Key words:
Gunnar Blix () was born in Lund in 1894. He was the son of a famous physiologist, Magnus Blix. After medical school, he started in research and defended his thesis entitled Studies on Diabetic Lipaemia in 1925 at the University of Lund. Previously he had been employed by the university as a research fellow in anatomy and medical chemistry, and for shorter periods of time he also practised as a physician.
Directly after his dissertation he moved to Uppsala, where he obtained a position in 1926 as associate professor (laborator) and four years later succeeded Carl Thore Mörner as professor in Medical and Physiological Chemistry. This professorship had been created in 1853. Gunnar Blix held the professorship for 31 years (1930–1961). During the years 1956–1961 he also served as Deputy Vice Chancellor in the university administration. He was Editor-in-Chief for Acta Societatis Medicorum Upsaliensis (from 1972 Upsala Journal of Medical Sciences) between 1943 and 1969. After his retirement he continued to work until 1970 as executive member of the Swedish Foundation for Nutritional Research (Stiftelsen Svensk Näringsforskning). Gunnar Blix died on 10 June 1981 at the age of 87 years.
Those who met and got to know Gunnar Blix remember him as an honest, dutiful, and humble person who generously gave of his ideas and devoted much of his time to teaching students in the medical school and graduate students in his research group. He became a highly worthy successor to his predecessors of the professorship (, ). His time as professor coincided with an important period in the development of the scientific field of medical and physiological chemistry. He became internationally well known and a leading scientist within his own research field.
Table I. Predecessors to Gunnar Blix for the professorship in Medical and Physiological Chemistry at Uppsala University.
Figure 2. Four generations of professors in Medical and Physiological Chemistry. Sitting from left: Carl Thore Mörner, Sven Gustaf Hedin, Olof Hammarsten. Standing: Gunnar Blix (1930).

Gunnar Blix enjoyed public confidence, and it was by no means accidental that he was assigned to numerous commissions of trust within his own faculty, within the university, and also nationally. He was a member of the organizing committees for the formation of both Gothenburg and Umeå Medical Schools in 1947 and 1958, respectively. In the case of Umeå, all existing universities in Sweden voted against the establishment of a medical school there. Blix, however, made a reservation against the negative attitude from Uppsala and thereby he succeeded in convincing the Swedish government to proceed with the plans, which later on led to the formation of Umeå University. The Faculty of Medicine in Uppsala initially became ‘mother faculty’ to the new medical faculties in both Gothenburg and Umeå and later on also in Linköping.
When Blix became a member of the Faculty of Medicine in Uppsala, his department of medical chemistry was housed in an old building called ‘Gamla Kemikum’ (Old chemistry institute) located in Engelska parken (The English park).
This building () was erected in 1853–1859 and in time became more and more worn out. The sanitary conditions, for instance, made it gradually impossible to continue to work there. The government therefore decided (in 1941) to construct a new building, to be shared by the Departments of Medical Chemistry and Pharmacology. Blix took a very active role in the planning of the various facilities and in the moving to the new building (). Due to the Second World War the construction materials were not of the best quality, and the whole building project was also delayed. Not until 1946 did the transfer actually take place.
Figure 4. The Departments of Medical Chemistry and Pharmacology, located at Stockholmsvägen 19 (later named Dag Hammarskjölds väg).

Gunnar Blix was also engaged as an inspector for two ‘student nations’ in Uppsala: 1938–1943 for ‘Gotlands nation’ and 1943–1961 for ‘Norrlands nation’. When he resigned from his inspectorship he was honoured with a Festschrift in which Blix’s instrumental role for the establishment of Umeå University is emphasized. In addition, the student dormitories ‘Norrlands-gårdarna’ were built during Blix’s time as inspector.
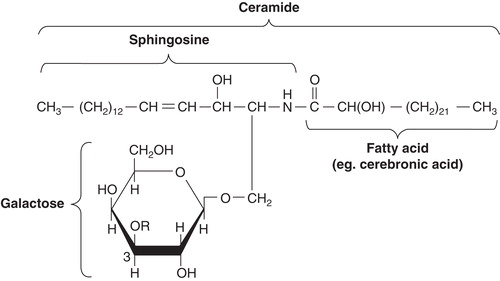
Blix covered a broad area in his research. His interest in lipid chemistry, which was documented already in his thesis, continued through the years. In this context two important discoveries should be mentioned. The first came already in 1933, in his studies of lipids in human brain tissue, when he isolated and characterized the first sulfatide known at this time. He showed that it was composed of sulfuric acid, cerebronic acid, sphingosine, and galactose () (Citation1).
Figure 5. Structure of a galactocerebroside (today usually called galactosphingolipid). In sulfatides the R at carbon 3 in galactose is a sulfate group. A glycosphingolipid containing sialic acid in the carbohydrate unit of the cerebroside is named ganglioside.
The other important discovery was made together with Arne Tiselius and Harry Svensson in 1941 when Blix demonstrated the existence of lipoproteins and that the blood lipids preferentially were transported in the β-globulin fraction obtained on serum electrophoresis (Citation2).
The most important discovery, however, was made in 1936. This year Gunnar Blix isolated a crystalline compound from bovine submaxillary mucin (Citation3). This compound was later designated ‘sialic acid’ by Blix. Much of the further work on sialic acid(s) was carried out during the 1950s and in competition with several groups worldwide.
Two of these important discoveries were published in Hoppe-Seyler’s Zeitschrift für Physiologische Chemie, published by the Springer Verlag in Germany. This journal had high impact, as we say today, and German was an important scientific language in those days.
Mainly during the 1960s, but also after his retirement, Blix was very interested in research related to nutrition, food intake, and public health. The importance of iron in food and the problem of sufficient iron intake in connection with a low total calorie intake was discussed in some of his publications. In his role as executive member in the Swedish Foundation for Nutritional Research and also chief editor for the Swedish journal Näringsforskning (Nutritional research) he was able to influence the quality of ongoing nutritional research in Sweden at the time.
Blix became a member of the Royal Swedish Academy of Science in 1956 and received ‘Björkén’s award’ (Björkénska priset) in 1960 from Uppsala university for his studies on sialic acid. In 1965 he was awarded an honorary doctoral degree in medicine at the University of Oslo.
The early years and the discovery
The discovery of sialic acid was, as mentioned above, Blix’s most important contribution to science. This made him known internationally. In a lecture that Blix presented at the IVth International Congress of Biochemistry in Vienna 1958 (Citation4) he looks back, and in a fascinating resumé he describes his view on how the discovery was made in 1936 and what actually happened during the following 22 years.
There were many initial difficulties and mistakes, partly due to the peculiar distribution and properties of the molecules, association to both proteins and lipids, ability to form mixed crystals, lability, and tendency to decompose.
On the other hand one remarkable circumstance greatly favoured the study: the molecules occur mainly in terminal positions of the parent molecules and are easy to split off. Furthermore materials could be found that contained up to 20% or more of sialic acid. However, not until an isolation procedure giving a sufficient yield had been developed would it become possible to perform a more detailed structural analysis.
In the mid-1930s Blix got interested in the chemistry of salivary mucin. At that time the knowledge was restricted to the fact that the mucin contained hexosamine. Blix prepared the mucin using a method that his predecessor Olof Hammarsten had developed as early as 1881. When Blix arrived in Uppsala as associate professor in 1926, Professor Hammarsten was around 85 years old. It is tempting to guess that he visited his old laboratory in the English park and inspired the young Blix to look a little closer into the chemistry of mucin. Hammarsten died in 1932 and never witnessed the international breakthrough for sialic acid.
Blix continues his story in the lecture and tells us that ordinary acid hydrolysis resulted in the formation of black humins and, as known before, free hexosamine. Different enzymes were tried with no results. However, when water-insoluble submaxillary mucin was heated at 100°C and at its isoelectric point (pH 2.5–3.5) some peptide material together with the bulk of the carbohydrate was dissolved. After fractionation of the methanol-soluble part of this material with less polar solvents, one day suddenly there was, unexpectedly, growth of nice crystal colonies on the walls of the vessel (, left).
Figure 6. Mixed crystals of sialic acid isolated from bovine submaxillary mucin by Blix in 1936 (Citation4) (left) and crystals of N-acetylneuraminic acid isolated by Svennerholm in 1956 (Citation5) (right).

Blix does not tell us how his discovery was celebrated and if anyone other than himself was present. We can only guess that his loyal assistant Ewert Lindberg was there and that probably no celebration took place; but a toast in champagne would certainly have been appropriate.
This was how it started. It might be added that Blix in the course of his intensive studies of saliva personally developed a technique to contract the muscles around one of his submaxillary glands. By doing so he was able to eject a jet of saliva straight into a test tube. His method might have simplified the purification and certified the origin of the saliva.
A positive thing with the original experiment when crystals of sialic acid were discovered was that an optimal pH (2.5–3.5) had been chosen. The sialic acid was released but not decomposed. On the other hand it was most unfortunate that bovine submaxillary mucin was chosen as it would turn out later. If instead sheep or pig mucin had been used, a relatively stable form of sialic acid would have been found directly, and crystals like those in (right) might have been seen. Bovine mucin, however, contains at least two extremely labile forms of sialic acid.
The crystals obtained were recrystallized and elementary analyses were performed. The results showed a composition of C14H23CNO11. Acetyl determination showed two groups, one probably bound to nitrogen. The molecule gave a pink colour with Ehrlich’s reagent (p-dimethyl-amino-benzaldehyde). Colorimetric analytical methods in studies of carbohydrates (hexoses, fucose, hexosamines, etc.) were standard procedures at a time long before nuclear magnetic resonance (NMR) and mass spectrometry (MS) became available. The analytical results pointed towards a disaccharide composed of N-acetylhexosamine and a poly-hydroxy-acid with six carbon atoms.
Mucin research temporarily put aside. International competition evolves
Since no methods were available for a large-scale preparation of sialic acid that would enable a detailed characterization, further research on mucin was put aside in Blix’s laboratory for about 10 years. Instead Blix resumed his lipid research, and in that context he found in 1938 (Citation6) a product with striking similarities to sialic acid. In his studies of normal brain lipids he found a subfraction that gave a beautiful violet colour with Bial’s reagent (contains orcinol that forms coloured complexes with different carbohydrates when shaken in iso-amylalcohol). The material was purified and hydrolysed with acid. Fatty acids, sphingosine, and carbohydrate were liberated, but in contrast to the behaviour of cerebrosides this material became black like in humin formation.
It turned out that Klenk a few years earlier (in 1935) (Citation7) had found a lipid in brain tissue from a patient with Niemann–Pick’s disease. Klenk’s lipid fraction also gave black humin formation in acid hydrolysis, which led Blix to test his purified brain lipid with Ehrlich’s and Bial’s reagents: pink and violet colours, respectively, were obtained with both the brain lipid and the previously isolated sialic acid from mucin. Klenk later gave his new glycolipid the name ganglioside ().
A few years later (in 1941) (Citation8) Klenk degraded his ganglioside in methanolic (5%) hydrochloric acid and succeeded in isolating a crystalline methoxy derivative of an acid that he named neuraminic acid. Methoxy-neuraminic acid was more stable than sialic acid and had in contrast to the latter a free amino group. It reacted in the same way with Ehrlich’s and Bial’s reagents as sialic acid and displayed the same tendency to humin formation. Obviously it was a matter of closely related or possibly identical compounds. Only many years later was the relationship between the two compounds clarified.
Because of the introduction of freeze-drying techniques Blix returned to the sialic acid problem. It became possible to obtain fairly good yields of the substance from bovine submaxillary mucin. It could be demonstrated that the mucin contained sialic acid and galactosamine in approximately equimolar amounts. It was at this time (in 1952) (Citation9) that Blix introduced the name sialic acid internationally and also suggested that neuraminic acid simply was identical to deacetylated sialic acid. This result was later confirmed by Klenk’s group.
Influenza virus releases a carbohydrate with properties similar to those of sialic acid
The development of the field after 1950 was very much influenced by the studies on influenza virus haemagglutination carried out in Frank M. Burnet’s laboratory in Melbourne. From their studies it was known that the influenza virus attaches itself to erythrocytes which thereby agglutinate. On incubating the agglutinated cells at 37°C, the virus is released in intact form and the cells disaggregate. At the same time they lose their ability to bind the virus (Citation10). Burnet et al. also coined the term RDE (receptor-destroying enzyme) for the activity (later identified as neruraminidase) of the virus (Citation11). Of special interest in this context was a work by Gottschalk and Lind (of 1949) (Citation12) where they described that the influenza virus was able to release a carbohydrate from ovomucin with properties similar to those of sialic acid.
Blix describes in a letter (in 1975) to a friend (Klaus Störiko, Behringwerke AG, Marburg, Germany) how he first came in contact with Gottschalk:
... I recall how I at the first international biochemist congress in Cambridge 1949 talked with Stacey and Gottschalk, when the latter described the general properties of the carbohydrate component in mucins that inhibited virus haemagglutination and asked for advice from Stacey. It struck me immediately that some of the properties Gottschalk mentioned strongly reminded me about those of sialic acid. When I got home I mailed Gottschalk what we had written about sialic acid and told him that we intended to test the matter closer. Gottschalk obviously did not, at this time, know about sialic acid, which outside Cologne and Uppsala still was of low interest. I had on my part limited knowledge about Burnet’s investigations. We agreed later on to study in each of our laboratories the carbohydrate component in the well defined Tamm–Horsfall urinary protein and to publish our results in 1952 simultaneously in Nature...
In 1952 Lars Odin in Blix’s group reported in addition to the Tamm–Horsfall urinary protein a long series of biological materials (blood serum, plasma glycoproteins, human tears, cow’s milk, various epithelial mucins, human saliva, and meconium) inhibiting virus haemagglutination and all containing sialic acid (as judged from colorimetric measurements) (Citation13). Odin also showed that bovine submaxillary mucin had a lower inhibitory ability than corresponding mucin from sheep, although the content of sialic acid was the same. The explanation to this phenomenon was that the submaxillary mucin from sheep, pig, horse, and cow all contained different forms of sialic acid, as was also demonstrated by different X-ray diffraction patterns. In sheep, N-acetylneuraminic acid was found, whereas in pig, N-glycolylneuraminic acid dominated. In horse and cow different forms of N- and O-diacetylated forms were isolated. Preparative chromatography carried out on special cellulose columns turned out to be an effective way to separate different forms of sialic acid. A review on all the achievements from Blix’s group was published in Acta Societatis Medicorum Upsaliensis in 1956 (Citation14).
Blix received many letters from research groups asking for samples of the new crystalline compounds. In general, Blix was very generous and sent out hundreds of vials with sialic acid to scientists around the world. This contributed to the intensive international race that took place in the mid-1950s to find out the correct structure. Beside Blix’s group in Uppsala there were the groups of Ernst Klenk in Cologne, Richard Kuhn in Heidelberg, Tamio Yamakawa in Tokyo, Alfred Gottschalk in Melbourne, and Noboro Hiyama in Sendai. Several suggestions were published, but finally Gottschalk reported a structure that would turn out to be correct (Citation15).
A short note was published in Nature in 1957 (Citation16) informing readers that Blix, Gottschalk, and Klenk had reached an agreement on the nomenclature of the substance, i.e.:
In order to avoid further confusion we propose to call the basic, unsubstituted compound neuraminic acid. Sialic acid is suggested as a group name for the acylated neuraminic acids (for example N-acetylneuraminic acid, N-glycolylneuraminic acid, diacetylneuraminic acids). For the enzyme which splits the glycosidic linkage joining the terminal sialic acid to the residual oligo- or polysaccharide the names neuraminidase and sialidase may be used synonymously (Citation16).
International breakthrough for sialic acid
Today it is nearly 80 years since Blix made his discovery, and about 60 years since its international breakthrough started. Around 1980, more than 30 types of sialic acid were known, and after the finding of 2-keto-3-deoxynononic acid (abbreviated Kdn) () the family has expanded to more than 50 members. Structures, biosynthesis, distribution, and biological roles have been studied by several groups.
Figure 7. Structures of N-acetylneuraminic acid (Neu5Ac), N-glycolylneuraminic acid (Neu5Gc), and 2-keto-3-deoxynononic acid (Kdn).
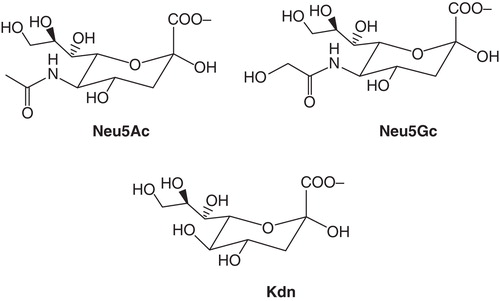
There are a number of recent and excellent review articles covering these fields (Citation17-22). A few aspects of general interest have been selected and are emphasized below. N-acetylneuraminic acid (Neu5Ac), N-glycolylneuraminic acid (Neu5Gc), and N-acetyl-9-O-acetylneuraminic acid (Neu5,9Ac2) are the three most frequently occurring forms. Only Neu5Ac is ubiquitous, while the others are not found in all species. The animal with the largest number of different sialic acids known so far is the cow. In its submandibular (previously: submaxillar) gland 15 types were detected. In man the number of different forms is much smaller, with Neu5Ac as the most abundant. Sialic acid has not been found in plants, despite intensive search. Neu5Ac and Neu5Gc () differ by a single oxygen atom, which is added to CMP-Neu5Ac via a reaction catalysed by the enzyme cytidine monophosphate N-acetylneuraminic acid hydroxylase (CMAH).
An interesting chemical difference exists between man and the great apes. In contrast to the latter, man has lost the expression of Neu5Gc due to a mutation in the gene encoding the hydroxylase (CMAH). The genes of these apes exhibit over 98% sequence homology with man. The event when the hydroxylase activity was lost is assumed to have occurred 2.5 million years ago. One can only speculate to what extent the lack of Neu5Gc has influenced the development of man and especially his brain.
Neu5Gc was first considered to be an onco-fetal antigen in humans since it was absent in normal adult tissues but found in fetal tissues and in some human tumours. Furthermore, antibodies directed against Neu5Gc were found in patients with cancer and certain infectious diseases. With sensitive techniques traces of Neu5Gc have now been found also in normal human tissues. It is generally believed, however, that this represents incorporation from dietary sources such as red meat and milk products. Neu5Gc is today considered to be a xeno-antigen in man, since most healthy humans have some circulating anti-Neu5Gc antibodies. The question has also been raised whether this might account for the higher frequency of atherosclerosis and epithelial cancers in humans, diseases that are considered to be correlated with red meat consumption.
General functions
The external position of sialic acid on glycoproteins and gangliosides and the high expression on outer cell membranes (e.g. more than 10 million molecules per human erythrocyte) imply a strong influence in cell biology. Some functions are certainly related to their strong electronegative charge, for instance stabilizing the conformation of proteins and enhancing the viscosity of mucins. Sialic acids are also involved in the binding and transport of positively charged molecules including pharmaceuticals as well as in the attraction and repulsion of cells and molecules.
Another important role is their ability to mask antigenic sites, receptors, and penultimate galactose residues. After desialylation, molecules and cells can be bound, for instance by macrophages and hepatocytes via galactose-recognizing receptors, and also be taken up and degraded. This has been extensively studied with serum glycoproteins and blood cells.
Industrial production of glycoprotein pharmaceuticals using recombinant techniques often results in incomplete glycosylation. The carbohydrate chains become truncated and are lacking the terminal sialic acid. The half-life of such molecules in circulation is short because of rapid uptake in the liver but can be extended again by correct sialylation, which can be carried out on an industrial scale.
The role of sialic acid in receptors for microbial lectins
Sialic acids themselves can also serve as receptors for a variety of microbial and animal lectins. Some of these lectins were first discovered in viruses (see above) because of their ability to agglutinate red cells in vitro, and by the loss of this ability after sialidase treatment of the target cells.
Most sialic acid-containing virus receptors contain terminal sialic acid attached to a penultimate galactose in either an α(2–6)- or an α(2–3)-linkage. The different tissue distribution of α(2–6)-and α(2–3)-linked sialic acid influences the tissue tropism of many viruses. For instance certain adenoviruses have a tropism for the eye and bind preferentially to α(2–3)-linked sialic acid which in fact is the most frequent linkage type in the corneal and conjunctival cells. Human influenza virus is another example where its haemagglutin (HA) preferentially binds to α(2–6)-linked sialic acid. This type of linkage is most abundant in the upper respiratory tract. Avian influenza HA on the other hand binds best to the α(2–3)-linked sialic acid, more frequent in the lower respiratory tract. Following virus replication in the infected cell, the neuraminidase also present in the influenza virus membrane removes its substrate (sialic acid) from infected cell surfaces so that newly made viruses are released to infect more cells. Many other viruses also attach to cells via sialic acid, the most prominent being corona, polyoma, paramyxo, and rota viruses.
Bacteria produce carbohydrate-specific adhesins located on their fimbriae or pili. Examples of colonization via sialic acid-containing receptors are certain strains of Escherichia coli, Streptococci, and Helicobacter pylori.
Bacterial toxins, such as cholera, tetanus, and diphtheria toxins, bind firmly to sialic acid-containing receptors, mostly those located on gangliosides, as basis for their pathophysiological activity.
The receptor–ligand interactions may be inhibited with soluble ligands such as sialic acid-containing oligosaccharides or glycoproteins. In human milk and especially in the colostrum there is a high content of sialic acid-rich oligosaccharides. The milk from each mammalian species contains different and more or less sialylated glycans. These carbohydrates are considered to regulate the species-specific colonization of the intestine by micro-organisms and to prevent the attachment of pathogenic bacteria, such as E. coli and Helicobacter strains, or pathogenic viruses, e.g. rota viruses, which is especially important in newborns.
Essential nutrients for brain development
In this context it should be noted that the high content of sialic acid in breast milk, especially during the first days after birth, was shown to increase the brain ganglioside content in rats, pigs, and man. It is considered important to have a sufficient amount of biochemical precursors available during the rapid neural growth that takes place postnatally. Polysialylated neural cell adhesion molecules (NCAM) and neural gangliosides both play critical roles in mediating cell–cell interactions which are important for neuronal outgrowth, synaptic connectivity, and memory formation. It has even been suggested that the sialic acid-rich glycans given to the child via breast milk permanently promote higher intelligence. Infant formulae, which are based on bovine milk, have in contrast to the human milk a much lower content of sialic acid. Furthermore human milk contains mainly Neu5Ac, whereas bovine milk has got both Neu5Ac and Neu5Gc.
The role of sialic acid in receptors for mammalian internal lectins
One example of sialic acid-binding molecules is the selectins, which belong to the group of internal lectins. They are located on endothelial cells and participate in the initial stage of adhesion of white blood cells to endothelia. In the course of this process the leukocytes roll along the vascular walls, adhere, and penetrate into the tissues below which have been damaged by the lack of oxygen (after transplants or infarcts for example) or in connection with an inflammation. Cytokines play an essential role in this context.
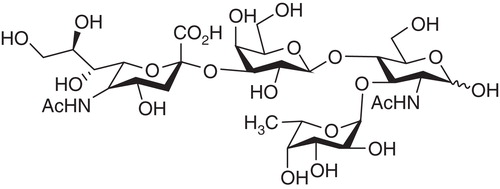
Selectins specifically recognize sialylated Lewis structures such as sialyl-Lex, which are bound to glycoproteins or glycolipids (). Since the sialyl-Lex structure is also present on the surface of some tumour cells, selectins can be involved in the formation of metastases. In order to prevent further tissue damage by evading leukocytes and to prevent metastases of tumour cells, great efforts have been made to prevent cell adhesion to endothelial cells by the application of either selectin antibodies or sialyl-Lex analogues.
Figure 8. Structure of sialylated Lewis X. A tetrasaccharide linked to a galactose unit in either glycoproteins or glycolipids. (The formula is: Neu5Ac-α(2,3)Galβ(1,4)(Fuc-α1,3)-GlcNAc).
Using ultrasensitive analysis by mass spectrometry of glycans linked to the human zona pellucida (ZP), it has been shown that this matrix is coated with a high density of complex type N-glycans terminated with the sialyl-Lex sequence, the universal selectin ligand. Subsequent inhibition studies showed that the free sialyl-Lex tetrasaccharide or neoglycoproteins terminated with this sequence inhibited sperm–ZP binding by 70% (Citation23). The results support the hypothesis that both lectin-like and protein–protein interactions play an essential role in human gamete interactions.
Another group of mammalian internal lectins are the Siglecs (sialic acid-binding immunoglobulin-like lectins). Many members of this family have been discovered during recent years, mainly due to the increased availability of expressed sequence tags (EST) and genomic DNA sequences. The first well-characterized type was macrophage sialoadhesin, involved in binding and nursing of maturing blood cells. Most other documented Siglecs participate in the interactions between B- and T-lymphocytes and are thereby regulators of the immune system. These lectins vary in the recognition of different sialic acids and can also distinguish between various glycosidic linkages.
Concluding remarks
In recent years carbohydrate chains, or glycans, have emerged from historical obscurity to the formation of the specialized field of Glycobiology, which refers to the molecular and cellular biology of glycans. The reason for the relatively late development of glycobiology is mainly technical, as glycans were more complex and difficult to study compared with nucleic acids, proteins, and lipids. The story around Blix’s discovery of sialic acid is a good illustration of this. This brief review provides only some examples of the role of sialic acid in glycobiology. Maybe we are only seeing ‘the tip of the iceberg’ and many more functions of sialic acids remain to be uncovered. Future research is much needed and will most likely increase our possibilities to fight against the many infectious, immunological, malignant, psychiatric, and degenerative diseases in which sialic acids are involved.
Declaration of interest: The author reports no conflicts of interest. The author alone is responsible for the content and writing of the paper.
References
- Blix G. Zur kenntnis der schwefelhaltigen Lipoidstoffe des Gehirns. Über Cerebronschwefelsäure. Z Physiol Chem. 1933;219:82–98.
- Blix G, Tiselius A, Svensson H. Lipids and polysaccharides in electrophoretically separated blood serum proteins. J Biol Chem. 1941;137:485–94.
- Blix G. Über die Kohlenhydratgruppen des Submaxillaris mucins. Z Physiol Chem. 1936;240:43–54.
- Blix G. Sialic acids. Proc Intern Congr Biochem 4th Vienna. 1958;1:94–106.
- Svennerholm L. On the isolation and characterization of N-acetyl-sialic acid. Acta Soc Med Upsalien. 1956;61:75–85.
- Blix G. Einige Beobachtungen über eine hexosaminhaltige Substanz in der Protagonfraktion des Gehirns. Scand Arch Physiol. 1938;80:46–51.
- Klenk E. Über die Natur der Phosphatide und anderer Lipoide des Gehirns und der Leber bei der Niemann-Pickschen Krankheit. Z Physiol Chem. 1935;235:24–36.
- Klenk E. Neuraminsäure, das Spaltprodukt eines neuen Gehirnlipoide. Z Physiol Chem. 1941;268:50–8.
- Blix G, Svennerholm L, Werner I. The isolation of chondrosamine from gangliosides and from submaxillary mucin. Acta Chem Scand. 1952;6:358–62.
- Hirst G. The agglutination of red cells by allantoic fluid of chick embryos infected with influenza virus. Science. 1941;94:22–3.
- Burnet FM, Stone JD. The receptor-destroying enzyme of V. cholerae. Aust J Biol Med Sci. 1947;3:227–33.
- Gottschalk A, Lind PE. Product of interaction between influenza virus enzyme and ovomucin. Nature. 1949;164:232–3.
- Odin L. Carbohydrate residue of a urine mucoprotein inhibiting influenza virus haemagglutination. Nature. 1952;170:662–3.
- Blix G, Lindberg E, Odin L, Werner I. Studies on sialic acids. Acta Soc Med Upsalien. 1956;61:1–25.
- Gottschalk A. Structural relationship between sialic acid, neuraminic acid and 2-carboxypyrrole. Nature. 1955;176:881–2.
- Blix G, Gottschalk A, Klenk E. Proposed nomenclature in the field of neuraminic and sialic acids. Nature. 1957;179:1088.
- Schauer R. Sialic acids: fascinating sugars in higher animals and man. Zoology. 2004;107:49–64.
- Schauer R. Sialic acids as regulators of molecular and cellular interactions. Curr Opin Struct Biol. 2009;19:507–14.
- Varki A, Schauer S. Sialic acids. In Varki A, Cummings RD, Esko JD, et al. editors. Essentials of glycobiology. 2nd ed. 2009. Chapter 14 Available at http://www.ncbi.nlm.nih.gov/books/NBK1920/#ch14.s1.
- Varki A. Sialic acids in human health and disease. Trends Mol Med. 2008;14:351–60.
- Gamblin SJ, Skehel JJ. Influenza hemagglutinin and neuraminidase glycoproteins. J Biol Chem. 2010;285:28403–9.
- Wang B. Molecular mechanism underlying sialic acid as an essential nutrient for brain development and cognition. 2012;3:465S–72S.
- Clark GF. The role of carbohydrate recognition during human sperm-egg binding. Hum Reprod. 2013;28:566–77.