Abstract
Objectives: We investigated the antibody levels against early antigens of Epstein–Barr virus (EBV), cytomegalovirus (CMV), and human herpesvirus 6 (HHV6) in systemic lupus erythematosus (SLE) patients and healthy controls, and further correlated these antibodies to haematology/biochemistry, serology, and disease activity measures.
Method: Immunoglobulin (Ig)M, IgG, and IgA levels against the DNA polymerase processivity factors of EBV, CMV, and HHV6, termed early antigen diffuse (EA/D), pp52, and p41, respectively, were determined in plasma samples from 77 SLE patients and 29 healthy controls by using enzyme-linked immunosorbent assays (ELISAs).
Results: IgM, IgG, and IgA levels against EBV EA/D, and IgG and IgA levels against CMV pp52, were significantly higher in SLE patients compared with healthy controls. Furthermore, EBV EA/D- and CMV pp52-directed IgG levels were inversely and positively associated, respectively, with lymphocyte counts in SLE patients. None of the findings seemed to be associated with use of immunosuppressive medication.
Conclusions: Our results suggest strong, but opposite, associations of lytic EBV and CMV infections with SLE. The amplified humoral responses to EBV EA/D and CMV pp52 in our SLE patient cohort probably reflect aberrant control of EBV and CMV reactivation. However, reactivation of EBV appeared to correlate with lymphopenic manifestations in SLE patients whereas CMV reactivation seemed to correlate with increments in lymphocyte levels.
Systemic lupus erythematosus (SLE) is an autoimmune disease of unknown aetiology that mainly occurs in women (90% of cases) of childbearing age. SLE is characterized by periodic flares (active disease) with production of autoantibodies against nuclear antigens, including ribonucleoproteins (RNPs), Ro, and double-stranded (ds)DNA (Citation1). Studies have suggested that several environmental factors, including viral infections, may trigger the disease in genetically predisposed individuals (Citation1–Citation4). Of interest in this regard is the alternating nature of active and inactive disease intervals, which strongly resembles the lytic and latent infectious properties of human herpesviruses (HHVs) (Citation4).
To date, eight viruses have been ascribed to the HHV family. These include Epstein–Barr virus (EBV), cytomegalovirus (CMV), and human herpesvirus 6 (HHV6), all of which are ubiquitous dsDNA viruses infecting the majority of adults worldwide (Citation5). The ability to shift between lytic (active/productive) and latent (non-productive) stages is the hallmark of all HHVs and enables the viruses to persist permanently in the host (Citation6). Lytic genes are divided into three groups, termed immediate-early, early, and late genes, according to their temporal order of expression. The early genes encode proteins essential for lytic replication, including DNA polymerase processivity factors, termed early antigen diffuse (EA/D), pp52, and p41, regarding EBV, CMV, and HHV6, respectively (Citation7–Citation10). Histories and current states of individual HHV infections are reflected in the humoral response patterns to various HHV antigens. The presence of antibodies to early antigens (EAs) is usually indicative of ongoing or recent lytic infections whereas class-switched antibodies to late or latency-associated antigens often suggest past exposure (Citation11–Citation14).
In previous serological studies, significantly elevated immunoglobulin (Ig)M, IgG, and IgA levels and/or positivity rates against EBV EA/D, and significantly elevated IgM levels against CMV antigens of unspecified classifications, were found in SLE patients relative to healthy controls or disease controls (Citation15–Citation22). These findings suggest higher rates of lytic EBV and CMV infections in subjects with SLE. The humoral responses to CMV pp52 and HHV6 p41 have not previously been elucidated in SLE patients. Moreover, HHV6 has been less explored in the context of SLE. However, significantly higher proportions of cell-free HHV6 serum viraemia were previously suggested in a group of patients with autoimmune connective tissue diseases (including SLE) compared with control subjects (Citation21). The lytic markers of EBV, CMV, and HHV6 have been shown to correlate with higher disease activities (regarding CMV and HHV6) (Citation21, Citation22) and the presence of certain autoantibodies and specific disease manifestations (regarding EBV) (Citation18). However, direct evidence for causative roles of the viruses in the development and/or exacerbation of SLE remains to be established.
Using enzyme-linked immunosorbent assays (ELISAs), the aims of this study were to compare plasma from SLE patients and healthy controls with respect to IgM, IgG, and IgA levels against EBV EA/D, CMV pp52, and HHV6 p41, and to further correlate these antibodies to haematology/biochemistry, serology, and disease activity measures, that is SLE Disease Activity Index (SLEDAI) scores. The findings from this study could assist in further substantiating the significance of lytic HHV infections in SLE.
Method
Samples
SLE patient plasma samples were obtained from 77 unrelated Danish SLE patients attending the Department of Rheumatology, Rigshospitalet, Copenhagen University Hospital, Denmark. Plasma samples from 29 healthy controls were obtained from volunteers at Statens Serum Institut, Copenhagen, Denmark. All SLE patients fulfilled the American College of Rheumatology (ACR) classification criteria for SLE (Citation23). Informed consent for the studies was obtained from all participants in accordance with the protocol as approved by the Scientific-Ethical Committee of the Capital Region of Denmark.
Laboratory analysis of SLE patient variables
The SLE patient plasma samples were screened for antinuclear antibodies (ANAs) by indirect immunofluorescence assays on HEp-2 cells. C3 and C4 levels were determined by ELISA whereas dsDNA-directed antibody levels were determined by the Crithidia luciliae immunofluorescence test (CLIFT) and ELISA. Haematology and C-reactive protein were obtained by routine biochemistry.
ELISA for EBV EA/D-, CMV pp52-, and HHV6 p41-directed antibodies
TTN buffer (0.05 M Tris, 1% Tween 20, 0.3 M NaCl, pH 7.5) was used for washing, blocking, and dilution of samples and secondary antibodies. Carbonate buffer (50 mM sodium carbonate, pH 9.6) was used for coating Nunc PolySorp microtitre plates (Thermo Fisher Scientific, Denmark) with recombinant CMV pp52 (Prospec, Ness-Ziona, Israel), recombinant EBV EA/D (Prospec), and recombinant HHV6 p41 (MyBioSource, San Diego, CA, USA) at a concentration of 1 µg/mL. A volume of 250 µL was used in each well for washing and blocking whereas 100 µL was used for incubation with diluted samples, secondary antibodies, and enzyme substrates. Both coated and non-coated wells were included; carbonate buffer with or without antigen was applied for coated and non-coated wells, respectively. The plates were subsequently incubated overnight at 4˚C. After incubation, the wells were washed for 3 × 1 min followed by blocking for 30 min. The samples were diluted 1:50/1:100/1:50 for detection of EBV EA/D-directed IgM/IgG/IgA. Dilutions of 1:100/1:100/1:50 and 1:20/1:100/1:20, respectively, were used for detection of CMV pp52- and HHV6 p41-directed IgM/IgG/IgA. All diluted samples were added to coated and non-coated wells in duplicate. The plates were subsequently incubated for 1 h at room temperature (RT), and wells were washed as described previously, followed by incubation with alkaline phosphatase (AP)-conjugated goat anti-human IgM, IgG, or IgA (Sigma-Aldrich, St Louis, MO, USA) (1:2000) for 1 h at RT. After three further washes, the plates were developed by adding AP substrate [p-nitrophenyl phosphate (p-NPP); Sigma-Aldrich] dissolved in AP substrate buffer (1 M diethanolamine, 0.5 mM MgCl2, pH 9.8) (1 mg/mL). A Versamax microplate reader (Molecular Devices, Sunnyvale, CA, USA) was used for reading the plates with a wavelength of 405 nm and a reference wavelength of 650 nm. The sample absorbance values of non-coated wells were subtracted from coated wells after averaging the duplicates. To enable intercomparison, all net absorbance values were normalized to standard curves derived from twofold serial dilutions of serum samples (CMV pp52-directed IgM, IgG, and IgA assays and HHV6 p41-directed IgM, and IgA assays) or serum pools (EBV EA/D-directed IgM, IgG, and IgA assays and HHV6 p41-directed IgG assays). The values were log10 transformed prior to normalization, and were back-transformed for statistical analyses.
Statistical analysis
Parametric statistics were inappropriate. The Mann–Whitney U test and Fisher’s exact test (two-tailed) were applied for two-group comparisons of continuous data and categorical data, respectively. The Kruskal–Wallis test (two-tailed) was used for comparison of continuous data among three or more groups (note that adjustment for multiplicity was not performed). Multiple linear regression models were included with both continuous and categorical patient variables as predictors. The validity of models was assessed from normal Q–Q plots of standardized residuals, histograms, residuals vs. fitted plots, and Cook’s distance. Furthermore, potential multicollinearity was evaluated with variance inflation factors. Cliff’s δ and odds ratios were applied as effect sizes for pairwise/two-group comparisons of continuous data and categorical data, respectively. Cliff’s δ of ±0.1, (≥) ± 0.35, and (≥) ± 0.5 represent small, medium, and large effects, respectively. A p-value < 0.05 was considered statistically significant. The statistical analysis was performed in GraphPad Prism software 5 (GraphPad Software Inc, San Diego, CA, USA) and R software 3.0.3 (The R Foundation for Statistical Computing, Vienna, Austria). The data are presented as medians and interquartile ranges unless stated otherwise.
Results
Characteristics of SLE patients and healthy controls
The median age of patients () and healthy controls () was 38 (range 20–76) and 42 (range 23–63) years, respectively. Females comprised 92.2% of the patient group and 75.9% of the control group. The median SLEDAI score was 4 (range 0–21), indicating a low disease activity SLE cohort.
Table 1. Characteristics of SLE patients.
Table 2. Characteristics of healthy controls.
Prevalence of IgM, IgG, and IgA isotypes against EBV EA/D, CMV pp52, and HHV6 p41
Significantly higher antibody levels against EBV EA/D and CMV pp52 in SLE patients relative to healthy controls
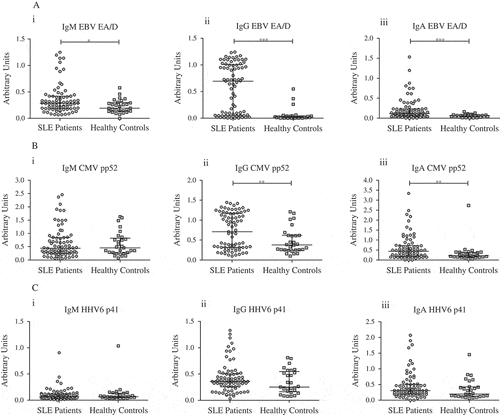
The SLE patients had significantly higher IgM, IgG, and IgA levels against EBV EA/D compared with healthy controls (p = 0.02, < 0.001, and < 0.001, respectively; –) (Cliff’s δ = 0.29, 0.72, and 0.49, respectively; Supplementary Table S1), and significantly higher IgG and IgA levels against CMV pp52 compared with healthy controls (p = 0.009 and 0.004, respectively; , ) (Cliff’s δ = 0.33 and 0.37, respectively). By contrast, no significant differences were detected in IgM, IgG, and IgA levels against HHV6 p41 (–) or in IgM levels against CMV pp52 between the two groups ().
Figure 1. Two-group comparisons of (A) EBV EA/D-, (B) CMV pp52-, and (C) HHV6 p41-directed (i) IgM, (ii) IgG, and (iii) IgA levels between SLE patients (n = 77) and healthy controls (n = 29). The antibody levels are presented in arbitrary units (AU). The horizontal bars represent medians and interquartile ranges. *p < 0.05, **p < 0.01, ***p < 0.001.
Antibody levels against EBV EA/D, CMV pp52, and HHV6 p41 in SLE patients with regard to intake of immunosuppressive medication
To ensure that the amplified humoral responses in SLE patients of this study were not resulting from treatment measures, the IgM, IgG, and IgA levels against EBV EA/D, CMV pp52, and HHV6 p41 were correlated to intake of immunosuppressive medication. Importantly, no significant differences were detected in these levels between patients receiving and patients not receiving immunosuppressive medication (data not shown). The results are therefore unlikely to be explained by deliberate immune suppression.
Comparison of antibody patterns against EBV EA/D and CMV pp52 with regard to SLE patient characteristics
To select for various indications of EBV and CMV infection status, the SLE patients were divided into four groups depending on their pattern of EBV EA/D- and CMV pp52-directed IgG levels (). SLE patients in group 1 (n = 20) and group 4 (n = 21) had lower IgG levels [i.e. < median arbitrary units (AU)] and higher IgG levels (i.e. ≥ median AU), respectively, against both EBV EA/D and CMV pp52 whereas SLE patients in group 2 (n = 18) and group 3 (n = 18) had higher/lower IgG levels and lower/higher IgG levels, respectively, against EBV EA/D/CMV pp52. For clarity, the IgG patterns in groups 1, 2, 3, and 4 are indicative of: no lytic activity, ‘isolated’ lytic EBV activity, ‘isolated’ lytic CMV activity, and concurrent lytic EBV/CMV activity, respectively.
Table 3. Patterns of EBV EA/D- and CMV pp52-directed IgG levels in SLE patients.
The IgG subclasses are regarded as the most consistent markers of lytic infections. Hence, to avoid abundant comparisons, the IgM and IgA levels were not considered. Moreover, because of the limited sample size, selection for HHV6 p41-directed IgG levels was not performed. The four groups were compared with regard to several characteristics.
The lymphocyte count and positivity rates for dsDNA-directed antibodies varied significantly among the groups (p = 0.004 and 0.034, respectively), with groups 2 and 3 having the lowest and highest median lymphocyte counts (0.65 and 1.45, respectively; Cliff’s δ = –0.57; Supplementary Table S2), whereas groups 1 and 4 had the highest and lowest positivity rates for dsDNA-directed antibodies (60% and 19.1%, respectively; odds ratio = 6.06).
Association of EBV EA/D- and CMV pp52-directed antibody levels with lymphocyte counts in SLE patients
The potential correlations of EBV EA/D- and CMV pp52-directed IgG levels with lymphocyte counts in SLE patients were further evaluated. To adjust for characteristics such as age, intake of immunosuppressive medication, and disease activity (based on SLEDAI scores), a multiple linear regression analysis was performed, with lymphocyte counts as the dependent variable. All 77 SLE patients were enrolled. Furthermore, HHV6 p41-directed IgG levels were additionally included as independent variables (note that IgG levels were included, exclusively, to avoid unreliable estimations due to potential multicollinearity between IgM, IgG, and IgA levels against each respective antigen). The regression output confirmed an inverse correlation between EBV EA/D-directed IgG levels and lymphocyte counts (B = –0.54 per AU increment, p = 0.009; Supplementary Table S3), and a positive correlation between CMV pp52-directed IgG levels and lymphocyte counts (B = 0.46 per AU increment, p = 0.031). Moreover, intake of immunosuppressive medication tended to correlate inversely with lymphocyte counts (B = –0.34, p = 0.095). No significant correlations were found between HHV6 p41-directed IgG levels and lymphocyte counts (B = –0.27 per AU increment, p = 0.401).
Discussion
To the best of our knowledge, this is the first study to investigate humoral responses to CMV pp52 and HHV6 p41 in subjects with SLE relative to healthy subjects. The results were generated using ELISAs, and revealed significantly higher IgM, IgG, and IgA levels against EBV EA/D, and higher IgG and IgA levels against CMV pp52, in SLE patients compared with healthy controls. By contrast, no significant differences were detected in antibody levels against HHV6 p41 between the two groups. Furthermore, lymphocyte counts were found to correlate negatively and positively, respectively, with EBV EA/D- and CMV pp52-directed IgG levels in SLE patients. None of the findings seemed to be associated with intake of immunosuppressive medication and are thus unlikely to be explained by deliberate immune suppression.
The significantly elevated EBV EA/D- and CMV pp52-directed antibody levels in SLE patients in this study suggest at least three scenarios. Subjects with SLE might exhibit increased susceptibility to EBV and/or CMV reactivation (i) with or (ii) without implication in disease exacerbation. Alternatively, (iii) increased susceptibility to EBV and/or CMV reactivation could precede SLE as a cause of disease development. Limited or impaired EBV-specific CD8+ T-cell responses were previously detected in subjects with SLE, whereas CD8+ T-cell responses against CMV seem to be normal (Citation24). Similar dysfunctions could explain the apparently higher susceptibility to lytic EBV infections in SLE patients in the present study.
Our findings for EBV EA/D-directed antibody levels in SLE patients relative to healthy controls substantiate several earlier findings (Citation15–Citation21), whereas all previous studies regarding antibodies to CMV addressed antigens with unspecified classifications (Citation16, Citation17, Citation20, Citation22). Moreover, only two of these studies revealed significantly higher IgM levels against CMV, suggesting higher rates of active CMV infections in SLE patients relative to healthy controls (Citation20) or disease controls (Citation22).
The absence of significant differences in HHV6 p41-directed antibody levels in our study indicates that HHV6 reactivation is equally prominent among the two groups. This lends support to the notion that the amplified humoral responses to EBV EA/D and CMV pp52 are specific to the activity of EBV and CMV, respectively, rather than a result of polyclonal B-cell hyperactivity. The finding, however, is inconsistent with a recent study that showed strong associations between SLE and lytic HHV6 infections, as judged from significantly higher proportions of cell-free HHV6 serum viraemia in SLE patients relative to control subjects (Citation21). However, in our study, potential cell-free EBV, CMV, and HHV6 serum DNA was not assessed.
With regard to the possible involvement of the viruses in the pathogenesis of SLE, it is pertinent to assess whether the EA-directed antibody levels are correlated with disease exacerbation or remission, or lack any associative pattern with these conditions.
In a previous study, elevated EBV EA/D-directed IgG levels correlated with the presence of antibodies against Ro and cutaneous symptoms in subjects with SLE (Citation18). The authors concluded that exposure to EBV infection is associated with mild SLE disease phenotypes. In our study, negative correlations were revealed between EBV EA/D-directed IgG levels and lymphocyte counts in SLE patients. Lower lymphocyte levels have been shown to correlate with higher disease activities and to be predictive of future flares (Citation25–Citation27). The SLEDAI scores were highest among subjects with ‘isolated’ EBV patterns but did not vary significantly. Nevertheless, our finding could provide a linkage between EBV reactivation and lymphopenic manifestations in SLE patients, with possible connection to present and/or forthcoming disease exacerbation.
There are several potential mechanisms, related to EBV, with explanatory power to this inverse axis. Previous in vitro studies found EBV to drive strong and persistent interferon (IFN)-α responses in plasmacytoid dendritic cells (pDCs) from healthy individuals (Citation28, Citation29). This is relevant, as IFN-α has been shown to correlate positively with SLEDAI scores and dsDNA-directed antibodies but inversely with lymphocyte levels in SLE patients, and is suspected of being a direct cause of lymphopenia in subjects with SLE (Citation30–Citation32). However, IFN-α levels were not measured in samples in this study, and thus there is no clear-cut evidence to support any viral modulation of this cytokine in our SLE patient cohort. Furthermore, as T cells are susceptible to EBV infection, it is possible that uncontrolled and chronic infections of this lymphocyte subset, with cell-destructive consequences, could serve as another explanation to our finding (Citation33). It is also possible that lower lymphocyte levels promote EBV reactivation and not vice versa. However, in this context, it would not explain why inverse correlations were not detected between lymphocyte counts and CMV pp52- and/or HHV6 p41-directed IgG levels.
In fact, as CMV pp52-directed IgG levels correlated positively with lymphocyte counts in our study, the potential influence(s) of active CMV in SLE patients seems opposite to that of active EBV. Of note, CMV has been shown to inhibit the production of IFN-α in pDCs from healthy individuals in vitro (Citation34, Citation35), opposite to the effect found for EBV.
It was surprising to discover that markedly fewer individuals with concurrent EBV/CMV patterns were positive for dsDNA-directed antibodies relative to subjects in the other groups. Moreover, SLEDAI scores were lowest among these individuals but were not significantly different from individuals with other antibody patterns. The reason for this finding is unclear. However, it seems unlikely that concurrent lytic activities of the viruses are implicated directly. As our study is non-longitudinal, the present picture could be ascribed to temporal variations in EBV and/or CMV reactivation starting points. Thus, the viruses might have been active for longer or shorter periods in a larger fraction of subjects with ‘isolated’ EBV and/or ‘isolated’ CMV patterns relative to individuals with concurrent EBV/CMV patterns.
In addition, the lymphocyte counts were markedly lower among individuals with ‘isolated’ EBV patterns relative to individuals with concurrent EBV/CMV and ‘isolated’ CMV patterns. The median lymphocyte count in this group corresponded to moderate to near-marked lymphopenia whereas median lymphocyte counts in the other two groups were near-normal values. This is of interest when considering the negative and positive correlations, respectively, of EBV EA/D- and CMV pp52-directed IgG levels with lymphocyte counts. Potentially, it suggests some sort of interplay between the viruses that may modulate the outcome of lymphocyte levels in SLE. For instance, if EBV is assumed to be involved in these lymphopenic manifestations, lytic collisions with CMV could perhaps disturb or counteract the underlying mechanism(s).
The potential influence(s) of EBV and CMV on the course of SLE disease and their possible interactions with each other should be further explored in future studies with longitudinal designs and larger SLE cohorts.
Our study has several limitations. First, quantitative assessment of cell-free viral DNA in our samples was not performed and hence lytic activity was only determined from indirect measures. Second, as samples were not screened for antibodies that indicate previous exposure, it is possible that lower fractions of EBV- and CMV-infected individuals in the control group could explain the weaker antibody reactivity among these subjects.
In conclusion, our results suggest strong, but opposite, associations of lytic EBV and CMV infections with SLE whereas lytic HHV6 infections do not seem to be associated with the disease. The amplified humoral responses to EBV EA/D and CMV pp52 in our SLE patient cohort probably reflect aberrant control of EBV and CMV reactivation. However, reactivation of EBV seems to be associated with lymphopenic manifestations in SLE patients whereas CMV reactivation seems to be associated with increments in lymphocyte levels.
Acknowledgements
We thank Jacob Sode, Department of Clinical Biochemistry, Immunology, and Genetics, Statens Serum Institut, Copenhagen, Denmark for donation of healthy control plasma samples, and Esin Djebir Güven and Dorte Olsen, Department of Clinical Biochemistry, Immunology, and Genetics, Statens Serum Institut, Copenhagen, Denmark for collecting and preparing blood samples. We are grateful to all the participants of this study.
References
- Gordon C, Li CK, Isenberg DA. Systemic lupus erythematosus. Medicine 2009;38:73–80.
- Tsao BP. The genetics of human systemic lupus erythematosus. Trends Immunol 2003;24:595–602.
- Sarzi-Puttini P, Atzeni F, Laccarino L, Doria A. Environment and systemic lupus erythematosus: an overview. Autoimmunity 2005;38:465–72.
- Draborg AH, Duus K, Houen G. Epstein-Barr virus and systemic lupus erythematosus. Clin Dev Immunol 2012;2012:370516.
- Braun DK, Dominguez G, Pellett PE. Human herpesvirus 6. Clin Microbiol Rev 1997;10:521–67.
- Penkert RR, Kalejta RF. Tegument protein control of latent herpesvirus establishment and animation. Herpesviridae 2011;2:3.
- Oster B, Hollsberg P. Viral gene expression patterns in human herpesvirus 6B-infected T cells. J Virol 2002;76:7578–86.
- Lin K, Ricciardi RP. The 41-kDa protein of human herpesvirus 6 specifically binds to viral DNA polymerase and greatly increases DNA synthesis. Virology 1998;250:210–19.
- Weiland KL, Oien NL, Homa F, Wathen MW. Functional analysis of human cytomegalovirus polymerase accessory protein. Virus Res 1994;34:191–206.
- Kiehl A, Dorsky DI. Bipartite DN A-binding region of the Epstein-Barr virus BMRF1 product essential for DNA polymerase accessory function. J Virol 1995;69:1669–77.
- De Paschale M, Cagnin D, Cerulli T, Manco MT, Agrappi C, Mirri P, et al. Search for anti-EA(D) antibodies in subjects with an ‘isolated VCA IgG’ pattern. Int J Microbiol 2010;2010:695104.
- Ooka T, Turenne-Tessier MD, Stolzenberg MC. Relationship between antibody production to Epstein-Barr virus (EBV) early antigens and various EBV-related diseases. Springer Semin Immunopathol 1991;13:233–47.
- Henle W, Henle G. EBV-specific serology in immunologically compromised individuals. Cancer Res 1981;41:4222–5.
- Musiani M, Carpi C, Zerbini M. Rapid detection of antibodies against cytomegalovirus induced immediate early and early antigens by an enzyme linked immunosorbent assay. J Clin Pathol 1984;37:122–5.
- Draborg AH, Jorgensen JM, Muller H, Nielsen CT, Jacobsen S, Iversen LV, et al. Epstein-Barr virus early antigen diffuse (EBV EA/D)-directed immunoglobulin A antibodies in systemic lupus erythematosus patients. Scand J Rheumatol 2012;41:280–9.
- Stratta P, Canavese C, Ciccone G, Santi S, Quaglia M, Ghisetti V, et al. Correlation between cytomegalovirus infection and Raynaud’s phenomenon in lupus nephritis. Nephron 1999;82:145–54.
- Esen BA, Yilmaz G, Uzun S, Ozdamar M, Aksö A, Kamali S, et al. Serologic response to Epstein-Barr virus antigens in patients with systemic lupus erythematosus: a controlled study. Rheumatol Int 2012;32:79–83.
- Zandman-Goddard G, Berkun Y, Barzilai O, Boaz M, Blank M, Ram M, et al. Exposure to Epstein-Barr virus infection is associated with mild systemic lupus erythematosus disease. Ann N Y Acad Sci 2009;1173:658–63.
- Huggins ML, Todd I, Powell RJ. Reactivation of Epstein-Barr virus in patients with systemic lupus erythematosus. Rheumatol Int 2005;25:183–7.
- Berkun Y, Zandman-Goddard G, Barzilai O, Boaz M, Sherer Y, Larida B, et al. Infectious antibodies in systemic lupus erythematosus patients. Lupus 2009;18:1129–35.
- Broccolo F, Drago F, Cassina G, Fava A, Fusetti L, Matteoli B, et al. Selective reactivation of human herpesvirus 6 in patients with autoimmune connective tissue diseases. J Med Virol 2013;85:1925–34.
- Su BY, Su CY, Yu SF, Chen CJ. Incidental discovery of high systemic lupus erythematosus disease activity associated with cytomegalovirus viral activity. Med Microbiol Immunol 2007;196:165–70.
- Hochberg MC. Updating the American College of Rheumatology Revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725.
- Larsen M, Sauce D, Deback C, Arnaud L, Mathian A, Miyara M, et al. Exhausted cytotoxic control of Epstein-Barr virus in human lupus. PLoS Pathog 2011;7:1371.
- Vilá LM, Alarcón GS, McGwin G Jr, Bastian HM, Fessler BJ, Reveille JD, et al. Systemic lupus erythematosus in a multiethnic US cohort, XXXVII: association of lymphopenia with clinical manifestations, serologic abnormalities, disease activity, and damage accrual. Arthritis Rheum 2006;55:799–806.
- Utsinger PD. Relationship of lymphocytotoxic antibodies to lymphopenia and parameters of disease activity in systemic lupus erythematosus. J Rheumatol 1976;3:175–85.
- Mirzayan MJ, Schmidt RE, Witte T. Prognostic parameters for flare in systemic lupus erythematosus. Rheumatology 2000; 39:1316–19.
- Quan TE, Roman RM, Rudenga BJ, Holers VM, Craft JE. Epstein-Barr virus promotes interferon-alpha production by plasmacytoid dendritic cells. Arthritis Rheum 2010;62:1693–701.
- Severa M, Giacomini E, Gafa V, Anastasiadou E, Rizzo F, Corazzari M, et al. EBV Stimulates TLR- and autophagy-dependent pathways and impairs maturation in plasmacytoid dendritic cells: implications for viral immune escape. Eur J Immunol 2013;43:147–58.
- Bengtsson AA, Sturfelt G, Truedsson L, Blomberg J, Alm G, Vallin H, et al. Activation of type I interferon system in systemic lupus erythematosus correlates with disease activity but not with antiretroviral antibodies. Lupus 2000;9:664–71.
- Kim T, Kanayama Y, Negoro N, Okamura M, Takeda T, Inoue T. Serum levels of interferons in patients with systemic lupus erythematosus. Clin Exp Immunol 1987;70:562–9.
- Schattner A. Lymphopenia in systemic lupus erythematosus: possible role of interferon. Arthritis Rheum 1983;26:1415.
- Groux H, Cottrez F, Montpellier C, Quatannens B, Coll J, Stehelin D. Isolation and characterization of transformed human T-cell lines infected by Epstein-Barr virus. Blood 1997;89:4521–30.
- Schneider K, Meyer-Koenig U, Hufert FT. Human cytomegalovirus impairs the function of plasmacytoid dendritic cells in lymphoid organs. PLoS One 2008;3:3482.
- Chang WLW, Barry PA, Szubin R, Wang D, Baumgarth N. Human cytomegalovirus suppresses type 1 interferon secretion by plasmacytoid dendritic cells through its interleukin 10 homolog. Virology 2009;390:330–7.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Supplementary Tables S1–3.
Please note: The editors are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries should be directed to the corresponding author for the article.

