Abstract
Background: The size of inhaled particles influences where they deposit and theoretically should be important for the development of airway inflammation and responsiveness. Our aim was to assess if sensitization to smaller-sized aeroallergens relates to higher prevalence of treated asthma, increased airway responsiveness, and airway and systemic inflammation.
Methods: Molecular-based IgE antibody determination was done in 467 subjects. Sensitized subjects were grouped based on the particle size of the aeroallergen: (1) Large particles only (mainly pollen); (2) Medium-sized particles (sensitized to mainly mite and mold and possibly to large particles); and 3) Small particles (sensitized to pet allergens and possibly to medium- and/or large-sized particles). Airway responsiveness to methacholine, exhaled nitric oxide (FENO), and serum eosinophil cationic protein (S-ECP) were measured. Asthma and rhinitis were questionnaire-assessed.
Results: Subjects sensitized to small particles had higher prevalence of treated asthma (35% versus 10%, P < 0.001), higher FENO50 (32 versus 17 ppb, P < 0.001), higher S-ECP (10 versus 7.5 ng/mL, P = 0.04), and increased bronchial responsiveness (dose-response slope, 5.6 versus 7.5, P < 0.001) compared with non-atopics. This was consistent after adjusting for potential confounders. Sensitization to only large or to medium and possibly also large aeroallergen particles was not related to any of these outcomes after adjustments.
Conclusions: Sensitization to smaller particles was associated with a higher prevalence of asthma under treatment, higher airway responsiveness, and airway and systemic inflammation. Mapping of IgE sensitization to small particles might help to detect subjects having increased airway and systemic inflammation and bronchial responsiveness, indicating increased risk of developing asthma.
Introduction
With the advance of multiplex, molecular-based technique it is possible to obtain information on profiles of IgE sensitization to more than 60 individual aeroallergens in one assay (Citation1). The profile of IgE sensitization is important as IgE sensitization against perennial aeroallergens is related to asthma, airway inflammation, and hyperresponsiveness stronger than seasonal aeroallergens. This association of allergic sensitization patterns with airway inflammation (assessed by the fraction of nitric oxide in exhaled air, FENO) can be found both in a general population and asthma subjects (Citation2).
Inflammation is a key feature in asthma (Citation3), and anti-inflammatory treatment in the form of inhaled corticosteroids is currently the most effective treatment for subjects with persistent atopic asthma. Recent studies highlight even the importance of systemic eosinophil inflammation for asthma outcomes such as asthma attacks or asthma-related emergency room visits (Citation4). We have recently reported (Citation2) an independent relation of IgE sensitization to aeroallergens (furry animals and mold) with systemic eosinophil inflammation (blood eosinophil counts) in patients with asthma. Serum eosinophilic cationic protein (S-ECP) is another marker of systemic eosinophil inflammation in asthma and allergic diseases (Citation5). To our knowledge only one previous study used the information form the multiplex IgE determination (animal-derived lipocalin, kallikrein, and secretoglobin) in order to relate to systemic eosinophil inflammation (blood eosinophils) in asthma (Citation6).
The dysfunction of the small airways (those with internal diameters of 2 mm or less) is associated with worse control of asthma and higher airway responsiveness (Citation6–8). Particle size is the most important aerosol characteristic that determines the site of airway deposition of inhaled drugs (Citation9), and several other studies (Citation10–12) demonstrated the deposition patterns of inhaled particles in relation to particle size and the importance of particle size of inhaled aeroallergens.
The alveolar region deposits mostly particles varying from 1 to 4 μm (Citation12–14), although even a proportion of 4% to 15% of large particles may deposit in the alveoli (Citation15). A majority of larger particles tend to deposit in the trachea and oropharyngeal tract (Citation12–14), and inhaled pollen particles with a diameter of 10 to 70 μm (Citation16) localize mainly in the tracheobronchial tree. Moreover, mathematical modeling of pulmonary NO dynamics can estimate fractional concentration of NO in the gas phase of the alveolar or acinar region (CANO). CANO has been investigated as a potential biomarker of small airways inflammation (Citation17), but the impact of IgE sensitization to allergen particles of small size on alveolar NO has been poorly studied (Citation18).
The aim of the present study was to examine the hypothesis that the size of inhaled particles influences their deposition area and therefore is also important for the development of airway inflammation and responsiveness. In particular, we aimed to investigate if there is a difference regarding levels of airways and systemic inflammation, airway responsiveness, and prevalence of asthma under treatment with inhaled corticosteroids, in relation to IgE sensitization to small compared to medium and large allergen particles.
Methods
Population
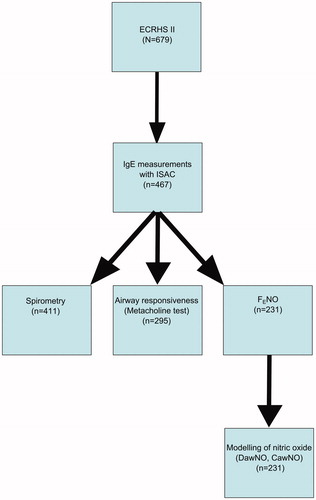
This study is a retrospective analysis of previous data based on subjects who participated in the European Community Respiratory Health Survey (ECRHS) II, which is the 10-year follow-up of ECRHS I. The design of ECRHS I and II has been published in detail (Citation19). In the Uppsala study center, 679 subjects were reinvestigated in ECRHS II. This study included 467 people who were examined with component-resolved IgE antibody determination, based on microarray techniques (ImmunoCap ISAC; Phadia/Thermo Fisher Scientific, Uppsala, Sweden), of whom 96 fulfilled criteria for current asthma. The characteristics of the 212 subjects who were not examined with ISAC are presented in Table E1 (available online). Those that were lost in the follow-up were more often females, younger, with lower prevalence of rhinitis and asthma. Lung function test results (forced expiratory volume in 1 s (FEV1) absolute levels and FEV% predicted) were available for 411 subjects. Eosinophil cationic protein (ECP) was measured in blood after our recent work (Citation2), where we correlated allergic sensitization patterns with systemic eosinophil inflammation (blood eosinophil counts) in patients with asthma. Therefore we wanted to analyze this correlation as well in a population-based material, and serum samples were available for measuring ECP. Moreover, in the present study we also analyzed nitric oxide (NO) flow-independent parameters, such as tissue concentration of NO of the airway wall (CawNO), airway transfer factor for NO (DawNO), and FENO (Citation20). These were available for 231 subjects. Methacholine challenge test results were available from 295 subjects, and ECP measurements for 404 subjects (). All these measurements were conducted randomly throughout the whole year both outside and during pollen and mold season. In the previously published results from the same material we grouped allergens as perennial/pollen/food allergens and had as outcomes asthma, bronchial responsiveness, and FENO (Citation1). No data were available on ECP, and no data on NO flow-independent parameters were used in our previous publication (Citation1).
Table I. Subjects sensitized to small particles were younger and used more inhaled corticosteroids than non-atopics. Sex, height, and weight did not differ in between groups.
IgE sensitization
The presence of IgE antibodies was examined using microarray chip technology (ImmunoCAP ISAC 103; Phadia/Thermo Fisher Scientific, Uppsala, Sweden) (Citation21,Citation22). The ImmunoCAP ISAC 103 has allergen components from 43 allergen sources and yields results as ISAC Standardized Units (ISU) for specific IgE, a semi-quantitative estimate of specific IgE antibody titers. Subjects were considered non-IgE-sensitized if the signal was non-measurable or very low (<0.3 ISU) for all allergen components. Previous air sampling studies (Citation23–26) have examined the particle size of various aeroallergens and have shown that approximately 20%–30% of airborne animal allergens are present on small particles of 1–5 μm diameter, in contrast to mite and cockroach allergens, which are carried on larger particles of 10–40 μm diameter (Citation23,Citation25). Airborne pollen allergen can vary because of the different atmospheric conditions (Citation24), but particles between 20 and 60 μm in diameter are often and can be carried in the wind causing nasal and ocular symptoms (allergic rhinoconjunctivitis) (Citation27).
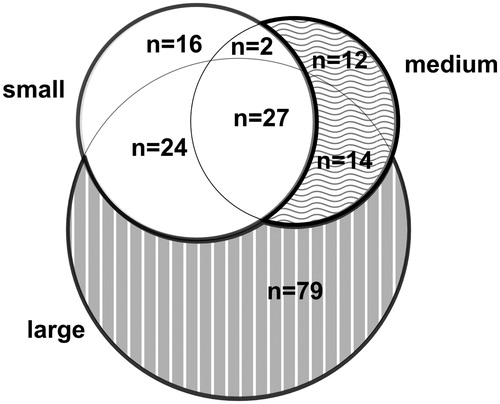
Detailed data on our subjects with regard to the different allergen components have been published elsewhere (Citation1), but for the clarity of this paper they have been presented here in a somewhat modified way (Table E2, available online). Subjects sensitized to aeroallergens were divided into three groups depending on the particle size of the allergen: (1) large particles only: subjects only sensitized to allergens with large-sized particles; (2) medium-sized: subjects sensitized to medium-sized particles, possibly even to large particles, but not to small particles; and (3) small-sized particles: subjects sensitized to small particles regardless of whether they were sensitized to medium- or large-sized particles. Subjects who were non-atopics were used as reference group. Those who were sensitized only to food allergens were omitted from analyses, and those sensitized to bee (nApi m 1), were grouped in one group named ‘others’ and omitted from analyses (Table E2, available online).
Table II. Subjects sensitized to small particles had lower FEV1, higher FENO50 levels, higher DawNO levels, higher CawNO levels, higher ECP levels, and higher degree of airway responsiveness than non-atopics.
Eosinophil cationic protein
Blood samples collected in ECRHS II (baseline) for the measurement of serum eosinophil cationic protein (S-ECP) were allowed to coagulate for 60 min at 24 °C before they were centrifuged. Sera were stored at −20 °C. The concentration of S-ECP was assayed using the ImmunoCAP system (Thermo Fisher Scientific, Uppsala, Sweden).
Measurements of exhaled NO
FENO measurements were performed according to American Thoracic Society/European Respiratory Society recommendations (Citation28), apart from the use of three additional flow-rates (5, 100, and 500 mL s−1). Mathematical modeling of pulmonary NO dynamics has been reviewed (Citation29), and in this study the Högman and Meriläinen algorithm (HMA) has been applied (Citation30). We used FENO collected at 5, 100, and 500 mL s−1 exhalation flow-rates and an iteration algorithm, which estimates NO parameters in two compartments: 1) the conducting airways, and 2) the alveoli. NO in the conducting airways is assessed in two variables: tissue concentration of NO of the airway wall (CawNO) and airway compartment diffusing capacity of NO from the airway wall to the gas stream (DawNO). NO in the alveoli is estimated by steady-state alveolar NO concentration (CANO). Correction for axial back-diffusion of NO into the alveolar site was not applied (Citation31). FENO at the exhalation flow-rate of 50 mL s−1 (FeNO50) is also presented throughout the article.
Lung function
Forced expiratory volume in 1 second (FEV1) was measured using a dry rolling-seal spirometer (Model 2130; SensorMedics, Anaheim, CA, USA). American Thoracic Society recommendations were followed (Citation32). The predicted values for FEV1 were calculated on the basis of European Coal and Steel Union reference values (Citation33,Citation34).
Methacholine challenge test
Details of the methacholine challenge test have been described elsewhere (Citation35). The level of airway responsiveness was expressed using a dose-response slope (Citation36) where a lower value indicates a higher degree of airway responsiveness.
Questionnaires
The ECRHS II main questionnaire (http://www.ecrhs.org) (Citation37) was used to obtain information about respiratory symptoms, exposure to investigated allergens, and smoking history.
Diagnosis of asthma or rhinitis
A person was recorded as having asthma if he/she had been diagnosed before with asthma and had had an asthma attack or one of the following symptoms during the last 12 months: nocturnal chest tightness, attack of shortness of breath, or chest wheezing or whistling (Citation38). Furthermore participants with asthma were recorded as having asthma under treatment if he/she had used inhaled steroids at any time during the last 12 months.
A person was recorded as having rhinitis if he/she answered yes to the question ‘Do you have any nasal allergies, including hay fever?’ Subjects with rhinitis were recorded as having rhinitis under treatment if he/she had used antihistamines or nasal steroids during the last 12 months.
Statistics
Statistical analyses were performed by using STATA 11.0 software (Stata Corp, 2001, TX, USA). FENO50, CawNO, DawNO, and CANO values were log-transformed before analysis. The chi-square test and unpaired t test were used when comparing non-atopics and sensitized subjects. Bonferroni correction was used to adjust for repeated comparisons. Linear regression was used when analyzing the correlation between the type of sensitization and FENO50 or CawNO, or DawNO or CANO, or FEV1 (%predicted), or FEV% or airway responsiveness or ECP. These models always included age, sex, height, weight, smoking history, presence of IgE sensitization to food allergens, and use of inhaled corticosteroids. Logistic regression was used in the bivariate analysis to analyze the correlation between the type of sensitization and asthma under treatment or rhinitis under treatment. The adjusted model included age, sex, height, weight, smoking history, IgE sensitization to food allergens, and use of inhaled corticosteroids (the last-mentioned was not included in the model with outcome treated asthma). A P value of <0.05 was considered statistically significant.
Ethics
All subjects gave their permission for the utilization of personal data for the purpose of this study. The study was approved by the Ethics Committee at the Medical Faculty at Uppsala University (Dnr 1998/495 and 1999/313).
Results
The study population consisted of 467 individuals (216 females) aged 29–55 years. The prevalence of atopy was 45% (n = 203), of whom 69 were sensitized only to large aeroallergen particles, 26 sensitized to medium- but not small-sized aeroallergen particles, 79 sensitized to small aeroallergen particles, and 37 only to food allergens. The overlap of sensitization to aeroallergen particles of different size is shown in .
Characteristics of participants grouped based on allergen particle size
Subjects sensitized to small particles (P = 0.01) and subjects sensitized only to large particles (P = 0.04) were younger than non-atopics (). Sex, height, and weight did not differ between groups. Smoking history was similar in all groups compared with non-atopics except for the group of subjects sensitized only to large particles which had more subjects who had never smoked. Subjects sensitized to small particles used more inhaled corticosteroids than non-atopics (P < 0.001), subjects sensitized to medium particles (P = 0.01), and subjects sensitized only to large particles (P < 0.001).
Lung function and inflammation parameters in relation to sensitization pattern
Subjects sensitized to small particles had lower FEV1 (%predicted) (P = 0.03) than non- atopics, while forced vital capacity (FVC) and FEV% did not differ between the groups (). Moreover, subjects sensitized to small particles (P < 0.001), subjects sensitized to medium-sized particles (P = 0.04), and subjects sensitized only to large particles (P = 0.02) had higher FENO50 levels than non-atopics. Likewise, subjects sensitized to small particles had higher DawNO levels (P = 0.001), CawNO levels (P = 0.01), and ECP levels (P = 0.01) than non-atopics. CANO levels were similar in all groups. Finally, subjects sensitized to small particles had a higher degree of airway responsiveness (lower slope which mirrors a steeper curve in the methacholine test) than non-atopics (P < 0.001) and subjects sensitized only to large particles (P < 0.001).
We examined the independent effects of sensitization to particles of different size towards FENO50, DawNO, CawNO, CANO, FEV1 (%predicted), FEV%, airway responsiveness, S-ECP, asthma under treatment, and rhinitis under treatment (, ). Sensitization to small particles was independently associated with higher ECP (P = 0.04), FENO50 (P < 0.001), CawNO (P = 0.001), and higher airway responsiveness (P < 0.001) when compared with non-atopics. No differences were observed for DawNO, CANO, FEV1 (%predicted), or FEV% (P > 0.05). Sensitization to small particles was associated with higher levels of FENO50 (P = 0.001), CawNO (P = 0.008), and higher airway responsiveness (P = 0.001) compared with large particles. These models were adjusted for age, sex, height, weight, smoking history, IgE sensitization to food allergens, month of examination, and use of inhaled corticosteroids. There were no differences between subjects sensitized to small and medium-sized aeroallergen particles with regard to FENO50, CawNO, DawNO, CANO, ECP, and airway responsiveness.
Figure 3. Independent effect of IgE sensitization to various groups (grouped according to their size) of aeroallergens (compared to non-atopics) to FENO and airway responsiveness (where a lower value indicates more responsiveness) and S-ECP. Adjusted for age, sex, height, weight, smoking history, presence of IgE sensitization to food allergens, month of examination, and use of inhalant corticosteroids.
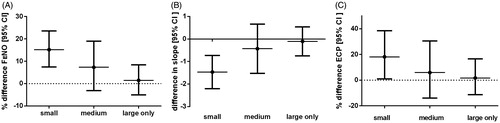
Table III. Sensitization to small particles is associated to higher tissue concentration of NO of the airway wall (CawNO) compared to non-atopics.
Prevalence of asthma and rhinitis in relation to sensitization pattern
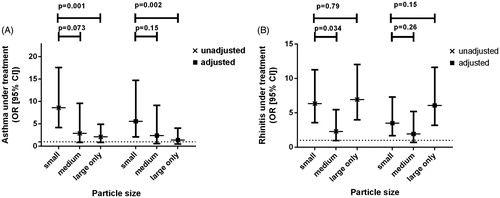
The prevalence of asthma (with or without ongoing treatment) was higher in subjects sensitized to small particles than non-atopics (P < 0.001, both) and subjects sensitized to large particles (P < 0.001 and P = 0.001, respectively). Rhinitis (with or without ongoing treatment) was more prevalent in subjects sensitized to small particles (P < 0.001, both) and to large particles (P < 0.001, both) compared with non-atopics. Furthermore the prevalence of rhinitis (with or without ongoing treatment) was higher in the group with sensitization to small particles than within the group with sensitization to medium-sized particles (P = 0.03, both) ().
Sensitization to small particles (P < 0.001) was independently associated with a higher risk of treated asthma after adjusting for age, sex, height, weight, smoking history, month of examination, and IgE sensitization to food allergens (). Sensitization to small particles was independently associated with a higher risk of having asthma under treatment compared with large particles both in unadjusted (P = 0.001) and adjusted (P = 0.001) models. Sensitization to small or large particles was independently associated with treated rhinitis in both the unadjusted and adjusted models (). In the non-adjusted model sensitization to small particles was related to a higher risk of treated rhinitis than medium-sized particles, but this difference disappeared in the adjusted model. The rhinitis models were adjusted for age, sex, height, weight, smoking history, IgE sensitization to food allergens, month of examination, and use of inhaled corticosteroids.
Figure 4. Independent effect of IgE sensitization to various groups of aeroallergens on risk for asthma (compared to non-atopics) and rhinitis. The adjusted model took into consideration age, sex, height, weight, smoking history, month of examination, use of inhaled corticosteroids (but not for the adjusted model of asthma under treatment), and presence of IgE sensitization to food allergens.
Discussion
The main finding of our study was that sensitization to small particles (for example cat and dog) was independently associated with a higher probability of having asthma under treatment, increased airway responsiveness, higher S-ECP, and higher exhaled NO and airway concentration of NO when compared with the non-atopic control group. Being sensitized to only large or medium-sized and/or large allergen particles was not related to any of these above-mentioned variables, after adjustments for potential confounders. Subjects sensitized to small allergen particles had higher FENO50, CawNO, airway responsiveness, and higher prevalence of asthma with ongoing treatment with inhaled corticosteroids than subjects sensitized only to large allergen particles.
According to previous studies (Citation36,Citation39,Citation40), IgE sensitization to perennial allergens (cat, dog, house dust mite) is the type of sensitization with the strongest relation to asthma, airway responsiveness, and exhaled NO. In the present study sensitization to perennial allergens like mite, mold, or mouse (also perennial allergens), grouped as medium-sized particles, was not related to asthma outcomes, as opposed to sensitization to small-sized allergens. This makes it unlikely that the relations we found for small-sized particles are mainly due to more exposure (perennial versus seasonal). However, in the absence of objective markers of exposure, it cannot be excluded that the differences in duration and amount of exposure contribute to this.
To our knowledge, few studies (Citation14,Citation41–43) examined the effects of IgE sensitization with regard to particle size on the airways responsiveness, lung function, and markers of systemic inflammation during bronchial challenge tests. Differential immediate and late allergic responses were reported in relation to particle size with small allergen particles leading to significantly lower FEF25–75 at 24 h after provocation compared with large particles (Citation42). Our study demonstrated a differential response to smaller allergen particles (for example cat and dog) leading to an increase of FENO50, mainly explained by an increase in airway concentration of NO but no effect on alveolar contribution to NO. This is consistent with results of our previous study where IgE sensitization to cat allergens was associated with higher levels of FENO50, CawNO, and DawNO, but not with CANO (Citation39). These results are also in line with previous data on this material, when only data on IgE sensitization to cat, timothy, mold, and mite were available and only IgE sensitization to cat extract independently related to FENO50, CawNO, and DawNO. Furthermore, ECP, a marker of systemic eosinophil inflammation and asthma severity (Citation44), was associated with sensitization to small particles in the present study. In a previous study (Citation5), ECP levels were higher in subjects sensitized to perennial allergens (including cat, dog, and mite allergens) than to seasonal allergens. Furthermore, exposure to small allergen particles produces an increase in interleukin-5 concentrations in sputum and blood (Citation43), which should result in more activation of eosinophils and increased ECP levels. However, in our material, no differences between sensitization to large and small particles were found with regard to ECP, contrary to the IL-5 findings (Citation43).
Subjects sensitized to small particles were more likely to have higher airway responsiveness compared with subjects sensitized only to large particles. That contradicts results of previous studies where larger aerosols of adenosine monophosphate (Citation41), methacholine (Citation14), cat allergen (Citation42), and mite allergen (Citation43) gave more immediate responses than smaller particles. The same quantity could be delivered in both large and small airways with the nebulizer, but the concentration relative to the area of deposition in the larger airways would be higher as deposition of the same dose in the small airways could be insufficient to achieve similar airway bronchoconstriction due to the larger area of the small airways. However, in our study we found that the methacholine reactivity was higher in patients sensitized to small particle allergens compared with patients sensitized to only large particle allergens, whereas FENO and FEV1 did not differ between these two groups. This may support the view that small particle allergens affect small airways more than large particle allergens. This hypothesis is further supported by Zeidler et al. showing that naturalistic exposure to cat allergen caused a significant decline in small airway function including air trapping and increased small airway responsiveness to methacholine, but not in large airway function (FEV1), in cat-sensitized asthmatics (Citation45).
Similar levels of alveolar NO were found in all patient groups, disregarding presence of IgE sensitization and allergen particle size. This might be due to the absence of an increase of small airway inflammation, or the limited utility of alveolar NO as a marker of small airway inflammation in the presence of obstruction (Citation46), which was not assessed. However, the present study was limited by not including other methods to assess small airway function, such as measurements of FEF25–75, impulse oscillometry, and nitrogen oxide washout (Citation7,Citation17). We were therefore not able to study small airway functional impairment in this cohort in relation to IgE sensitization and allergen particle size. Moreover, our results were consistent also when further adjustment for the month of taking measurements was introduced in the multivariate analyses.
One strength of the present study was the availability of multiplex, molecular-based IgE antibody determinations of a large number of allergen components, which made possible the formation of subject groups with regard to allergen particle size. One more strength was the combined measurement of lung function, airway responsiveness, ECP, and airway inflammation, both as a global measure (FENO) and extended measurements, allowing assessment of bronchial and alveolar inflammation. In the present study we focused on the particle size and therefore assessed independently the effect of pet IgE sensitization (small allergen particles) versus mite, mold, and mouse sensitization (medium-sized allergen particles). Furthermore, we analyzed the relation of the different sensitization patterns with regard to asthma and rhinitis under treatment, a marker of systemic eosinophilic inflammation (ECP) (Citation1) and nitric oxide (NO) flow-independent parameters which reflect NO contribution from central and peripheral airways, outcomes that were not used in our previous study.
However, it has to be acknowledged as a weakness that the group of subjects sensitized to medium-sized allergen particles was relatively small, and therefore we cannot exclude that these specific analyses were underpowered. Moreover, the present results can be difficult to transfer to a more general population due to the enrichment of the studied people with subjects with respiratory symptoms and due to the loss of eligible subjects due to lack of blood samples or different tests (Citation1). Our groups were heterogeneous, and most of the significant differences were observed in comparisons with non-asthmatic, non-atopic controls but not between groups of sensitized individuals, stratified according to the particle size division.
In conclusion, this study demonstrated that sensitization to small aeroallergens, as assessed by means of multiplex techniques, was associated with more local airway and systemic inflammation, airway responsiveness, and higher prevalence of asthma. Interestingly, sensitization to medium-sized and/or large allergen particles was not related to these asthma characteristics. However, we were not able to find a relation between the size of allergen particles and inflammation in the small airways, as assessed by alveolar NO. Future studies should incorporate functional methods to look at the small airways as well as longitudinal studies to assess if small allergen particles are more likely to lead to the development of asthma and airway responsiveness than medium-sized or large allergen particles.
Declaration of interest
Kjell Alving is a minority shareholder of Aerocrine AB and has received funds for research from the same company. Magnus Borres is an employee of Thermo Fisher Scientific (which manufactures kits to measure IgE antibodies against allergens). The rest of the authors had no financial or other conflicts of interest.
This study was supported financially by the Swedish Heart and Lung Foundation, the Vårdal Foundation for Health Care Science and Allergy Research, the Swedish Association against Asthma and Allergy, Thermo Fisher Scientific, Agnes and Mac Rudberg’s Foundation, the Bror Hjerpstedt Foundation, FoU region Kronoberg, and Södra Sjukvårdsregionen.
References
- Patelis A, Gunnbjornsdottir M, Malinovschi A, Matsson P, Onell A, Hogman M, et al. Population-based study of multiplexed IgE sensitization in relation to asthma, exhaled nitric oxide, and bronchial responsiveness. J Allergy Clin Immunol. 2012;130:397–402 e2.
- Patelis A, Janson C, Borres MP, Nordvall L, Alving K, Malinovschi A. Aeroallergen and food IgE sensitization and local and systemic inflammation in asthma. Allergy. 2014;69:380–7.
- Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31:143–78.
- Malinovschi A, Fonseca JA, Jacinto T, Alving K, Janson C. Exhaled nitric oxide levels and blood eosinophil counts independently associate with wheeze and asthma events in National Health and Nutrition Examination Survey subjects. J Allergy Clin Immunol. 2013;132:821–7 e1–5.
- Tomassini M, Magrini L, De Petrillo G, Adriani E, Bonini S, Balsano F, et al. Serum levels of eosinophil cationic protein in allergic diseases and natural allergen exposure. J Allergy Clin Immunol. 1996;97: 1350–5.
- van der Wiel E, ten Hacken NH, Postma DS, van den Berge M. Small-airways dysfunction associates with respiratory symptoms and clinical features of asthma: a systematic review. J Allergy Clin Immunol. 2013;131:646–57.
- Wagner EM, Bleecker ER, Permutt S, Liu MC. Direct assessment of small airways reactivity in human subjects. Am J Respir Crit Care Med. 1998;157:447–52.
- Downie SR, Salome CM, Verbanck S, Thompson B, Berend N, King GG. Ventilation heterogeneity is a major determinant of airway hyperresponsiveness in asthma, independent of airway inflammation. Thorax. 2007;62:684–9.
- Dolovich MA. Influence of inspiratory flow rate, particle size, and airway caliber on aerosolized drug delivery to the lung. Respir Care. 2000;45:597–608.
- Yeh HC, Phalen RF, Raabe OG. Factors influencing the deposition of inhaled particles. Environ Health Perspect. 1976;15:147–56.
- Usmani OS, Biddiscombe MF, Barnes PJ. Regional lung deposition and bronchodilator response as a function of beta2-agonist particle size. Am J Respir Crit Care Med. 2005;172:1497–504.
- Darquenne C. Aerosol deposition in health and disease. J Aerosol Med Pulm Drug Deliv. 2012;25:140–7.
- Rubin BK. Inhaled corticosteroids: devices and deposition. Paediatr Respir Rev. 2004;5(Suppl A):S103–6.
- Naji N, Keung E, Beaudin S, Kane J, Killian KJ, Gauvreau GM. The effects of particle size on measurement of airway hyperresponsiveness to methacholine. Ann Allergy Asthma Immunol. 2013;110:359–63.
- Svartengren M, Falk R, Linnman L, Philipson K, Camner P. Deposition of large particles in human lung. Exp Lung Res. 1987;12:75–88.
- Michel FB, Marty JP, Bousquet J. [Penetration of solid particles in the respiratory system. Secondary immunity reactions to the inhalation of a dry aerosol (author’s transl)]. Poumon Coeur. 1979;35:375–8. [In French]
- Contoli M, Bousquet J, Fabbri LM, Magnussen H, Rabe KF, Siafakas NM, et al. The small airways and distal lung compartment in asthma and COPD: a time for reappraisal. Allergy. 2010;65:141–51.
- Heijkenskjöld-Rentzhog C, Nordvall L, Janson C, Borres MP, Alving K, Malinovschi A. Alveolar and exhaled NO in relation to asthma characteristics – effects of correction for axial diffusion. Allergy. 2014;69:1102–11.
- Burney PG, Luczynska C, Chinn S, Jarvis D. The European Community Respiratory Health Survey. Eur Respir J. 1994;7:954–60.
- Hogman M, Drca N, Ehrstedt C, Merilainen P. Exhaled nitric oxide partitioned into alveolar, lower airways and nasal contributions. Respir Med. 2000;94:985–91.
- Hiller R, Laffer S, Harwanegg C, Huber M, Schmidt WM, Twardosz A, et al. Microarrayed allergen molecules: diagnostic gatekeepers for allergy treatment. FASEB J. 2002;16:414–16.
- Jahn-Schmid B, Harwanegg C, Hiller R, Bohle B, Ebner C, Scheiner O, et al. Allergen microarray: comparison of microarray using recombinant allergens with conventional diagnostic methods to detect allergen-specific serum immunoglobulin E. Clin Exp Allergy. 2003;33:1443–9.
- Custovic A, Green R, Fletcher A, Smith A, Pickering CA, Chapman MD, et al. Aerodynamic properties of the major dog allergen Can f 1: distribution in homes, concentration, and particle size of allergen in the air. Am J Respir Crit Care Med. 1997;155:94–8.
- Spieksma FT. Aerobiology of common environmental allergens: sizes of allergen carrying particles. Asian Pac J Allergy Immunol. 1993;11:93–4.
- Luczynska CM, Li Y, Chapman MD, Platts-Mills TA. Airborne concentrations and particle size distribution of allergen derived from domestic cats (Felis domesticus). Measurements using cascade impactor, liquid impinger, and a two-site monoclonal antibody assay for Fel d I. Am Rev Respir Dis. 1990;141:361–7.
- Zeldin DC, Eggleston P, Chapman M, Piedimonte G, Renz H, Peden D. How exposures to biologics influence the induction and incidence of asthma. Environ Health Perspect. 2006;114:620–6.
- Shah R, Grammer LC. Chapter 1: an overview of allergens. Allergy Asthma Proc. 2012;33(Suppl 1):S2–5.
- American Thoracic Society; European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–30.
- George SC, Hogman M, Permutt S, Silkoff PE. Modeling pulmonary nitric oxide exchange. J Appl Physiol. 2004;96:831–9.
- Hogman M, Holmkvist T, Wegener T, Emtner M, Andersson M, Hedenstrom H, et al. Extended NO analysis applied to patients with COPD, allergic asthma and allergic rhinitis. Respir Med. 2002;96:24–30.
- Hogman M, Thornadtsson A, Hedenstierna G, Merilainen P. A practical approach to the theoretical models to calculate NO parameters of the respiratory system. J Breath Res. 2014;8:016002.
- Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152:1107–36.
- Standardized lung function testing. Report working party. Bull Eur Physiopathol Respir. 1983;19(Suppl 5):1–95.
- Roca J, Burgos F, Sunyer J, Saez M, Chinn S, Anto JM, et al. References values for forced spirometry. Group of the European Community Respiratory Health Survey. Eur Respir J. 1998;11:1354–62.
- Chinn S, Burney P, Sunyer J, Jarvis D, Luczynska C. Sensitization to individual allergens and bronchial responsiveness in the ECRHS. European Community Respiratory Health Survey. Eur Respir J. 1999;14:876–84.
- Chinn S, Heinrich J, Anto JM, Janson C, Norback D, Olivieri M, et al. Bronchial responsiveness in atopic adults increases with exposure to cat allergen. Am J Respir Crit Care Med. 2007;176:20–6.
- European Community Respiratory Health Survey II Steering Committee. The European Community Respiratory Health Survey II. Eur Respir J. 2002;20:1071–9.
- Olafsdottir IS, Gislason T, Thjodleifsson B, Olafsson I, Gislason D, Jogi R, et al. C reactive protein levels are increased in non-allergic but not allergic asthma: a multicentre epidemiological study. Thorax. 2005;60: 451–4.
- Malinovschi A, Janson C, Holmkvist T, Norback D, Merilainen P, Hogman M. IgE sensitisation in relation to flow-independent nitric oxide exchange parameters. Respir Res. 2006;7:92.
- Sears MR, Herbison GP, Holdaway MD, Hewitt CJ, Flannery EM, Silva PA. The relative risks of sensitivity to grass pollen, house dust mite and cat dander in the development of childhood asthma. Clin Exp Allergy. 1989;19:419–24.
- Cohen J, Postma DS, Douma WR, Vonk JM, De Boer AH, ten Hacken NH. Particle size matters: diagnostics and treatment of small airways involvement in asthma. Eur Respir J. 2011;37:532–40.
- Lieutier-Colas F, Purohit A, Meyer P, Fabries JF, Kopferschmitt MC, Dessanges JF, et al. Bronchial challenge tests in patients with asthma sensitized to cats: the importance of large particles in the immediate response. Am J Respir Crit Care Med. 2003;167:1077–82.
- Casset A, Purohit A, Birba E, Chenard MP, Uring Lambert B, Bahram S, et al. Bronchial challenge test in asthmatics sensitized to mites: role of particle size in bronchial response. J Aerosol Med. 2007;20:509–18.
- Koh GCH, Shek LPC, Goh DYT, Van Bever H, Koh DSQ. Eosinophil cationic protein: is it useful in asthma? A systematic review. Respir Med. 2007;101:696–705.
- Zeidler MR, Goldin JG, Kleerup EC, Kim HJ, Truong DA, Gjertson DW, et al. Small airways response to naturalistic cat allergen exposure in subjects with asthma. J Allergy Clin Immunol. 2006;118:1075–81.
- Verbanck S, Kerckx Y, Schuermans D, Vincken W, Paiva M, Van Muylem A. Effect of airways constriction on exhaled nitric oxide. J Appl Physiol. 2008;104:925–30.