Abstract
Background. Hyaluronan (HA) is the dominant glycosaminoglycan in the renomedullary interstitium. Renomedullary HA has been implicated in tubular fluid handling due to its water-attracting properties and the changes occurring in parallel to acute variations in the body hydration status.
Methods. HA production was inhibited by 4-methylumbelliferone (4-MU in drinking water for 5 days, 1.45 ± 0.07 g/day/kg body weight) in rats prior to hydration.
Results. Following hypotonic hydration for 135 min in control animals, diuresis and osmotic excretion increased while sodium excretion and glomerular filtration rate (GFR) remained unchanged. The medullary and cortical HA contents were 7.85 ± 1.29 ng/mg protein and 0.08 ± 0.01 ng/mg protein, respectively. Medullary HA content after 4-MU was 38% of that in controls (2.98 ± 0.95 ng/g protein, p < 0.05), while the low cortical levels were unaffected. Baseline urine flow was not different from that in controls. The diuretic response to hydration was, however, only 51% of that in controls (157 ± 36 versus 306 ± 54 µl/g kidney weight/135 min, p < 0.05) and the osmolar excretion only 47% of that in controls (174 ± 47 versus 374 ± 41 µOsm/g kidney weight/135 min, p < 0.05). Sodium excretion, GFR, and arterial blood pressure were similar to that in control rats and unaltered during hydration.
Conclusions. Reduction of renomedullary interstitial HA using 4-MU reduces the ability of the kidney to respond appropriately upon acute hydration. The results strengthen the concept of renomedullary HA as a modulator of tubular fluid handling by changing the physicochemical properties of the interstitial space.
Introduction
The glycosaminoglycan hyaluronan (HA) dominates in the extracellular matrix of the renal medulla. The specific physicochemical properties of HA enable a unique hydration capacity (Citation1). In the last decade it was revealed that in the interstitium of the renal medulla the HA levels change depending on the body hydration status while that of the cortex remains unchanged at very low levels (Citation2-5). The rapid changes in HA occurring in response to acute hydration require intact nitric oxide and prostaglandins, while dehydration for 24 h primarily seems to affect the mRNA expression of hyaluronan synthase 2 (Citation4). The renomedullary interstitial cell (RMIC) is a major producer of HA, and the turnover depends on the osmolality of the growth media and classical hormones involved in renal fluid handling, such as vasopressin and angiotensin II (Citation6-8). The involvement of HA in pathological kidney function has also been well described where severely elevated levels have been found during for example ischemia-reperfusion injury, transplant rejection, experimentally induced diabetes, and tubulointerstitial inflammation (Citation9-14). The proinflammatory and water-attracting properties of HA have been implicated in the organ dysfunction associated with kidney disease (Citation5).
Since the renomedullary HA content varies with hydration status and the RMIC turnover of HA depends on osmotic conditions and fluid regulatory hormones, it has been suggested that the physicochemical properties of HA will affect tubular fluid handling by regulating the properties of the interstitial space of the renal medulla where fine-tuning of fluid volume occurs. This system would thus function in parallel with vasopressin-regulated aquaporins. To test the concept of an involvement of renomedullary interstitial HA in renal fluid handling, we reduced the synthesis of HA by administering 4-methylumbelliferone (4-MU) to rats in the drinking water during five days and then challenging them with an acute hypotonic hydration.
Materials and methods
All chemicals were from Sigma-Aldrich (Saint Louis, MI, USA) and at the highest grade available unless otherwise stated. All experiments were performed in accordance with the NIH guidelines for use and care of laboratory animals and approved by the Animal Care and Use Committee of Uppsala University. All animals were kept in a room with controlled temperature of 24°C and a 12 h dark–light cycle and had free access to standard rat chow and water.
Male Sprague-Dawley rats (Charles River, Sulzfeld, Germany; n = 15, body weight 274 ± 32 g) received the HA synthesis inhibitor 4-MU (1.45 ± 0.07 g/kg body weight/24 h) in the drinking water or the corresponding vehicle for five consecutive days. The drinking water was made fresh every day with 4-MU. The dose was calculated after the experiments from the concentration in the drinking water (15 mg/ml) and by measuring daily water consumption.
After five days the rats were anaesthetized with an intraperitoneal injection of thiobutabarbital (Inactin, 5-ethyl-5-(1-methyl-propyl)-2-thio-barbiturate sodium, 120 mg/kg body weight) and were placed on a servo-controlled heating pad to maintain the core temperature at 37.5°C.
Surgery
After tracheotomy, polyethylene catheters were inserted into the right femoral vein and artery, the former for infusion of isotonic saline (0.9% NaCl) and hypotonic glucose-saline (0.25% NaCl, 0.5% glucose) containing 3H-inulin, and the latter for measurement of mean arterial blood pressure (MAP). The urinary bladder was catheterized through a suprapubic incision for urine sampling.
Protocol
After a post-surgery equilibration period of 45 minutes, another 45-minute period followed for determination of the baseline glomerular filtration rate (GFR) estimated from inulin clearance. Therefore, 3H-inulin (185 kBq/ml; Bionuclear Scandinavian AB, Bromma, Sweden) dissolved in isotonic saline was infused (5 ml/h/kg body weight) intravenously from the start of the equilibration period. Urine and arterial blood samples were taken for subsequent analyses. After this control period the rats were hydrated by changing the infusion to a hypotonic glucose-saline solution and increasing the infusion rate (15 ml/h/kg body weight). Then three consecutive periods followed lasting 45 minutes each, resulting in 135 minutes of hypotonic infusion corresponding to hydration of the animal.
After completion of the experimental procedure, the kidneys were excised and weighed, and samples from cortex and inner medulla (papilla) were taken and frozen for subsequent analysis of HA content.
Measurement of blood and urine parameters for GFR
GFR was estimated from the clearance of 3H-inulin. The radioactivity of 3H-inulin in plasma (10 μL) and urine (1 μL) was measured by liquid scintillation counting. Urine volumes were measured gravimetrically, osmolality by use of a freezing point technique (Model 210, The Fiske Micro-Sample Osmometer Advanced Instruments, Boston MA, USA), and urinary sodium concentrations by use of flame photometry (IL943, Instrumentation Lab, Milan, Italy).
Measurement of HA content
Excised and frozen samples of kidney tissue were dried at 68°C over night, then disrupted in 0.5 M NaCl (FP120, Thermo Electron Corporation, Marietta, Ohio, USA). Protein content was measured using a commercial assay (DC Protein Assay, Bio-Rad Laboratories, Hercules, CA, USA), and samples were analysed for HA content using a commercial ELISA kit (Echelon Biosciences, Inc., Salt Lake City, UT, USA).
Statistical analysis
All values are expressed as mean ± SEM. One-way analysis of variance (ANOVA) and Dunnett's multiple comparison tests were used for comparing effects over time within each group. Bonferroni's multiple comparison tests were used for comparisons of treatment effects against controls at each time point. A p-value of less than 0.05 was considered statistically significant.
Results
During the five days of treatment prior to acute experiments the animals ingested similar amounts of fluid suggesting similar hydration (control group 21.8 ± 1.4 ml/day; 4-MU group 25.7 ± 1.2 ml/day).
In the control group (n = 7; body weight 283 ± 11 g; kidney weight 1.10 ± 0.03 g) the medullary HA content was 7.85 ± 1.29 ng/mg protein while that of the cortex was 0.09 ± 0.01 ng/mg protein (). During hydration, GFR, sodium excretion, and arterial blood pressure remained unchanged, while urine flow increased (). The excreted urine volume during hydration was 306 ± 54 µL/g kidney weight/135 min (), and the excretion of osmotically active particles during hydration was 374 ± 41 µOsm/g kidney weight/135 min ().
Figure 1. Hyaluronan (HA) content in cortex and inner medulla (papilla) in control rats and in rats treated with the hyaluronan synthesis inhibitor 4-MU. *p < 0.05 versus control.
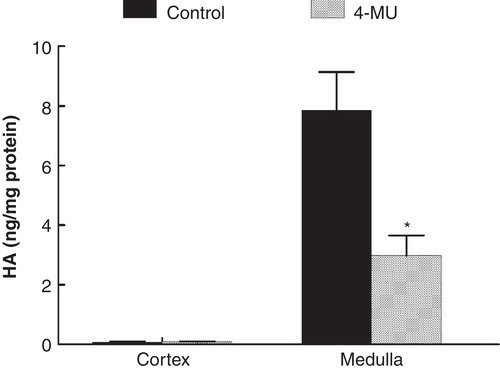
Table I. Glomerular filtration rate (GFR), mean arterial blood pressure (MAP), urine flow rate, and sodium excretion in control rats (C) and in rats treated with the hyaluronan synthesis inhibitor 4-MU. Each period corresponds to 45 min in consecutive order, p < 0.05 versus Control period of same group. Values are means ± SEM.
Figure 2. Accumulated urine excretion during hydration in control rats and in rats treated with the hyaluronan synthesis inhibitor 4-MU. *p < 0.05 versus control. (kw = kidney weight).
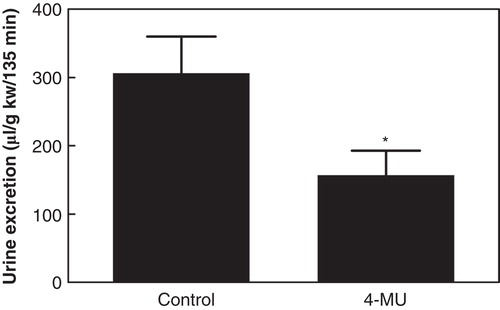
Figure 3. Accumulated osmolar excretion during hydration in control rats and in rats treated with the hyaluronan synthesis inhibitor 4-MU. *p < 0.05 versus control. (kw = kidney weight).
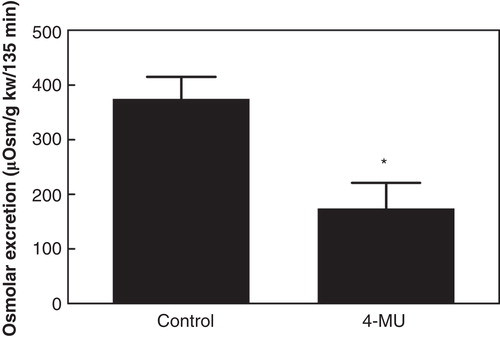
In the group treated with the HA synthesis inhibitor 4-MU (n = 8; body weight 266 ± 4 g; kidney weight 1.10 ± 0.02 g) the renomedullary HA content was 2.98 ± 0.95 ng/g protein, while that of the cortex was 0.08 ± 0.01 ng/mg protein (). The renomedullary HA content was thus only 38% of that in the control animals (p < 0.05), whereas it was similarly low in the cortex. Baseline GFR, urine flow, and arterial blood pressure were similar to that in the control group (). The excreted urine volume during hydration was 157 ± 36 µL/g kidney weight/135 min (), which was only 51% of that in the control animals (p < 0.05). The excretion of osmotically active particles during hydration was 174 ± 47 µOsm/g kidney weight/135 min, which was only 47% of that in the control animals (, p < 0.05).
Discussion
The present study demonstrates that if the renomedullary HA level is reduced with the HA synthesis inhibitor 4-MU the ability to respond to a hydration challenge is suppressed. This suggests that the interstitial matrix component HA changes the physicochemical characteristics of the medullary interstitial space, which directly affects fluid transport from the tubular lumen through the interstitial space. Such a suggestion is corroborated by earlier findings on changes in medullary HA in relation to hydration status (Citation2,3,5). Interstitial HA is elevated during hydration which will reduce reabsorption in conjunction with reduced vasopressin-regulated aquaporins. In contrast, during dehydration lower interstitial HA and up-regulated aquaporins will increase fluid transport across the interstitial space of the medulla, leading to increased reabsorption.
The concept of an involvement of HA in renal water handling was first proposed by Ginetzinsky in 1958 (Citation15). He suggested that the action of the antidiuretic hormone arginine vasopressin (ADH) in the renal medulla is exerted through activation of hyaluronidases, which changes the interstitial properties for fluid transport by reducing the amounts of glucosaminoglycans. These findings have been forgotten, and the discovery of aquaporins (Citation16) has further shifted the interest from a possible involvement of the interstitial matrix component HA in renal fluid regulation. We have previously demonstrated that ADH infusion not only reduces renomedullary HA levels in vivo but also reduces the HA content in the supernatant of cultured RMIC (Citation8). It should be noted that these cells are the major producers of HA in the renal medulla. The effect primarily involves the V1-receptor, since the V2-receptor agonist desmopressin did not alter the HA levels (Citation2). It would thus seem that ADH exerts its antidiuretic effect via two separate pathways: First, by changing the permeability of the apical membranes of the medullary tubular system through regulation of the number of aquaporins over the V2-receptor; and second, by changing the physicochemical characteristics of the interstitial space which will reduce or increase the permeability for fluid by changing the HA content over the V1-receptor.
The present study suggests that HA directly participates in renal water handling by changing the physicochemical characteristics of the interstitial matrix of the medulla and possibly by affecting the interstitial hydrostatic pressure (Citation2,17,18). It could thereby function as an important regulator of water diffusion in the interstitium regardless of organ origin. A possible mechanistic view of the effects is the following: 1) the repulsion between the negatively charged carboxylate groups of the sugar moieties in HA (glucuronic acid) protrude outward at regular intervals and contribute to the structure and size of the HA molecule; 2) the negatively charged gel attracts positive ions and increases osmosis, which will attract water into the gel matrix. When water is immobilized within the gel, further water reabsorption is antagonized. Furthermore, the medullary thick ascending limb of the loop of Henle (mTAL), which generates the medullary osmotic gradient, may be functionally compromised when interstitial diffusion characteristics are changed due to elevated HA. Also, the efficiency of the vasa recta (‘counter-current exchanger’), which help to maintain the osmotic gradient by recirculating fluid and electrolytes in the medulla, may also be affected when diffusion characteristics are changed. This would also result in altered urine concentration capacity. Finally, the interstitial swelling (‘functional oedema’), which occurs in response to elevations in HA, increases the diffusion distances between the tubules and blood vessels, which also will affect the reabsorption rate. In the present study, 4-MU reduced the medullary HA content which corresponded to increased ability to reabsorb water demonstrated by a reduced diuretic response upon hydration.
The mechanisms underlying the reduced HA production by 4-MU are not completely understood. However, it is clear that 4-MU has no direct influence on the HA synthases as studied in vitro (Citation19). Quite in contrast, the substance has been suggested to deplete the cellular stores of the substrate UDP-glucuronic acid which will reduce HA synthesis (Citation20).
In summary, when renomedullary HA is reduced using the HA synthesis inhibitor 4-MU, the ability to respond appropriately to hydration is suppressed. The study strengthens the concept that the properties of the medullary interstitium are modulated by the glycosaminoglycan HA, which directly affects the transport of fluid.
Acknowledgements
The technical assistance of Angelica Fasching is gratefully acknowledged.
Declaration of interest: Support for this study was provided by the Swedish Research Council. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Fraser JR, Laurent TC, Laurent UB. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med. 1997;242:27–33.
- Hansell P, Göransson V, Odlind C, Gerdin B, Hällgren R. Hyaluronan content in the kidney in different states of body hydration. Kidney Int. 2000;58:2061–8.
- Göransson V, Johnsson C, Nylander O, Hansell P. Renomedullary and intestinal hyaluronan content during body water excess: a study in rats and gerbils. J Physiol. 2002;542:315–22.
- Rugheimer L, Olerud J, Johnsson C, Takahashi T, Shimizu K, Hansell P. Hyaluronan synthases and hyaluronidases in the kidney during changes in hydration status. Matrix Biol. 2009;28:390–5.
- Stridh S, Palm F, Hansell P. Renal interstitial hyaluronan—functional aspects during normal and pathological conditions. Am J Physiol Regul Integr Comp Physiol. 2012;302:R1235–49.
- Hansell P, Maric C, Alcorn D, Göransson V, Johnsson C, Hällgren R. Renomedullary interstitial cells regulate hyaluronan turnover depending on growth media osmolality suggesting a role in renal water handling. Acta Physiol Scand. 1999;165:115–16.
- Göransson V, Hansell P, Moss S, Alcorn D, Johnsson C, Hällgren R, et al. Renomedullary interstitial cells in culture; the osmolality and oxygen tension influence the extracellular amounts of hyaluronan and cellular expression of CD44. Matrix Biol. 2001;20:129–36.
- Rugheimer L, Johnsson C, Maric C, Hansell P. Hormonal regulation of renomedullary hyaluronan. Acta Physiol (Oxf). 2008;193:191–8.
- Hällgren R, Gerdin B, Tufveson G. Hyaluronic acid accumulation and redistribution in rejecting rat kidney graft. Relationship to the transplantation edema. J Exp Med. 1990;171:2063–76.
- Johnsson C, Tufveson G, Wahlberg J, Hällgren R. Experimentally-induced warm renal ischemia induces cortical accumulation of hyaluronan in the kidney. Kidney Int. 1996;50:1224–9.
- Nilsson AB, Johnsson C, Friberg P, Hansell P. Renal cortical accumulation of hyaluronan in adult rats exposed neonatally to angiotensin-converting enzyme inhibition. Acta Physiol Scand. 2001;173:343–50.
- Göransson V, Johnsson C, Jacobson A, Heldin P, Hällgren R, Hansell P. Renal hyaluronan accumulation and hyaluronan synthase expression after ischaemia-reperfusion injury in the rat. Nephrol Dial Transplant. 2004;19:823–30.
- Rugheimer L, Carlsson C, Johnsson C, Hansell P. Renal hyaluronan content during experimental uncontrolled diabetes in rats. J Physiol Pharmacol. 2008;59:115–28.
- Stridh S, Kerjaschki D, Chen Y, Rügheimer L, Astrand AB, Johnsson C, et al. Angiotensin converting enzyme inhibition blocks interstitial hyaluronan dissipation in the neonatal rat kidney via hyaluronan synthase 2 and hyaluronidase 1. Matrix Biol. 2011;30:62–9.
- Ginetzinsky AG. Role of hyaluronidase in the reabsorption of water in renal tubules: the mechanism of action of the antidiuretic hormone. Nature. 1958;182:1218–19.
- Carbrey JM, Agre P. Discovery of the aquaporins and development of the field. Handb Exp Pharmacol. 2009;190:3–28.
- Lai-Fook SJ, Brown LV. Effects of electric charge on hydraulic conductivity of pulmonary interstitium. J Appl Physiol. 1991;70:1928–32.
- Zawieja DC, Garcia C, Granger HJ. Oxygen radicals, enzymes, and fluid transport through pericardial interstitium. Am J Physiol. 1992;262:H136–43.
- Kakizaki I, Takagaki K, Endo Y, Kudo D, Ikeya H, Miyoshi T, et al. Inhibition of hyaluronan synthesis in Streptococcus equi FM100 by 4-methylumbelliferone. Eur J Biochem. 2002;269:5066–75.
- Kakizaki I, Kojima K, Takagaki K, Endo M, Kannagi R, Ito M, et al. A novel mechanism for the inhibition of hyaluronan biosynthesis by 4-methylumbelliferone. J Biol Chem. 2004;279:33281–9.