Abstract
Current use, misuse, and overuse of antibiotics raise dangers and ethical dilemmas that cannot be solved in isolation, exclusively within a health system building block or even within the health sector only. There is a need to tackle antibiotic resistance emergence and containment on levels ranging from individuals, households, and the communities, to health care facilities, the entire health sector, and finally to national and global levels. We analyse emergence of antibiotic resistance based on interdependencies between health systems resources. We further go beyond the health system building blocks, to look at determinants of antibiotic resistance referring to wider global dynamics. Multi-level governance is the key for successful action in containment strategies. This will involve, in a comprehensive way, patients, health facilities where they receive care, health systems to which these facilities pertain, and the wider national context as well as the global community that influences the functioning of these health systems. In order to be effective and sustainable in both high and low-resource settings, implementation of containment interventions at all these levels needs to be managed based on existing theories and models of change. Although ministries of health and the global community must provide vision and support, it is important to keep in mind that containment interventions for antibiotic resistance will target individuals, consumers as well as providers.
Background
In a future without antibiotics, which the World Health Organization (WHO) has warned us about (Citation1), processes that are fundamental for the functioning of health systems would be unavailable or unsafe. Examples of services that could no longer be delivered safely without effective antibiotics are major surgery, cancer treatment, and prophylaxis in caesarean sections, not to mention the treatment of pneumonia. Through such mechanisms, antibiotic resistance has the potential to fundamentally change the functioning of health systems as we know them. As a consequence of these and other potentially damaging effects of antibiotics resistance, it is important to apply a health systems perspective when analysing what we know about what can be currently done to contain this phenomenon, as well as the difficulties and the obstacles to implement what we know.
Health systems are defined by the WHO as the totality of ‘organizations, people and actions whose primary intent is to promote, restore or maintain health’ (Citation2). The WHO health systems strengthening framework identifies the building blocks of a health system as related to governance, financial arrangements, medicines and technologies, health information systems, human resources, and health service delivery. Systems thinking for health systems strengthening applies elements of complex adaptive systems theory to that framework. In doing so, the interactions within and between these building blocks are looked upon as self-organizing, constantly changing, tightly linked, governed by feed-back, non-linear, history-dependent, counter-intuitive, and resistant to change (Citation3). The practical relevance of using systems thinking in analysing health systems functioning is apparent in the shift in our understanding of health towards an explicit systems perspective. The WHO definition dating back from 1948 already implied that factors outside the health sector would influence health and well-being. Currently, the concept of one health, although not yet operationalized, emphasizes how ‘sustainable health solutions that respect the right of future and current generations’ cannot be achieved in isolation (Citation4). Antibiotics are one health system resource whose current use risks its future. As such, antibiotic use raises dangers and ethical dilemmas that cannot be tackled by looking at current dynamics in isolation, exclusively within a health system building block or even within the health sector (Citation5).
Four paradigm shifts for looking at antibiotic resistance from a systems perspective
A framework applying a health systems perspective on access to essential medicines such as antibiotics has recently been developed (Citation6). It is based on three paradigm shifts that are also adopted here: 1) placing people at the core of the system, 2) analysing dynamic interdependencies between health system resources, and 3) understanding national and global contexts that might influence access to medicines. In the case of antibiotic resistance, however, adding to that complexity is the fact that antibiotics constitute a special class of medicines in that they are—and need to be seen as—a non-renewable resource whose use by individuals has important influences, both positive and negative, not only on individuals’ health, but also on population health, currently and in the future (Citation7). Thus, a fourth paradigm shift necessary to analyse antibiotic resistance from a systems perspective stems from the fact that 4) current benefits of antibiotics must be balanced against their use leading to resistance.
These four paradigm shifts translate into a need to discuss and tackle antibiotic resistance emergence and containment on levels ranging from the individual, the household, and the community, to health care facilities, to the entire health sector, and finally to national and global levels, and to do so in an integrated way. This vertical view of the use and overuse of antibiotics includes important, but diverse and not obviously linked, determinants of access to medicines (innovation, transparency, donors’ agendas, market forces), as well as determinants of antibiotic use and overuse in the animal sector and the environment. Horizontally, it means sharing authority and accountability for containment of antibiotic resistance between different state and non-state actors at each level. This type of governance, emerging from the interactions between a range of both public and private stakeholders operating at different system levels and having different degrees of authority, is known as multi-level governance (Citation8). As in the access to essential medicines framework, governance is an overarching determinant for antibiotic resistance emergence and containment seen from a systems perspective. As such, it can be relevant specifically within the health sector, but equally so when discussing antibiotic resistance as a whole-of-society and whole-of-government problem.
Emergence of antibiotic resistance—interdependencies between health system resources
Access to high-quality antibiotics is a key function of the medicines and technology building block, which is threatened by the continued overuse of antibiotics across societies and the resulting antibiotic resistance. Especially in high-income countries (HICs), this overuse has led to a necessary shift to more expensive, second- and third-generation antibiotics—which are then rapidly losing their effectiveness (Citation9). At the same time, patients in low- and middle-income countries (LMICs) often do not have access to these types of antibiotics (Citation10). In these latter settings, lack of regulations for pharmaceutical production or poor implementation of existing regulations leads to another major problem: counterfeit or low-quality products reaching the patients (Citation11). Not only have such products been shown to contribute to antibiotic resistance emergence, but their use further increases the need for second- and third-generation antibiotics (Citation12).
Financial arrangements for the provision of antibiotics can contribute to emergence of antibiotic resistance both in HICs and LMICs. Financial incentives that link medicines sales with health provider revenues are often counter-productive by encouraging antibiotics overprescription and thus contributing to emergence of resistance. In order to reduce overconsumption of antibiotics while ensuring access, realigning financial incentives between health insurers, pharmaceutical companies, and prescribers and dispensers is needed (Citation13). This is difficult to achieve especially in LMICs, where revenues are often still linked with prescription and sale of medicines (e.g. in China); to add insult to injury, lack of access to health care or to fast and specific diagnostic tests once seeking care, partly due to financial constraints, contributes to both increases in non-prescription antibiotics consumption and irrational prescribing (Citation14). Lack of resources for antibiotic resistance containment at the community and hospital levels further contributes to the emergence of resistance (Citation15) and increases the financial burden that antibiotic resistance puts on health care facilities (Citation16). Although there is little evidence on the economic burden of antibiotic resistance in LMICs, it is expected that it is widely underestimated, given the higher incidence of infectious diseases in these settings (Citation10). Even in HICs, where economic evaluations are more common, it is believed that the financial burden of antibiotic resistance is underestimated by not accounting for costs associated with routine health care services becoming unsafe (Citation17).
In order to put in context the financial arrangements that influence access to and excess of antibiotics, it is important to understand the availability and use of another important system resource and building block: information. Containment of antibiotic resistance cannot be successful without using health information systems to monitor resistant strains and rational use of antibiotics, as well as update standard treatment guidelines though a feedback loop within health service delivery. Paucity of timely surveillance data on resistance trends and on the magnitude of this resistance breaks this feedback loop and contributes to irrational prescription of antibiotics (Citation18). This is a problem particularly in LMICs, where basic data collection and analysis infrastructure or diagnostic infrastructure is often lacking (Citation19). In such settings, where infection control surveillance systems are not functioning, with repercussions for all health system resources and processes, outbreaks by resistant pathogens have been registered (Citation15).
Patterns of antibiotics resistance emergence are importantly explained by demand- and supply-side determinants of antibiotic use, overuse, and misuse. The role of human resources for health, another health system building block, is key to understanding supply-side factors. The emergence of antibiotic resistance, combined with patients’ expectations to receive antibiotics, often misaligned financial incentives that encourage prescription, physicians’ mistrust of available diagnostic tests, or at times unprofessional conduct, all contribute to the irrational prescription of broad-spectrum antibiotics (Citation20,21). In LMICs, other factors related to human resources for health refer to the lack of awareness of the problem of antibiotic resistance or inappropriate training for rational use of antibiotics. For example, in LMICs, where laboratory capacity and standard treatment guidelines, if existing, could be outdated, syndromic management is often not used, so that patients are treated for all major causes of a particular syndrome (Citation22).
Where syndromic management is used, as is the case for community health workers making presumptive diagnoses on acute lower respiratory infections in children, unnecessary antibiotic treatment still often occurs and could be avoided by better diagnostic technologies (Citation23). Even when health professionals prescribe rationally, according to existing guidelines, this might lead to overuse of antibiotics since these guidelines, even in HICs, do not recommend diagnostic testing before initiation of antibiotic therapy or recommend rapid initiation of antibiotic treatment (Citation22). On the demand-side, individuals, households, and communities have a strong impact on antibiotic resistance. Use of non-prescription antibiotics is a major driver of emergence of resistance in both HICs and LMICs (Citation24). Also in both settings, non-compliance and using or sharing leftover antibiotics are common (Citation25). There is evidence suggesting that patients can misunderstand the effect of antibiotics (which might in turn lead to non-compliance), although attitudes towards this category of medicines are generally positive (Citation26). In LMIC settings, the problems are aggravated by the fact that advertising pressure tends to be higher and that the general public might have lower levels of education and be misinformed (Citation27).
Emergence of antibiotics resistance—beyond the health system building blocks
The larger national context has significant influence on antibiotic resistance emergence and the strain it puts on health systems. In LMICs, where both the burden of infectious diseases and the prevalence of multidrug-resistant pathogens are higher, poverty contributes to antibiotic resistance by being linked with poor infection control at hospital and community levels. Poverty within the community might also mean a poor nutritional status of individuals or poor sanitation, leading to higher susceptibility to infections (Citation28). Furthermore, antibiotic resistance in the environment is a cause of major concern due to the increased use of antibiotics in animal medicine where, just as in human medicine, the balance between access and excess is problematic. Antibiotics are also increasingly used for growth promotion and disease prevention in agriculture, horticulture, and aquaculture (Citation29). Links with the food industry means that global market forces such as trade policies or consumers’ expectations can influence use of antibiotics and consequently antibiotic resistance emergence in ways that are difficult to monitor (Citation22). The dangers of antibiotic resistance emergence in the animal sector and the environment have been discussed in detail elsewhere in this special issue on antibiotic resistance (Citation30,31).
Insufficient technical innovation efforts for development of new antibiotics, largely caused by scientific and financial bottlenecks (Citation32), are another important factor that, although not strictly pertaining to the health systems building blocks, exacerbates emergence of resistance. In addition, there is no consensus on the type of diagnosis research and development should aim for. When new diagnostic tests are developed, clinical factors and investigations on effectiveness in terms of antibiotic resistance, antibiotics use, or patient outcomes are less emphasized in their assessment in favour of efficacy considerations. There is also lack of clarity about how HICs and LMICs might take up these new diagnostic tests, in the context of different speed, robustness of system, cost, or user-friendliness. The reimbursement mechanisms in different health care models need to be taken into account as well. In the meantime, existing, simple tests are still not widely used (Citation22). All of the above-mentioned circumstances contribute to the emergence of antibiotic resistance by enabling overuse of antibiotics (especially broad-spectrum antibiotics) in hospitals as well as in outpatient settings.
Market forces influence the direction of the innovation efforts (i.e. the prioritization of certain products over others in the research and development pipeline), as well as the way they are financed. The existing model has placed research for antibiotics at a low rank in the priority list. If the same model is used, then extended data exclusivity and premium pricing could be used as incentives to stimulate innovation. This would be counter-productive since, at the health sector level, it would lead to both promotion of sales for new antibiotics (contributing to emergence of resistance) and decrease in access to antibiotics due to higher prices (Citation13). The same model has contributed, for example, to advertising pressures for antibiotics prescription and use from community to health system level (Citation27). However, such practices are outdated and counter-productive and should not be used for a non-renewable resource such as antibiotics.
Multi-level governance and change management for antibiotic resistance containment
Good multi-level governance is a necessity for successful action in antibiotic resistance containment. Global governance is key in redesigning financial arrangements for provision of effective antibiotics. Already, global public–private partnerships have contributed towards financing and ensuring access to essential technologies, such as vaccines, that are instrumental in curbing emergence of resistance (Citation33). As a way of strengthening such initiatives through which the public and private sectors redesign their mechanisms for collaboration, calls have been made for the development of a global antibiotic resistance governance coalition. Such a coalition would be instrumental in, for example, setting up global funding mechanisms for the research and development of the technologies needed in containment of antibiotic resistance (new antibiotics, combination therapies, vaccines, and new diagnostic tests) (Citation22). In order to support not only innovation, but also access to and rational use of antibiotics through such new business models, public funding could be secured to buy out patents, and local manufacturers could be licensed to produce only the quantities of new antibiotics that are appropriate for rational use (Citation13). Such mechanisms would be an important step forward towards ensuring global accountability for emergence and containment of resistance and towards globally emphasizing the primacy of the public health rather than of the economic function of antibiotics (Citation34). Furthermore, containment efforts need to be made with involvement of all relevant stakeholders, including those outside the health sector, since human, animal, and environmental health contribute to one health. For example, monitoring of antibiotics use in the animal sector and the environment is needed, combined with the phasing out of preventive and growth promotion use (Citation35). Although some countries have put in place mechanisms to achieve such goals (Citation36), commitment to containment efforts at the global level is needed to ensure that supranational market forces will not continue to take precedence over public health considerations.
At the national level, good national and health system governance is key for ensuring appropriate financing for containment strategies, with recommendations to create a national body or task force dealing with antibiotic resistance at the highest decision-making levels. Such a task force is needed in order to oversee the scale-up of evidence-informed interventions for containment, collection of surveillance data, and assessment and review of interventions (Citation22). This approach was taken in Sweden, where the Swedish Strategic Programme for the Rational Use of Antimicrobial Agents and Surveillance of Resistance (Strama) was created already in 1995. As a multidisciplinary, centrally co-ordinated programme, Strama has contributed to maintaining low antibiotic prescription rates and has consistently tracked and effectively slowed down emergence of antibiotic resistance (Citation37). In a high-resource setting such as Sweden, it is reasonable to expect that resources for antibiotic resistance containment are allocated and co-ordinated through a national programme that can also contribute to the development of national multi-sectoral plans.
On the contrary, such commitments and funding for antibiotic resistance containment are rare in lower-resource settings. Still, especially in those settings, where health systems are weaker, championship at central level is essential for improvement of cash flows towards health information systems, laboratory infrastructure, and human resources training (Citation18). In Romania, a middle-income country where non-prescription antibiotics use is among the highest in Europe, despite regulation prohibiting dispensing without prescription (Citation24), national political commitment for tackling the problem of antibiotic resistance is low. This contributes to the perpetuation of overuse of antibiotics throughout the health system and results in high rates of resistance (Citation38). As an example of the importance of regional governance for antibiotic resistance containment, the main objectives for the Romania–WHO collaboration in 2012–2013 refer to conducting a comprehensive situation assessment on emergence of resistance, as well as establishing a national mechanism and developing action plans for containment of antibiotic resistance (Citation39). Good multi-level governance has also contributed to antibiotic resistance containment strategies in Vietnam. Based on a situation analysis conducted in 2010, a public–private partnership has paved the way for continued engagement in global partnerships, collaboration with other sectors in the country, review of policies and guidelines based on health information systems, and pushing legislation for antibiotics resistance containment nationally (Citation40). These examples show how good multi-level governance for antibiotics resistance is instrumental in planning for effective containment of resistance through context-specific efforts aimed at creating a bridge between what is recommended globally and what can be put into practice locally.
Involvement of health facility and community representatives in high-level decision-making through a bottom-up model is recommended, as action for containment of resistance ultimately targets specific health facilities and communities (Citation15). In high- as well as low- resource settings, good governance at health facility level means that individual institutions need to commit to and invest in antibiotic resistance containment, training human resources for health, and maintaining appropriate records on antibiotic resistance. Standardized reporting of resistant hospital- and community-acquired pathogens should be done electronically and thus be available throughout the system. In terms of human resources, this requires involvement from microbiologists, infectious disease specialists, clinical pharmacists, laboratory staff, as well as IT experts and hospital administrators. Multi-disciplinary antibiotic resistance containment teams that ensure good governance are instrumental in creating and implementing flexible and enforceable regulatory frameworks for balancing access and excess of antibiotics (Citation41). Meeting such targets in low-income settings can seem impossible, given that, in such contexts, providing printed guidelines and reporting protocols, together with appropriate audit mechanisms, are needed just as much as validation and provision of new rapid diagnostics test (Citation22). However, carefully managing interventions according to theories of change () could provide support in adapting to such complex contextual realities when implementing containment interventions. In Vietnam, the above-mentioned public–private partnership implemented a capacity development project for containment of resistance in hospitals. This project, which was co-ordinated by local health professionals, went through orientation, insight, and acceptance of the needed changes among key stakeholders as the first steps of implementing change. The priorities of the interventions were put on formulating the first evidence-based standard treatment guidelines in the country, installing and testing surveillance tools, building on existing point-of-prevalence surveys to identify priorities in infection control, and providing laboratories with equipment and software as well as adapted testing guidelines and external quality assurance (Citation40).
Figure 1. A 10-step model for inducing change in professional behaviour. (Source: Grol R, Wensing M. What drives change? Barriers to and incentives for achieving evidence-based practice. Med J Aust. 2004;180:S57–60.)
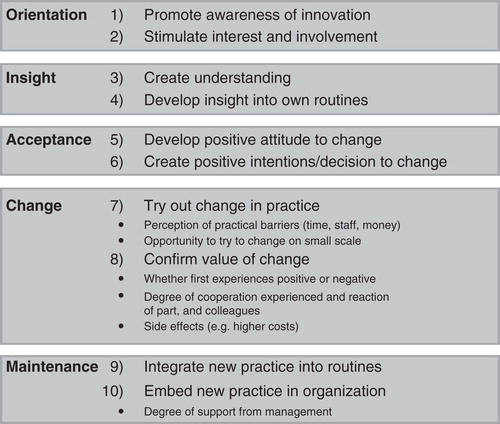
Innovative strategies that promote integration between health care facility and community roles for containment of resistance need to be designed and implemented in both high- and low-income settings. If patients are enabled to become ‘expert patients’ (Citation6), they could actively contribute to containment strategies. In this process of change for containment of antibiotic resistance, patients’ and communities’ awareness and understanding of the importance of their roles in the safe use of antibiotics, but also their acceptance of the needed changes, are instrumental in order to embed new practices within health service delivery. One example refers to the treatment of otitis media, for which interventions designed to teach patients to use prescribed antibiotics if symptoms worsen after 48 hours were proven to be effective in decreasing use of antibiotics and were implemented in Sweden (Citation42). The national programme for antibiotics containment in Sweden, Strama, was instrumental in developing this and other new standard treatment guidelines (Citation37). Thus, after confirming the value of change, Strama has moved to integrate and embed constant surveillance and action for containment of antibiotic resistance throughout the Swedish health system. Similarly, management according to theories of change is needed, for example, in the development and evaluation of interventions aimed at improvement of compliance, for which mobile phones show great potential, especially in low- and middle-income countries such as Vietnam and Romania (Citation43,44). These are just some examples of the redesign of health delivery systems needed for the containment of antibiotic resistance (Citation22). They show why the integration of theories and practice of change management is needed in interventions aimed at tackling antibiotic resistance. Indeed, a process that begins by creating awareness, interest, and understanding () is much more likely to lead to acceptance and integration of the new knowledge into daily practice at all system levels and thus contribute to successful and sustainable containment (Citation27).
Educational campaigns for the general public that deal with some of the demand-side determinants of antibiotic overuse and misuse may also be effective (Citation45). For example, a survey of European countries showed that the general public in Romania often have inaccurate knowledge concerning appropriate use of antibiotics, which is a strong argument for national information campaigns (Citation46). However, since telling people what to do often proves insufficient in changing their behaviours, understanding people’s perceptions, needs, and concerns is a must in order to be successful in the process of change towards curbing overuse and misuse of antibiotics. The contextual imperatives of antibiotics resistance containment demand that health systems research, behavioural science, and social marketing are used to clarify barriers to the uptake of new technologies and models of care and scale-up and implementation of recommended interventions (Citation22). Furthermore, the benefits of participatory education and research need to be explored, both at community level and within health service delivery, in order to increase social awareness of the importance of containment of antibiotics resistance through participatory action. Thus, taking a less passive view on potential patients’ roles and exploring the potential of household- and community-level interventions can guide the design of innovative programmes for antibiotic resistance containment (e.g. school-based art programmes) (Citation47). In that way, social innovation is just as needed as technological innovation.
Conclusions
This analysis of antibiotic resistance emergence and containment from a systems perspective shows that although action needs to be focused and targeted, it also needs to be designed based on a clear understanding of interdependencies within and between systems. Thus, in order to effectively balance access to, misuse, and overuse of antibiotics at health service delivery level, resources, regulations, and incentives need to be aligned at all system levels, creating feedback loops that promote access to, but curb overuse of antibiotics. Furthermore, good multi-level governance for containment of antibiotic resistance will involve the patients, the health facilities where they receive care, the health systems to which these facilities pertain, and the wider national context as well as the global community that influences the functioning of these health systems. These hospitals and communities pertain to contexts and health systems that are significantly different in low-income, middle-income, or high-income countries. To respond to contextual imperatives, changes for antibiotic resistance containment need to be managed appropriately, based on existing theories and models of change. Although ministries of health and the global community must provide vision and support, it is important to keep in mind that containment interventions will target individuals, consumers as well as providers.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Kaplan W, Laing R. Priority medicines for Europe and the world. Geneva: World Health Organization; 2004.
- World Health Organization. Everybody’s business–strengthening health systems to improve health outcomes: WHO’s framework for action. Geneva: World Health Organization; 2007.
- de Savigny D, Adam T. Systems thinking for health systems strengthening. Geneva: World Health Organization; 2009.
- Marais B, Crawford J, Iredell J, Ward M, Simpson S, Gilbert L, et al. One world, one health: beyond the Millennium Development Goals. Lancet. 2012;380:805–6.
- Leibovici L, Paul M, Ezra O. Ethical dilemmas in antibiotic treatment. J Antimicrob Chemother. 2012;67:12–16.
- Bigdeli M, Jacobs B, Tomson G, Laing R, Ghaffar A, Dujardin B, et al. Access to medicines from a health system perspective. Health Policy Plan. 2013;28:692–704.
- Cars O, Hogberg LD, Murray M, Nordberg O, Sivaraman S, Lundborg CS, et al. Meeting the challenge of antibiotic resistance. BMJ. 2008;337:a1438.
- Tomson G. The impact of global processes on health systems in Europe. Global Health Europe. 2010;2:1–47.
- Goossens H, Ferech M, Vander Stichele R, Elseviers M, Group EP. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet. 2005;365:579–87.
- Okeke IN, Laxminarayan R, Bhutta ZA, Duse AG, Jenkins P, O’Brien TF, et al. Antimicrobial resistance in developing countries. Part I: recent trends and current status. Lancet Infect Dis. 2005;5:481–93.
- Newton PN, Green MD, Fernández FM, Day NPJ, White NJ. Counterfeit anti-infective drugs. Lancet Infect Dis. 2006;6:602–13.
- Planta MB. The role of poverty in antimicrobial resistance. J Am Board Fam Med. 2007;20:533–9.
- So AD, Ruiz-Esparza Q, Gupta N, Cars O. 3Rs for innovating novel antibiotics: sharing resources, risks, and rewards. BMJ. 2012;344:e1782.
- Laxminarayan R, Heymann DL. Challenges of drug resistance in the developing world. BMJ. 2012;344:e1567.
- Zaidi AK, Huskins WC, Thaver D, Bhutta ZA, Abbas Z, Goldmann DA. Hospital-acquired neonatal infections in developing countries. Lancet. 2005;365:1175–88.
- Allegranzi B, Bagheri Nejad S, Combescure C, Graafmans W, Attar H, Donaldson L, et al. Burden of endemic health-care-associated infection in developing countries: systematic review and meta-analysis. Lancet. 2011;377:228–41.
- Smith R, Coast J. The true cost of antimicrobial resistance. BMJ. 2013;346:f1493.
- Okeke IN, Klugman KP, Bhutta ZA, Duse AG, Jenkins P, O’Brien TF, et al. Antimicrobial resistance in developing countries. Part II: strategies for containment. Lancet Infect Dis. 2005;5:568–80.
- Ashley EA, Lubell Y, White NJ, Turner P. Antimicrobial susceptibility of bacterial isolates from community acquired infections in Sub-Saharan Africa and Asian low and middle income countries. Trop Med Int Health. 2011;16:1167–79.
- Radyowijati A, Haak H. Improving antibiotic use in low-income countries: an overview of evidence on determinants. Soc Sci Med. 2003;57:733–44.
- Davey P, Brown E, Charani E, Fenelon L, Gould IM, Holmes A, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. 2013;4:CD003543.
- Laxminarayan R, Duse A, Wattal C, Zaidi AKM, Wertheim HFL, Sumpradit N, et al. Antibiotic resistance—the need for global solutions. Lancet Infect Dis. 2013;13:1057–98.
- Lim YW, Steinhoff M, Girosi F, Holtzman D, Campbell H, Boer R, et al. Reducing the global burden of acute lower respiratory infections in children: the contribution of new diagnostics. Nature. 2006;444:9–18.
- Morgan DJ, Okeke IN, Laxminarayan R, Perencevich EN, Weisenberg S. Non-prescription antimicrobial use worldwide: a systematic review. Lancet Infect Dis. 2011;11:692–701.
- Zarb P, Goossens H. Human use of antimicrobial agents. Rev Sci Tech. 2012;31:121–33.
- Hawkings NJ, Butler CC, Wood F. Antibiotics in the community: a typology of user behaviours. Patient Educ Couns. 2008;73:146–52.
- Nordberg P, Stålsby-Lundborg C, Tomson G. Consumers and providers – could they make better use of antibiotics? Int J Risk Safe Med. 2005;17:117–25.
- Byarugaba DK. A view on antimicrobial resistance in developing countries and responsible risk factors. Int J Antimicrob Agents. 2004;24:105–10.
- World Health Organization. Critically important antimicrobials for human medicine: categorization for the development of risk management strategies to contain antimicrobial resistance due to non-human antimicrobial use. Report of the Second WHO Expert Meeting Copenhagen: World Health Organization; 2007.
- Bengtsson B, Greko C. Antibiotic resistance—consequences for animal health, welfare, and 3 food production. Upsala J Med Sci. 2014; [Epub ahead of print].
- Larsson DGJ. Antibiotics in the environment. Upsala J Med Sci. 2014; [Epub ahead of print].
- So AD, Gupta N, Brahmachari SK, Chopra I, Munos B, Nathan C, et al. Towards new business models for R&D for novel antibiotics. Drug Resist Updat. 2011;14:88–94.
- Levin A, Kaddar M. Role of the private sector in the provision of immunization services in low- and middle-income countries. Health Policy Plan. 2011;26:i4–12.
- Newton PN, Amin AA, Bird C, Passmore P, Dukes G, Tomson G, et al. The primacy of public health considerations in defining poor quality medicines. PLoS Med. 2011;8:e1001139.
- Marshall BM, Levy SB. Food animals and antimicrobials: impacts on human health. Clin Microbiol Rev. 2011;24:718–33.
- Grave K, Jensen VF, Odensvik K, Wierup M, Bangen M. Usage of veterinary therapeutic antimicrobials in Denmark, Norway and Sweden following termination of antimicrobial growth promoter use. Prev Vet Med. 2006;75:123–32.
- Molstad S, Erntell M, Hanberger H, Melander E, Norman C, Skoog G, et al. Sustained reduction of antibiotic use and low bacterial resistance: 10-year follow-up of the Swedish Strama programme. Lancet Infect Dis. 2008;8:125–32.
- European Centre for Disease Prevention and Control. Antimicrobial resistance surveillance in Europe 2012. Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net). Stockholm: ECDC; 2013.
- World Health Organization Regional Office for Europe. Countries - Romania - Areas of Work. Available at http://www.euro.who.int/en/countries/romania/areas-of-work. accessed 24 November 2013.
- Wertheim HF, Chandna A, Vu PD, Pham CV, Nguyen PD, Lam YM, et al. Providing impetus, tools, and guidance to strengthen national capacity for antimicrobial stewardship in Viet Nam. PLoS Med. 2013;10:e1001429.
- World Health Organization. The evolving threat of antimicrobial resistance: options for action. Geneva: World Health Organization; 2012.
- Pshetizky Y, Naimer S, Shvartzman P. Acute otitis media–a brief explanation to parents and antibiotic use. Fam Pract. 2003;20:417–19.
- Suffoletto B, Calabria J, Ross A, Callaway C, Yealy DM. A mobile phone text message program to measure oral antibiotic use and provide feedback on adherence to patients discharged from the emergency department. Acad Emerg Med. 2012;19:949–58.
- Nglazi MD, Bekker LG, Wood R, Hussey GD, Wiysonge CS. Mobile phone text messaging for promoting adherence to anti-tuberculosis treatment: a systematic review protocol. Syst Rev. 2013;2:6.
- Huttner B, Goossens H, Verheij T, Harbarth S; CHAMP Consortium. Characteristics and outcomes of public campaigns aimed at improving the use of antibiotics in outpatients in high-income countries. Lancet Infect Dis. 2010;10:17–31.
- European Commission. Antimicrobial resistance. Special Eurobarometer 338. 2010. Available at http://ec.europa.eu/health/antimicrobial_resistance/docs/ebs_338_en.pdf. accessed 23 November 2013.
- Management Sciences for Health. Building local coalitions for containing drug resistance: a guide 2012. Available at http://www1.msh.org/projects/sps/SPS-Documents/upload/AMR-guide-English_FINAL.pdf. accessed 24 November 2013.
