Abstract
Background. Cardiovascular mortality is high in hemodialysis (HD) patients. Early arterial pressure wave reflections predict mortality in HD patients, and HD acutely improves the central pressure waveform. Potassium (K) plays a crucial role in cardiac electrophysiology, and patients with end-stage kidney disease depend on HD for neutral K balance. We aimed to study the impact of dialysate K concentrations on central arterial pressure waveform.
Methods. Thirty-three chronic HD patients were studied before and after a HD session, and the prescribed dialysate K concentration was recorded. In a subset of 23 patients without arrhythmias, pulse wave analysis was performed on radial arteries. Nine patients had dialysate K set to 1 mmol/L (group 1), and 14 patients had K set to 2 or 3 mmol/L (group 2). Augmentation index (AIx), defined as difference between the second and first systolic peak divided by central pulse pressure, was used as a measure of arterial stiffness.
Results. HD reduced the AIx in group 1 only (p = 0.0005). Likewise, central systolic pressure was reduced in group 1 only (p = 0.006). The relative reduction of AIx post-HD was significantly higher in group 1 compared with group 2 (p < 0.0001). The association between low dialysate K and AIx reduction remained statistically significant after adjustment for variables including the change in central and peripheral systolic pressure and mean arterial pressure.
Conclusion. Low dialysate K is strongly and independently associated with the acute improvement of AIx.
Introduction
Chronic kidney disease (CKD) is associated with high mortality from cardiovascular disease (CVD), and patients with end-stage kidney disease are exposed to the highest risk (Citation1). Currently, our possibilities to reduce the risk of CVD in this population are limited because the few treatment trials targeting established CVD risk factors provide no convincing effect on outcome (Citation2-6). Hemodialysis (HD) patients have a cardiac mortality pattern that differs from the general population. Notably, cardiac arrest accounts for approximately a quarter of all deaths and is more common on the day before dialysis after the weekend HD hiatus (Citation7).
Potassium (K) plays a crucial role in cardiac electrophysiology, and patients with end-stage kidney disease depend on HD to maintain neutral K balance. In order to avoid pre-dialysis hyperkalemia, dialysate K is usually adjusted to suboptimal. However, dialysate K of 1 mmol/L is not recommended as a standard in order to avoid high blood-to-bath K gradient (Citation8).
Patients in HD have stiff arteries and impaired endothelial function. Left ventricular hypertrophy (LVH) is common and convincingly associated with mortality (Citation1,9,10). Pulse wave analysis (PWA) provides a non-invasive assessment of central pressure waveform, the shape of which is influenced by ventricular ejection, arterial stiffness, and smooth muscle tone that influences pressure wave reflection. Increased arterial wave reflections from the periphery leading to augmentation of central systolic blood pressure (BP) increase left ventricular afterload and predispose to LVH (Citation11). The effect of a HD session on the improvement of augmentation index (AIx) has previously been reported (Citation12-16). A rapid decrease in serum K during the initial stage of HD has been shown to translate into a decrease in peripheral resistance (Citation17). To our knowledge, the impact of dialysate K on AIx changes has not been described. Therefore, we aimed to investigate the association in a single HD session.
Methods
Subjects
All chronic HD patients in Uppsala who had been on HD for two months or more and had one fistula-free arm were invited to participate. The study was carried out in two dialysis centers in Uppsala, Sweden, in 2003–2004. Criteria for exclusion were: unwillingness to participate, atrial fibrillation, hemodynamic instability at >5% of dialyses, HIV, B or C hepatitis-positivity, severe anemia, or aortic valve insufficiency. The study was conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent, and the Regional Ethics Review Board in Uppsala approved the trial.
Pulse wave analysis
Measurements were performed before the initiation of HD and 10 min after HD. Resting brachial BP was measured in the non-fistula arm using Omron automated sphygmomanometer. PWA to assess arterial stiffness was performed non-invasively using the SphygmoCor apparatus (AtCor Medical, Sydney, Australia). All measurements were taken at least in duplicate in the radial artery of the non-fistula arm using a micromanometer (SPC-301; Millar Instruments, Houston, TX, USA) applying the principle of applanation tonometry to flatten the artery by gentle pressure. The recording place on the wrist was marked to guarantee the repeated measurement from the same site. The aortic pressure waveform was generated from an averaged radial artery waveform (derived from 20 sequentially recorded radial artery waveforms) using a validated transfer function (Citation18). The software allowed to control for the objectivity of measurements by setting quality control parameters on the radial artery waveform recordings. If any of the parameters on a given recording were outside the predetermined acceptable limits (<100 mV for pulse height, >5% for systolic and diastolic variability), the recording was excluded. The reproducibility of the method in kidney patients has been previously described (Citation19). Computerized analysis of the central waveform allows determination of central BP, augmentation pressure, and AIx that is augmentation pressure expressed as percent of the central pulse pressure. Augmentation pressure is an increase in aortic pressure after the peak of blood flow and is determined by ventricular ejection, pulse wave velocity, and reflective properties of the peripheral vessels (Citation20). Taking into consideration the influence that HD may have on heart rate (HR), AIx was normalized by software to a HR of 75 beats per min.
Statistical analysis
Data are expressed as mean ± SD for normally distributed variables and median (range) for data with a non-normal distribution. Skewed data was log-normalized. Fisher’s exact test was used to detect differences in proportions, and t test was used to detect differences in means in the groups. Two-tailed paired t test was used to detect the effects of HD. The correlation between variables was evaluated using Pearson’s correlation coefficients. p < 0.05 was regarded as significant.
Results
In total, 33 chronic HD patients participated in the study. The dialysis bath buffer consisted of bicarbonate, dialysate calcium range was 1.25–1.5 mmol/L, and K range 1–3 mmol/L. Seven patients were dialyzed with high-flux membranes. All dialysis settings were kept as prescribed with no modifications made for study purposes.
The full set of pre- and post-HD PWA could be obtained from 23 patients, because 10 patients presented with arrhythmias leading to PWA readings outside the predetermined acceptable quality limits.
Nine patients had dialysate K set to 1 mmol/L (group 1, n = 9), 10 patients had K set to 2 mmol/L, and four patients had K set to 3 mmol/L. Due to the small number of patients with dialysate K 3 mmol/L, the patients with dialysate K 2 and 3 mmol/L were studied together as group 2, n = 14.
Patients in group 1 had longer dialysis vintage, higher pre-HD creatinine, albumin, and triglycerides, and lower HDL-cholesterol (). A single HD session significantly reduced AIx in group 1 (p = 0.0005) but not in group 2 (p = 0.5179) (). There was an effect of HD on peripheral systolic BP and mean arterial pressure (MAP) reduction in group 1 (p = 0.0238 and p = 0.009, respectively) but not in group 2 (). Likewise, HD effected a decrease of the central systolic BP in group 1 only (p = 0.006).
Table I. Characteristics of patients treated with hemodialysis, divided in two groups by concentration of dialysate potassium.
Table II. Hemodynamic parameters in patients treated with hemodialysis, divided in two groups by concentration of dialysate potassium.
The relative reduction of AIx post-HD was higher in group 1 when compared with group 2 (). Also, the relative reduction of central systolic BP and MAP was higher in group 1 when compared with group 2 ().
Figure 1. Relative change of augmentation index in the two study groups (Group 1 = dialysate potassium 1 mmol/L; Group 2 = dialysate potassium 2 or 3 mmol/L) is presented using a boxplot with the square representing the 25th–75th percentile, the T-lines the 10th–90th percentile, and the circles representing observations <10th and >90th percentile.
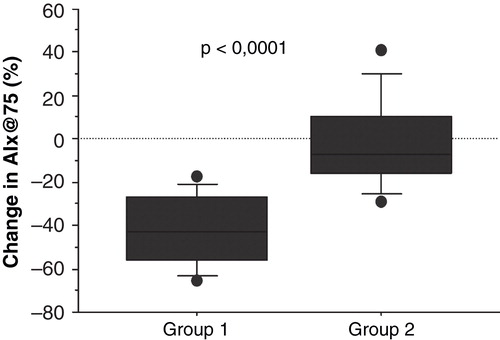
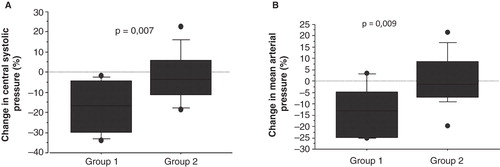
Figure 2. Boxplots of relative change of central systolic pressure and mean arterial pressure in the two study groups. A: Relative change of central systolic pressure. B: Relative change of mean arterial pressure. (Group 1 = dialysate potassium 1 mmol/L; Group 2 = dialysate potassium 2 or 3 mmol/L). The square represents the 25th–75th percentile, the T-lines the 10th–90th percentile, and the circles representing observations <10th and >90th percentile.
A more detailed analysis of the effect of HD on AIx revealed that the relative reduction in AIx was higher in patients with dialysate K 1 mmol/L compared with patients with dialysate K 2 mmol/L (p < 0.0001) and compared with the group with dialysate K 3 mmol/L (p = 0.0129). There was no difference between dialysate groups K 2 and 3 mmol/L (p = 0.1405).
After multiple bivariate adjustments (one model per adjusting factor) for dialysis vintage, age, post-HD calcium, HDL-cholesterol, triglycerides, pre-dialysis creatinine, albumin, and changes in central and peripheral systolic BP and MAP, the association between low dialysate K and AIx reduction remained statistically significant (p < 0.004 in all models).
Change in AIx correlated positively with dialysis vintage (p = 0.01). However, the association lost its statistical significance after adjustment for HDL-cholesterol or albumin.
Discussion
These observational data demonstrate an independent association between low dialysate K concentration and acute improvement of AIx in prevalent HD patients. To our knowledge, the association has not been described previously, and studies investigating the acute effects of HD on AIx have not accounted for dialysate K (Citation12-16).
Mardare et al. investigated the change in AIx intradialytically and found a fall in AIx to be the largest during the first two hours of the dialysis session (Citation12). Although dialysate K was not reported in that study, extracellular K reduction is, as a rule, most pronounced at the beginning of the dialysis session. The authors also reported that plasma K pre-HD did not correlate with a decrease in AIx (Citation12).
Clearly, low dialysate K is not recommended, and our study is based on historical data from the period when the current recommendations had not yet been adopted (Citation8). The pronounced improvement of AIx in the low dialysate K group in the current study should be considered as an acute phenomenon and not extrapolated to long-term benefit. In fact, pre-HD AIx in the study groups did not differ, and a return of AIx to pre-HD levels at 24–48 h after HD has previously been described (Citation13).
The mechanisms leading to the reduction of AIx when extracellular potassium is acutely lowered remain unclear. In animal models, acute hypokalemia results in vasoconstriction mediated by the endothelium and an increase in myocardial contractility, and an increase in plasma K reverses the effect (Citation21,22). Patients dialyzed with low K have been described to develop prolonged QT interval which, in turn, exposes the patients to the risk for sudden cardiac death (Citation23). Longer QT interval is associated with higher AIx in the general population and therefore cannot explain the association between low dialysate K and pronounced AIx reduction (Citation24).
A previous study investigating hemodynamic consequences of rapid K lowering in HD reports a decrease in systolic and mean BP, mediated by a decrease in peripheral resistance (Citation17). Clearly, BP is a critical factor affecting the amplitude of the wave reflected at peripheral sites and thereby AIx. In the current study, the association between low dialysate K and AIx reduction remained statistically significant after adjustment for changes in central and peripheral systolic BP or MAP. This indicates that the decrease in AIx may be caused by alterations at the level of small arteries resulting in elevated wave reflection coefficients, without modification of the total peripheral resistance (Citation16). It has previously been demonstrated that HD does not affect pulse wave velocity (PWV) (Citation16). We have no data concerning systemic vascular resistance or PWV in the studied cohort and, therefore, can only speculate that factors other than large artery stiffness contribute.
The observational nature of the study is an obvious limitation. It is conceivable that low dialysate K was prescribed to patients with highest pre-HD plasma K. This, in turn, may indicate that these patients were better nourished. We adjusted for factors related to nutrition status, but the association between dialysate K and AIx remained significant. We have no data on plasma K pre- and post-HD, and the lack of registration of blood-to-bath K gradient in parallel with serial recordings of AIx and pH in the course of HD is also a limitation. Ideally, a prospective cross-over design with dialysate K modifications would have been preferred; however, results from this small cross-sectional study were robust.
In conclusion, low dialysate K is strongly and independently associated with the acute improvement of AIx. The pathophysiological mechanisms responsible for the acute phenomenon remain to be elucidated. Future studies investigating AIx in HD patients will hopefully clarify the impact of dialysate K concentrations.
Funding
The study was supported by grants from the Swedish Society of Nephrology and Swedish Kidney Association.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108:2154–69.
- Holdaas H, Fellstrom B, Jardine AG, Holme I, Nyberg G, Fauchald P, et al. Effect of fluvastatin on cardiac outcomes in renal transplant recipients: a multicentre, randomised, placebo-controlled trial. Lancet. 2003;361:2024–31.
- Wanner C, Krane V, Marz W, Olschewski M, Mann JF, Ruf G, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238–48.
- Fellstrom BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360:1395–407.
- Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181–92.
- EVOLVE Trial Investigators. Chertow GM, Block GA, Correa-Rotter R, Drueke TB, Floege J, et al. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012;367:2482–94.
- Ostermann M. Cardiac arrests in hemodialysis patients: an ongoing challenge. Kidney Int. 2008;73:907–8.
- Weisberg LS, Rachoin JS. The safety of low-potassium dialysis. Semin Dial. 2010;23:556–60.
- London G, Guerin A, Pannier B, Marchais S, Benetos A, Safar M. Increased systolic pressure in chronic uremia. Role of arterial wave reflections. Hypertension. 1992;20:10–19.
- Joannides R, Bakkali EH, Le Roy F, Rivault O, Godin M, Moore N, et al. Altered flow-dependent vasodilatation of conduit arteries in maintenance haemodialysis. Nephrol Dial Transplant. 1997;12:2623–8.
- Vlachopoulos C, Aznaouridis K, O’Rourke MF, Safar ME, Baou K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. Eur Heart J. 2010;31:1865–71.
- Mardare NG, Goldsmith DJ, Gusbeth-Tatomir P, Covic A. Intradialytic changes in reflective properties of the arterial system during a single hemodialysis session. Hemodial Int. 2005;9:376–82.
- Covic A, Goldsmith DJ, Panaghiu L, Covic M, Sedor J. Analysis of the effect of hemodialysis on peripheral and central arterial pressure waveforms. Kidney Int. 2000;57:2634–43.
- Cross JM, Donald A, Vallance PJ, Deanfield JE, Woolfson RG, MacAllister RJ. Dialysis improves endothelial function in humans. Nephrol Dial Transplant. 2001;16:1823–9.
- Soveri I, Lind L, Wikstrom B, Zilmer M, Zilmer K, Fellstrom B. Improvement in central arterial pressure waveform during hemodialysis is related to a reduction in asymmetric dimethylarginine (ADMA) levels. Nephron Clin Pract. 2007;106:c180–6.
- Georgianos PI, Sarafidis PA, Malindretos P, Nikolaidis P, Lasaridis AN. Hemodialysis reduces augmentation index but not aortic or brachial pulse wave velocity in dialysis-requiring patients. Am J Nephrol. 2011;34:407–14.
- Gabutti L, Salvade I, Lucchini B, Soldini D, Burnier M. Haemodynamic consequences of changing potassium concentrations in haemodialysis fluids. BMC Nephrol. 2011;12:14.
- O’Rourke MF, Gallagher DE. Pulse wave analysis. J Hypertens Suppl. 1996;14:S147–57.
- Savage MT, Ferro CJ, Pinder SJ, Tomson CR. Reproducibility of derived central arterial waveforms in patients with chronic renal failure. Clin Sci (Lond). 2002;103:59–65.
- O’Rourke MF, Staessen JA, Vlachopoulos C, Duprez D, Plante GE. Clinical applications of arterial stiffness; definitions and reference values. Am J Hypertens. 2002;15:426–44.
- Amberg GC, Bonev AD, Rossow CF, Nelson MT, Santana LF. Modulation of the molecular composition of large conductance, Ca(2+) activated K(+) channels in vascular smooth muscle during hypertension. J Clin Invest. 2003;112:717–24.
- Prasad K, Koob R. Cardiovascular function in dogs with acute hypokalemia. Angiology. 1978;29:589–600.
- Severi S, Grandi E, Pes C, Badiali F, Grandi F, Santoro A. Calcium and potassium changes during haemodialysis alter ventricular repolarization duration: in vivo and in silico analysis. Nephrol Dial Transplant. 2008;23:1378–86.
- Tabara Y, Takahashi Y, Kohara K, Setoh K, Kawaguchi T, Terao C, et al. Association of longer QT interval with arterial waveform and lower pulse pressure amplification: the Nagahama Study. Am J Hypertens. 2013;26:973–80.
