Abstract
Objective: To assess morning stiffness in rheumatoid arthritis (RA) patients switched from immediate-release (IR) to delayed-release (DR) prednisone.
Method: Circadian Administration of Prednisone in Rheumatoid Arthritis-1 (CAPRA-1) is a 12-week, randomized, multicentre, active-controlled study of morning stiffness that consisted of a double-blind phase and a 9-month open-label extension. Patients receiving IR prednisone with no significant improvement after the double-blind study were switched to DR prednisone. Morning stiffness duration and median absolute and relative changes in pain and global assessment were evaluated (3, 6, and 9 months).
Results: In patients switched from IR to DR prednisone (n = 110), statistically significant reductions in morning stiffness occurred over 3 months and were sustained for 9 months. Absolute reduction of morning stiffness was ~50 min with > 40% relative reduction at each visit. Interleukin (IL)-6 levels were reduced by the same amount. Statistically significant and clinically meaningful mean reductions in morning stiffness were maintained at > 67 min at each visit along with significant improvements in pain and patient global assessment. There was no evidence of tachyphylaxis seen over the 9-month study.
Conclusions: Patients receiving disease-modifying anti-rheumatic drugs (DMARDs) and IR prednisone who had not had significant reductions in morning stiffness demonstrated statistically significant and clinically meaningful improvements when switched to DR prednisone.
There is circadian variation in the signs and symptoms associated with rheumatoid arthritis (RA), with the physical impact of the disease typically peaking, along with inflammatory cytokines, during early morning hours (Citation1–Citation3). Cytokine levels, particularly interleukin (IL)-6, correlate with morning disease activity and often decrease with treatment (Citation3, Citation4).
Considering the early morning rise in pro-inflammatory cytokines and subsequent morning stiffness, timed delivery of glucocorticoids (GCs) can maximize effectiveness (Citation5). Although delivery of GCs at the optimal delivery time (02:00 h) can be accomplished with conventional-release GCs, it requires the patient to awaken and may be inconvenient (Citation5). A delayed-release (DR) prednisone tablet enables timed delivery of prednisone to target the circadian pattern of inflammatory mediators when taken just before bedtime (around 22:00 h).
Results from the double-blind Circadian Administration of Prednisone in Rheumatoid Arthritis-1 (CAPRA-1) study in patients with moderately to severely active RA on stable disease-modifying anti-rheumatic drug (DMARD) therapy showed that DR prednisone given at night reduced the duration of morning stiffness compared with immediate-release (IR) prednisone given in the morning (Citation6, Citation7).
The objective of this new analysis was to assess morning stiffness during the 9-month open-label extension phase of the CAPRA-1 study in patients who had responded inadequately to IR prednisone and who were switched to DR prednisone.
Method
Study design and patients
Patients from the double-blind portion of CAPRA-1 previously randomized to IR prednisone were switched to DR prednisone. During the open-label extension phase, visits occurred at the beginning of this phase and at months 3, 6, and 9; IL-6 samples were taken at baseline and at month 9.
Diary entries documented waking time, stiffness, time of resolution, and intensity of pain measured by a visual analogue scale (100-mm VAS) during the day. All patient diary entries ±4 weeks of each visit were compared with baseline at switch, thus increasing the number of observations from the original trial. Patients who had at least one diary measurement in the open-label study were included.
Outcomes
We analysed absolute and relative duration of morning stiffness (min) in the patients who switched from IR to DR prednisone. Secondary outcome measures included pain according to VAS, patient global assessment (PGA-VAS), Disease Activity Score based on 28 joint counts (DAS28), and IL-6 levels.
Patients
The patient population has been described previously in detail (Citation7). This study protocol was approved by responsible administrators and ethics committees and was conducted in accordance with the Declaration of Helsinki at 29 centres in Germany and Poland and was registered with ClinicalTrials.gov; NCT00146640.
Analysis
This analysis was conducted in a modified intent-to-treat efficacy population that included patients with diary entries at baseline and at least one additional visit during the open-label extension phase. Median absolute (paired t-test) and relative changes (Wilcoxon rank sum test) in duration of morning stiffness (min, at 3, 6, and 9 months), pain VAS, PGA-VAS, and IL-6 plasma concentration (IU/L) were measured at baseline and 9 months. Safety has been reported previously (Citation7).
Results
Patient disposition and demographics
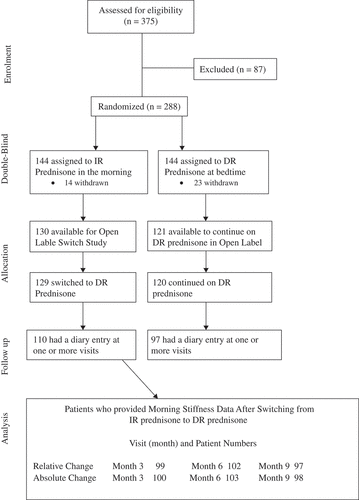
Patient disposition is detailed in . Patient demographics have been described previously (Citation7). Eighty-five per cent of the patients who switched to DR prednisone completed the open-label analyses. Concomitant DMARDs included: 76% methotrexate, 14% lefunomide, and 12% sulfasalazine (< 5% were on more than one DMARD).
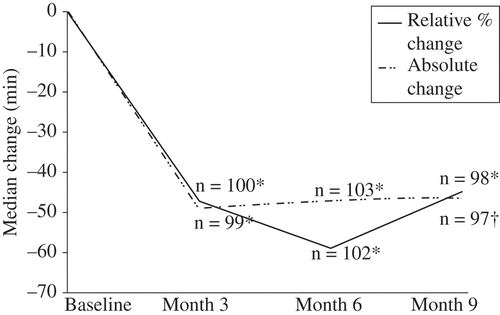
Morning stiffness
The mean absolute reduction in morning stiffness was significantly reduced at 3, 6, and 9 months (). This represented about a 50-min reduction from a baseline of 143.5 min. Similarly, about a 50% relative reduction in morning stiffness was observed at 3, 6, and 9 months after switching treatment regimens (). Patients switched to DR prednisone essentially ‘caught up’ with those continuing on DR prednisone within the first 3 months, as represented by a significantly greater relative reduction in minutes of morning stiffness (–47.2% vs. –28.5%, p = 0.0011) compared to those continuing on DR prednisone and similar decreases thereafter. The largest relative reduction in duration of morning stiffness was reported at 6 months (). At each visit, there was at least a 67-min reduction in the mean duration of morning stiffness in patients who switched.
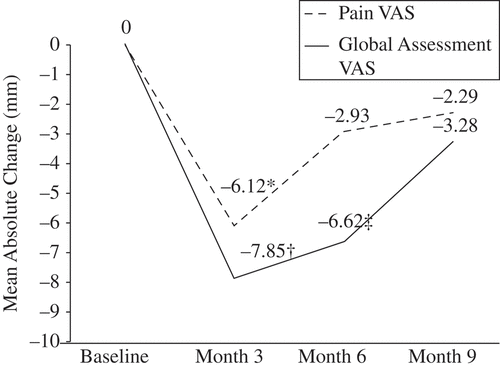
Secondary efficacy results
After the switch from IR to DR prednisone, there was a significant improvement in pain VAS (mean absolute change –6.1; p = 0.002) at 3 months followed by stabilization with a non-significant reduction at 9 months (). PGA-VAS also improved significantly at 3 (mean absolute change –7.9; p < 0.0001) and 6 months (–6.6; p = 0.008), with a non-significant reduction at 9 months. Median IL-6 levels (IU/L) showed a significant reduction (–53%; p < 0.001) from baseline (1055, range 200–22 700) to 9 months (500, range 200–5380) following the treatment switch from IR to DR prednisone.
Disease activity
In those patients switched to DR prednisone, 12.2% had low disease activity (DAS28 ≤ 3.2) at the end of the switch study period (9 months), which represented a 75% improvement in the number of patients meeting this threshold. Likewise, there was a decrease in the number of patients with high disease activity (DAS28 > 5.1) from 53.5% to 46%.
Safety
Detailed safety results have been reported previously (Citation7).
Discussion
The symptoms of RA are often at their worst in the morning after sleep/immobility and regularly affect quality of life, making it difficult for patients to begin their day comfortably. A GC formulation that releases prednisone when the inflammatory processes are the highest may be an appropriate treatment strategy to alleviate increased stiffness and pain in the waking hours (Citation1, Citation2, Citation5).
The efficacy and safety of DR prednisone, when added to DMARD therapy, has been established in double-blind, randomized trials, both in those previously receiving prednisone (CAPRA-1) and in those not previously receiving prednisone (CAPRA-2) (Citation6–Citation8).
In this analysis, statistically significant reductions in absolute and relative changes in morning stiffness occurred within 3 months after patients who had not responded to IR prednisone were switched to DR prednisone. The responses were similar to those patients continuing on DR prednisone from the double-blind phase of the study and reductions were maintained throughout the 9-month open-label extension phase. Furthermore, patients previously treated with DR prednisone who continued on DR prednisone maintained the efficacy demonstrated in the double-blind phase, indicating no tachyphylaxis. Following prior therapy with IR prednisone, patient-reported pain and global assessment improved after switching to DR prednisone. Both pain VAS and PGA-VAS were significantly different at 3 months; and at 9 months there was a relative reduction of approximately 16.0% in pain (p = 0.1318). Given that patients were previously treated for more than 3 months with IR prednisone therapy and had been on stable doses of GCs coming into the CAPRA-1 trial, it would not be entirely unexpected that VAS-measured pain would not differ dramatically. This finding may reinforce patients’ assessment of the hierarchical importance and differentiation of pain vs. morning stiffness (Citation9). The baseline pain was almost identical in both groups, and both groups experienced statistically significant decreases in absolute pain VAS from the beginning of the open-label evaluation to the end of the analysis 9 months later (p < 0.006). After 9 months of treatment with DR prednisone, there was a greater than 50% reduction in levels of IL-6, a pro-inflammatory cytokine that is elevated in patients with uncontrolled RA and is associated with morning symptoms (Citation2, Citation10).
Patients who switched from IR to DR prednisone in the open-label phase achieved and maintained reductions in morning stiffness that were comparable to those of patients who received DR prednisone continuously. These reductions were statistically and clinically significant (Citation11). This indicates that patients still experiencing morning stiffness while receiving IR prednisone may benefit from switching to DR prednisone. Data from a recently published 4-month open-label, observational study similarly suggest that switching patients from IR prednisone or 6-methyl-prednisone to DR prednisone can markedly decrease the duration of morning stiffness (Citation10). Patients who switched to DR prednisone showed improvements in duration of morning stiffness, numerical rating scale pain scores, and global assessment scores (Citation10).
This novel analysis has some strengths and weakness; it mirrors the real-world clinical scenario where patients often switch from ineffective therapies over time and offers insights for everyday clinical practice situations in which physicians are considering alternatives for their patients who have not responded adequately to conventional GC formulations. Only patients who had at least one diary measurement in the open-label extension phase were included in this analysis and thus represent an analysis of completers, which may bias the results.
DR prednisone was effective in reducing the duration of morning stiffness in patients previously treated with IR prednisone, and the results were maintained for 9 months. Considering the emotional and physical burden experienced by patients with RA, bedtime treatment with DR prednisone may offer patients a convenient way to benefit from early morning administration of GCs, as suggested by initial improvements in morning stiffness, pain, and global assessment following the switch from an ineffective 3-month course of IR prednisone, administered traditionally upon awakening.
Acknowledgements
We thank Amanda McGeary Tollen of JK Medical Communications, Inc, Conshohocken, PA, USA, for medical writing support; this editorial support and the study were funded by Horizon Pharma, Inc, USA.
References
- Spies CM, Cutolo M, Straub RH, Burmester GR, Buttgereit F. Prednisone chronotherapy. Clin Exp Rheumatol 2011;29:S42–5.
- Cutolo M, Seriolo B, Craviotto C, Pizzorni C, Sulli A. Circadian rhythms in RA. Ann Rheum Dis 2003;62:593–6.
- Perry MG, Kirwan JR, Jessop DS, Hunt LP. Overnight variations in cortisol, interleukin 6, tumour necrosis factor alpha and other cytokines in people with rheumatoid arthritis. Ann Rheum Dis 2009;68:63–8.
- Arvidson NG, Gudbjörnsson B, Elfman L, Rydén AC, Tötterman TH, Hällgren R. Circadian rhythm of serum interleukin-6 in rheumatoid arthritis. Ann Rheum Dis 1994;53:521–4.
- Arvidson NG, Gudbjörnsson B, Larsson A, Hällgren R. The timing of glucocorticoid administration in rheumatoid arthritis. Ann Rheum Dis 1997;56:27–31.
- Buttgereit F, Doering G, Schaeffler A, Witte S, Sierakowski S, Gromnica-Ihle E, et al. Efficacy of modified-release versus standard prednisone to reduce duration of morning stiffness of the joints in rheumatoid arthritis (CAPRA-1): a double-blind, randomised controlled trial. Lancet 2008;371:205–14.
- Buttgereit F, Doering G, Schaeffler A, Witte S, Sierakowski S, Gromnica-Ihle E, et al. Targeting pathophysiological rhythms: prednisone chronotherapy shows sustained efficacy in rheumatoid arthritis. Ann Rheum Dis 2010;69:1275–80.
- Buttgereit F, Mehta D, Kirwan J, Szechinski J, Boers M, Alten RE, et al. Low-dose prednisone chronotherapy for rheumatoid arthritis: a randomised clinical trial (CAPRA-2). Ann Rheum Dis 2013;72:204–10.
- Orbai AM, Smith KC, Bartlett SJ, De Leon E, Bingham CO 3rd. “Stiffness has different meanings, I think, to everyone”: Examining stiffness from the perspective of people living with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2014;66:1662–72.
- Cutolo M, Iaccarino L, Doria A, Govoni M, Sulli A, Marcassa C. Efficacy of the switch to modified-release prednisone in rheumatoid arthritis patients treated with standard glucocorticoids. Clin Exp Rheumatol 2013;31:498–505.
- Iqbal I, Dasgupta B, Taylor P, Heron L, Pilling C. Elicitation of health state utilities associated with differing durations of morning stiffness in rheumatoid arthritis. J Med Econ 2012;15:1192–200.