification of the ligamentum flavum of the thoracic spine. J Neurosurg Spine. 2006;5:133–9.
- Kudo S, Ono M, Russell WJ. Ossification of thoracic ligamenta flava. AJR Am J Roentgenol. 1983;141:117–21.
- Hiraoka S. Ossification of ligament flava at intervertebral foramina. Geka no Ryouiki (Journal of Surgery). 1955;3:6–11; in Japanese.
- Yoon SH, Kim WH, Chung SB, Jin YJ, Park KW, Lee JW, Clinical analysis of thoracic ossified ligamentum flavum without ventral compressive lesion. Eur Spine J. 2011. 20:216–23.
- Li KK, Chung OM, Chang YP, So YC. Myelopathy caused by ossification of ligamentum flavum. Spine. 2002;27:E308–12.
- Koga H, Sakou T, Taketomi E, Hayashi K, Numasawa T, Harata S, Genetic mapping of ossification of the posterior longitudinal ligament of the spine. Am J Hum Genet. 1998;62:1460–7.
- Maeda S, Koga H, Matsunaga S, Numasawa T, Ikari K, Furushima K, Gender-specific haplotype association of collagen alpha2 (XI) gene in ossification of the posterior longitudinal ligament of the spine. J Hum Genet. 2001;46:1–4.
- Aizawa T, Sato T, Sasaki H, Kusakabe T, Morozumi N, Kokubun S. Thoracic myelopathy caused by ossification of the ligamentum flavum: clinical features and surgical results in the Japanese population. J Neurosurg Spine. 2006;5:514–19.
- Guo JJ, Luk KD, Karppinen J, Yang H, Cheung KM. Prevalence, distribution, and morphology of ossification of the ligamentum flavum: a population study of one thousand seven hundred thirty-six magnetic resonance imaging scans. Spine. 2010;35:51–6.
- Beamer YB, Garner JT, Shelden CH. Hypertrophied ligamentum flavum. Clinical and surgical significance. Arch Surg. 1973;106:289–92.
- Morimoto N. Ossification of the ligamentum flavum. Seikeigeka (Orthopaedic Surgery). 1986;37:231–40; in Japanese.
- Miyasaka K, Kaneda K, Ito T, Takei H, Sugimoto S, Tsuru M. Ossification of spinal ligaments causing thoracic radiculomyelopathy. Radiology. 1982;143:463–8.
- Ben Hamouda K, Jemel H, Haouet S, Khaldi M. Thoracic myelopathy caused by ossification of the ligamentum flavum: a report of 18 cases. J Neurosurg. 2003;99:157–61.
- Sakou T, Tomimura Y, Maehara T, Tomimura K, Maehara T, Morimoto N, Pathological status of ligament flava ossification. Rinshou Seikeigeka (Clinical Orthopaedic Surgery). 1977;12:368–76; in Japanese.
- Iihara K, Hanakita J, Suwa H, Nishihara K, Sakaida H. Ossification of the thoracic ligamentum flavum presenting with intercostal neuralgia. Neurol Med Chir (Tokyo). 1991;31:999–1002; in Japanese.
- Shiokawa K, Hanakita J, Suwa H, Saiki M, Oda M, Kajiwara M. Clinical analysis and prognostic study of ossified ligamentum flavum of the thoracic spine. J Neurosurg. 2001;94:221–6.
