Abstract
Objectives: This phase IIIB study compared the efficacy and safety of febuxostat and allopurinol in gout patients with or without tophi who were HLA-B*5801 negative.
Method: Eligible patients were randomized to a febuxostat group (80 mg QD) or an allopurinol group (300 mg QD). Following an initial 2-week washout period, over the next 12 weeks we made five measurements of serum urate levels along with assessments of adverse events (AEs).
Results: Forty-three out of 152 screened subjects (28.3%) were ineligible either because of the presence of the HLA-B*5801 allele or for various other reasons. The febuxostat group (n = 54) and the allopurinol group (n = 55) had no significant differences in demographic or baseline characteristics. From week 2 to week 12, the febuxostat group had a significantly lower serum urate level than the allopurinol group (p ≤ 0.001 for all comparisons) and significantly more patients with serum urate levels less than 6.0 mg/dL. The serum urate levels of the febuxostat group declined by more than 40% from week 2 to week 12 and this decrease was greater than that in the allopurinol group (~30%). The two groups were similar in terms of AEs.
Conclusions: Febuxostat was more effective than allopurinol in reducing the serum urate levels of Han Chinese patients with gout or tophaceous gout who were HLA-B*5801 negative, without causing any serious skin reactions. Febuxostat should be considered for treatment of Han Chinese patients with gout who are HLA-B*5801 negative.
Gout is an inflammatory arthritis induced by accumulation of microscopic crystals of monosodium urate monohydrate and the deposition of aggregated monosodium urate crystals (tophi) in synovial fluid and other tissues (Citation1). Urolithiasis (renal stones composed of uric acid) may accompany gout or occur independently when renal urate excretion is inadequate. If untreated, gout can lead to painful and destructive arthropathy, urolithiasis, and renal failure (Citation2). The prevalence of hyperuricaemia and gout varies among different populations and racial groups. For example, gout has a high prevalence (11.7%) among Taiwan aborigines (Citation3) but a low prevalence (0.025%) in the Uygur population of Xinjiang (Citation4). Although hyperuricaemia and gout have become increasingly common in China during the past 30 years (Citation5, Citation6), the prevalence of gout is lower in Han Chinese (~0.2–1.2%) (Citation7, Citation8) than in residents of Western countries (~2%) (Citation9). In addition, the frequency of the HLA-B*5801 allele, which is associated with severe cutaneous adverse reactions to allopurinol, varies significantly among different ethnic groups (Citation10). The prevalence of the HLA-B*5801 allele in the Han Chinese (~20%) is higher than that in other populations (Citation10).
The goals of treatment are to control pain and lower the level of serum urate. Gout may be managed by dietary and other lifestyle changes or by various medications that block the synthesis or increase the excretion of uric acid (Citation9). Allopurinol is commonly used to treat gout as the first-line medication (Citation11). Allopurinol and its natural metabolite oxypurinol are purine analogues that inhibit xanthine oxidase (XO) and block uric acid production. Probenecid may also be used to treat gout (Citation11), although it is associated with significant liver and kidney toxicity. Probenecid binds to the renal organic anion transporter and inhibits the reabsorption of urate, thereby increasing the excretion of uric acid.
Allopurinol is associated with multiple side-effects, including increased toxicity when the glomerular filtration rate is reduced, and this may cause bone marrow depression, allopurinol hypersensitivity syndrome (AHS), hepatotoxicity, and Stevens–Johnson syndrome (Citation11). AHS is a rare but serious condition, with a mortality rate of 20–30%, and occurs at a higher frequency in patients with renal impairment and those receiving thiazide diuretics (Citation12).
Febuxostat is a 2-arylthiazole derivative that inhibits XO and was recently approved for the treatment of gout. Unlike allopurinol, febuxostat is a non-purine inhibitor of XO (Citation13). Thus, febuxostat differs from purine-like XO inhibitors, such as allopurinol and oxypurinol, in its structure, selectivity, and potency. Recent studies in the USA (Citation14–Citation16) and Japan (Citation17, Citation18) indicate that febuxostat compares favourably with allopurinol in the treatment of gout.
The aim of the current study was to compare the efficacy and safety of oral febuxostat (80 mg QD) and allopurinol (300 mg QD) over a 12-week period in a population of Han Chinese patients diagnosed with gout.
Method
Study design
This was a phase IIIB, multicentre, open-label, randomized, allopurinol-controlled, parallel-design study that evaluated the safety and efficacy of febuxostat vs. allopurinol in Han Chinese patients with gout. Eligible patients were randomized (1:1) and treated with febuxostat (80 mg QD) or allopurinol (300 mg QD) for 12 weeks. The febuxostat dose was based on previous clinical trials of populations in other geographic regions and the allopurinol dose was according to the Taiwan Guideline for the Management of Gout and Hyperuricaemia. The study included an initial screening visit (washout/run-in for 2 weeks) and five visits during the treatment period (day 1 and at the end of weeks 2, 4, 8, and 12). Colchicine (0.5 mg BID) was used for prophylaxis of gout flares. The subjects were treated at the Taipei Veterans General Hospital (VGH-TP), the Taichung Veterans General Hospital (VGH-TC), Chang Gung Medical Foundation – Linkou (CGMH-LK), Chang Gung Medical Foundation – Kaohsiung (CGMH-KS), National Taiwan University Hospital (NTUH), or the Tri-Service General Hospital (TSGH) and all gave signed written informed consent. This study was approved by all local Institutional Review Boards.
Patients who were taking allopurinol or uricosuric agents prior to study onset discontinued these agents at the initial screening visit, and began taking prophylactic medication to prevent flares. Patients who were not taking allopurinol or uricosuric agents began prophylactic treatment at the initial screening visit. All patients continued prophylactic medication throughout the entire 12-week study period.
Enrolment criteria
Enrolled subjects were 20–65 years old and diagnosed with gout based on the American College of Rheumatology (ACR) criteria (Citation19). These criteria consider clinical symptoms, laboratory results, and X-ray findings. All enrolled subjects who were not taking urate-lowering agents had serum urate levels ≥ 8.0 mg/dL at the screening visit.
The following exclusion criteria were applied to all subjects: breastfeeding or pregnancy, history of xanthinuria, allopurinol intolerance (i.e. hypersensitivity, Stevens–Johnson syndrome, topical epidermal necrolysis), use of allopurinol at > 300 mg/day and serum urate level > 8 mg/dL at the screening visit, presence of the HLA-B*5801 allele, use of thiazide diuretic therapy, secondary hyperuricaemia, requirement for concurrent therapy with any systemic or topical medication (prescribed or non-prescribed) that contained aspirin or other salicylates at the screening visit or during the study [although stable, low-dose aspirin (325 mg/day) was allowed], requirement for therapy with prednisone of at least 10 mg/day during the study period, change in hormone replacement or oral contraceptive therapy within 3 months of the screening visit, alcohol intake of 14 or more drinks per week or alcohol abuse within the previous 5 years, requirement for concurrent therapy with any urate-lowering agent, active liver disease or hepatic dysfunction [alanine aminotransferase (ALT) and aspartate aminotransferase (AST)] more than 1.5 times the upper limits of normal), serum creatinine of 1.5 mg/dL or more at the screening visit, inability to take the protocol-required gout flare prophylactic medication of colchicine due to intolerance, hypersensitivity, active gastric ulcer disease, renal impairment, and/or changes in liver enzymes, presence of any other significant medical condition that would interfere with treatment, safety, or compliance (e.g. clinically significant electrocardiography result), history of cancer (other than basal cell carcinoma), use of any systemic cancer chemotherapy within 5 years prior to the screening visit, participation in a clinical study in which febuxostat was administered, or participation in another investigational trial in the 30 days prior to the screening visit.
Outcomes
The primary end-point was the proportion of subjects whose last serum urate levels were below 6.0 mg/dL. The secondary end-point was the percentage reduction in serum urate level. Adverse events (AEs) due to febuxostat and allopurinol were also collected during the entire study period.
Sample size calculation
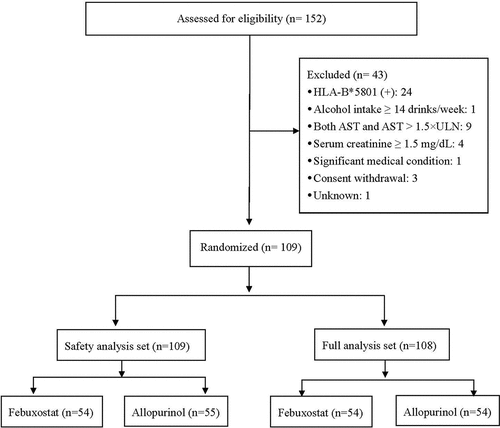
We determined that 120 patients (60 patients in each group) were needed to achieve 80% power and meet the 26% difference between two groups (22% for the control group and 48% for the febuxostat group) for the primary efficacy variable at an alpha level of 0.05. Based on local clinical experiences with febuxostat and allopurinol in terms of efficacy and safety, we assumed a dropout rate of 25% due to HLA-B*5801 positivity and other factors during the screening period. Thus, 150 patients with gout were needed for the initial screening. We enrolled 152 subjects, 43 of whom were ineligible based on the screening criteria (43/152, 28.2%). Ultimately, 109 patients were randomized, 54 subjects in the febuxostat group and 55 in the allopurinol group ().
Statistical analysis
All efficacy variables were analysed based on data from the full analysis set. Missing data in the analysis of serum urate levels were imputed by use of the last observation carried forward (LOCF) method (including the baseline value, if necessary). Continuous variables are presented as means and standard deviations and were compared by Student’s t-test. The number and percentage of patients are presented as categorical variables and the two groups were compared with a χ2 test or Fisher’s exact test.
Changes in serum urate levels over time in the two groups were compared by analysis of covariance (ANCOVA), with the level at baseline (day 1) as the covariate. The proportion of subjects in the two groups whose serum urate levels fell below 6.0 mg/dL and who had AEs were compared by the χ2 test. Non-inferiority between the treatment groups was declared if the absolute value of the lower bound of the 95% confidence interval (CI) did not exceed 15%. Subgroup analysis of subjects with and without tophi at baseline was performed for efficacy assessments. All statistical analyses were performed with SAS Version 9.3 on the MS Windows platform. All statistical comparisons were made using two-sided tests, and a p-value of 0.05 was considered statistically significant.
Results
A total of 152 subjects were initially enrolled, and 43 of these subjects (28.3%) were deemed ineligible due to the presence of the HLA-B*5801 allele (n = 24), ALT and AST levels more than 1.5-fold above the upper limit of normal (n = 9), serum creatinine ≥ 1.5 mg/dL (n = 4), withdrawal of consent (n = 3), and other reasons (n = 3). The remaining 109 subjects were randomly assigned to the febuxostat group (n = 54) or the allopurinol group (n = 55).
shows the demographic and baseline characteristics of the two treatment groups. There were no significant differences in all parameters measured. The mean age was 46.0 ± 11.0 years in the febuxostat group and 45.2 ± 12.0 years in the allopurinol group. The mean body mass index (BMI) was 26.8 ± 3.7 kg/m2 in the febuxostat group and 27.8 ± 4.2 kg/m2 in the allopurinol group. The proportion of subjects with serum urate levels ≥ 9 mg/dL at baseline was 83.3% in the febuxostat group and 80.0% in the allopurinol group. The two groups also showed no significant differences in the use of alcohol, tobacco, and caffeine, and no significant differences in all measured urinalysis parameters (uric acid, creatinine, and creatinine clearance).
Table 1. Demographic and baseline characteristics of enrolled gout patients after the washout period (safety analysis set *).
shows the percentage change in serum urate levels throughout the study period. Relative to day 1, the febuxostat group had a mean decline of serum urate of at least 40% from week 2 to week 12 and this decrease was greater than that in the allopurinol group (~30%) at all times (p < 0.001 for all comparisons). We observed the same results for all subjects, subjects with tophi, and subjects without tophi. The mean serum urate levels of the two groups were recorded at the initial screening, at study onset (day 1), and at the end of weeks 2, 4, 8, and 12. These results show that the febuxostat group had a mean serum urate level less than 6 mg/dL from week 2 to week 12 and that these levels were significantly lower than those of the allopurinol group at all times (p < 0.001 for all comparisons, data not shown), while shows the proportion of subjects in each group whose serum urate levels decreased to less than 6.0 mg/dL. There were significantly more subjects in the febuxostat group who had serum urate levels less than 6.0 mg/dL from week 2 to week 12 (p < 0.001 for all comparisons) and we observed the same results for all subjects, subjects with tophi, and subjects without tophi (p < 0.025 for all comparisons). Eight patients (7.3%) had changes in the number of tophi at 3 months: two patients in the allopurinal group had fewer tophi and two had more tophi; four patients in the febuxostat group had fewer tophi (data not shown). We would expect more significant differences between the two treatment groups if the study period was longer.
Figure 2. Percentage change in serum urate level (mean ± sd) of gout patients before and at various times after initiation of treatment (day 1), full analysis set. * Significant difference (p < 0.05) based on a χ2 test.
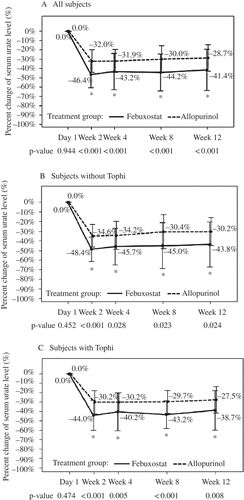
Table 2. Number (percentage) of enrolled gout patients with a serum urate level less than 6.0 mg/dL at different times after initiation of treatment (full analysis set *).
compares the AEs of the two treatment groups. A total of 38 subjects (70.4%) in the febuxostat group and 35 subjects (63.6%) in the allopurinol group experienced at least one AE during the study period (p = 0.587). There were also no significant differences in any of the individual AEs (p ≥ 0.124 for all comparisons). During the study period, the incidence of gout flare was 40.7% in the febuxostat group and 34.6% in the allopurinol group (data not shown). Drug-related AEs occurred in eight subjects (15%) in the febuxostat group and seven subjects (13%) in the allopurinol group. For both groups, the most frequent drug-related AE was an abnormal liver function test result, but all of these abnormalities were all mild. Severe AEs occurred in two subjects in the febuxostat group (cellulitis from a ruptured tophi wound and a duodenal ulcer) and one subject in the allopurinol group (malignant tongue neoplasm), but these events were not drug-related. The incidence of skin rash was lower in subjects receiving febuxostat than allopurinol (5.6% vs. 16.4%), but this difference was not statistically significant (p = 0.124). Among subjects with skin rashes, none experienced a serious reaction, such as a severe cutaneous adverse reaction, and we considered causal relationships with use of the study drug as possible for one subject (1.9%) in the febuxostat group and five subjects (9.1%) in the allopurinol group. Two subjects (3.6%) discontinued allopurinol treatment due to skin rashes.
Table 3. Adverse events (AEs) of enrolled gout patients during the 12-week study period (safety analysis set).
shows the changes in serum creatinine during the study period. There were significant reductions in serum creatinine in both groups. There were no statistically significant differences between the groups but there was a tendency for a greater decline in the febuxostat group (from 1.08 ± 0.18 to 1.01 ± 0.17 vs. from 1.09 ± 0.18 to 1.04 ± 0.17) after 12 weeks.
Table 4. Changes in serum creatinine (safety analysis set).
Discussion
The present study was a phase IIIB comparison of the safety and efficacy of allopurinol (300 mg QD) and febuxostat (80 mg QD) in lowering the serum urate levels of Chinese patients with gout. The results indicate that the febuxostat group had significantly lower serum urate levels and significantly more patients with serum urate levels less than 6.0 mg/dL at week 12. In addition, relative to baseline, the serum urate levels of the febuxostat group had a mean decline of more than 40% from week 2 to week 12 and this decline was greater than in the allopurinol group (~30%). There were no significant differences between the two groups in overall AEs or in any specific type of AE. There was a greater increase in gout flares in the febuxostat group because of the rapid decline in serum urate level in this group, which may indicate that significant fluctuations in the serum urate level can cause this AE. We suggest that clinicians perform short-term co-administration of low-dose colchicine or another anti-inflammatory analgesic to prevent or minimize acute gout attacks.
Most other studies that have compared allopurinol and febuxostat in the treatment of hyperuricaemia (with or without gout) have studied Western populations. For example, Chohan (Citation14) assessed the effect of febuxostat in 13 gout patients with severe adverse effects following allopurinol treatment, and reported that febuxostat was safe and effective in 12 of these patients. Becker et al (Citation15) studied 762 patients with gout and showed that febuxostat (80 or 120 mg QD) more effectively lowered serum urate levels than allopurinol (300 mg QD). Schumacher et al (Citation20) compared the effect of febuxostat (80, 120, or 240 mg QD), allopurinol (300 or 100 mg QWD), and placebo on the serum urate levels of 1072 gout patients and found that febuxostat was more effective than allopurinol or placebo. Becker et al (Citation16) compared the effect of febuxostat (40 or 80 mg QD) and allopurinol (200 or 300 mg) in 2269 subjects with gout and reported that 80 mg QD febuxostat was the most effective, and that the different treatment groups were similar in terms of AEs.
Several Japanese studies have compared the safety and efficacy of allopurinol and febuxostat. One study (Citation17) examined 247 patients with hyperuricaemia and reported that febuxostat (40 mg QD) was more effective than allopurinol (200 mg QD) in lowering serum urate and that these two agents had similar safety profiles. Kamatani et al (Citation21) studied 40 patients with hyperuricaemia and found that febuxostat was safe at the two tested doses (40 and 60 mg QD) and had equal or greater efficacy than allopurinol (300 mg QD) in lowering serum urate levels. A phase III study (Citation18) enrolled 104 patients with hyperuricaemia and compared the efficacy of febuxostat (20 or 40 mg QD) and placebo. The results indicated that febuxostat at either dose effectively lowered serum urate over the course of 8 weeks, and that this drug had an acceptable safety profile. Finally, a recent meta-analysis of 10 studies from the USA and Japan (Citation22) reported that febuxostat more effectively reduced serum urate in patients with hyperuricaemia at doses of 40 or 120 mg QD compared with allopurinol at 100 or 300 mg QD, and that these two drugs had similar tolerability.
The present study is the first rigorous clinical trial to compare the safety and efficacy of allopurinol and febuxostat in a homogeneous population of Chinese subjects with hyperuricaemia and a diagnosis of gout based on ACR criteria. The prevalence of gout (Citation23) and the frequency of the HLA-B*5801 allele (Citation10) vary significantly among different populations. The high screening failure rate of subjects who were HLA-B*5801 positive in our study (15.8%) is due to the high prevalence of this allele among the Han Chinese. In addition, treatment with urate-lowering drugs is essential for gout patients because a high serum uric acid level has been found to increase the risk of cardiovascular disease and metabolic syndrome in the Chinese population (Citation24–Citation26). Thus, the clinical effects of febuxostat in previous studies, most of which enrolled Western patients, may or may not apply to Chinese patients. Nevertheless, the results presented here clearly indicate that febuxostat was more effective than allopurinol for short-term control of hyperuricaemia in patients diagnosed with gout, and that these two drugs had similar safety profiles. This indicates that febuxostat is a safe and effective alternative to allopurinol for the treatment of gout in Chinese patients, and that febuxostat may be particularly suitable for patients who cannot tolerate allopurinol.
Several recent studies agree with our results. Hatoum et al (Citation27) examined a database of adults with newly diagnosed gout and concluded that febuxostat was effective in the management of gout. Maekawa et al (Citation28) reported that allopurinol, febuxostat, and benzbromarone therapy reduced serum urate levels to below 6 mg/dL in the treatment of refractory gout. Huang et al (Citation29) reported that the urate-lowering efficacy of febuxostat at 80 mg QD was greater than that of febuxostat at 40 mg QD and allopurinol at 300 mg QD.
The present phase IIIB study had certain limitations that should be noted. First, we excluded patients who had the HLA-B*5801 allele, a significant risk factor for severe allergic skin reactions from allopurinol in certain populations (Citation10), so we cannot draw any conclusions about the efficacy and safety of these two medications in treatment of these patients. We also excluded patients with impaired renal function (serum creatinine ≥ 1.5 mg/dL at screening), even though renal complications are often a consequence of severe gout (Citation30). Thus, our results cannot be applied to gout patients with serious kidney malfunction. Finally, we only measured serum urate levels for 12 weeks and did not include a placebo group; however, a high serum urate level is associated with cardiovascular disease and metabolic syndrome. Thus, on an ethical basis, to include a placebo group when established treatments are available is considered unethical. Hence, our results are of limited use for the assessment of the safety and efficacy of these medications in the treatment of chronic gout.
The major finding of this study is that 80 mg QD febuxostat is superior to 300 mg QD allopurinol in reducing serum urate levels in Han Chinese patients with gout or tophaceous gout. The superiority of febuxostat is indicated by the significantly higher percentage of patients in the febuxostat group who had serum urate levels less than 6.0 mg/dL and the significantly greater mean reduction of serum urate in the febuxostat group. These two drugs had similar safety profiles. In addition, for patients with mild or moderately impaired renal function, our measurements of serum creatinine throughout the entire study period indicate that there was no need to adjust the dose of febuxostat, making this drug convenient for clinical use. Furthermore, both groups had a statistically significant decrease in serum creatinine after 12 weeks. Several other studies have also reported that serum creatinine decreased after treatment with urate-lowering agents (Citation30, Citation31). Thus, the decline in serum creatinine is associated with the decline in serum urate, although the changes in serum creatinine appeared to take much longer, so it is possible that they are not directly related. Nevertheless, our results do indicate that improvement in renal function is achieved by using urate-lowering agents in subjects with gout.
Based on the results of this study, we conclude that febuxostat is an effective, safe, and well-tolerated treatment for lowering the serum urate levels of Han Chinese patients with gout or tophaceous gout.
Acknowledgements
We thank the following investigators at the clinical sites of this study: W-N Huang, C-W Hsieh, T-Y Hsieh, Y-H Chen, Y-C Chen, T-T Cheng, Y-J Su, S-C Hsieh, K-J Li, and C-H Chen. We also thank S-I Hung for assisting with the HLA-B*5801 genotype screening.
This study was funded by Astellas Pharma Taiwan, Inc and is registered as NCT01736514 on clinicaltrials.gov. Medical writing and editorial support were provided by MedCom Asia Medical Writing and funded by Astellas Pharma Taiwan, Inc.
References
- Crittenden DB, Pillinger MH. New therapies for gout. Annu Rev Med 2013;64:325–37.
- Roddy E, Zhang W, Doherty M. The changing epidemiology of gout. Nat Clin Pract Rheumatol 2007;3:443–9.
- Chou CT, Lai JS. The epidemiology of hyperuricaemia and gout in Taiwan aborigines. Br J Rheumatol 1998;37:258–62.
- Wu LJ, Song XY. Epidemiological study on hyperuricemia and gout in Uygur population in Turpan area of Xinjiang [in Chinese]. Beijing Da Xue Xue Bao 2012;44:250–3.
- Richette P, Bardin T. Gout. Lancet 2010;375:318–28.
- Zeng QY, Chen R, Darmawan J, Xiao ZY, Chen SB, Wigley R, et al. Rheumatic diseases in China. Arthritis Res Ther 2008;10:R17.
- Xiang YJ, Dai SM. Prevalence of rheumatic diseases and disability in China. Rheumatol Int 2009;29:481–90.
- Chen JH, Yeh WT, Chuang SY, Wu YY, Pan WH. Gender-specific risk factors for incident gout: a prospective cohort study. Clin Rheumatol 2012;31:239–45.
- Brook RA, Forsythe A, Smeeding JE, Lawrence Edwards N. Chronic gout: epidemiology, disease progression, treatment and disease burden. Curr Med Res Opin 2010;26:2813–21.
- Hung SI, Chung WH, Liou LB, Chu CC, Lin M, Huang HP, et al. HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci U S A 2005;102:4134–9.
- Gois PH, Souza ER. Pharmacotherapy for hyperuricemia in hypertensive patients. Cochrane Database Syst Rev 2013;1:CD008652.
- Ramasamy SN, Korb-Wells CS, Kannangara DR, Smith MW, Wang N, Roberts DM, et al. Allopurinol hypersensitivity: a systematic review of all published cases, 1950–2012. Drug Saf 2013;36:953–80.
- Tayar JH, Lopez-Olivo MA, Suarez-Almazor ME. Febuxostat for treating chronic gout. Cochrane Database Syst Rev 2012;11:CD008653.
- Chohan S. Safety and efficacy of febuxostat treatment in subjects with gout and severe allopurinol adverse reactions. J Rheumatol 2011;38:1957–9.
- Becker MA, Schumacher HR Jr, Wortmann RL, MacDonald PA, Eustace D, Palo WA, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med 2005;353:2450–61.
- Becker MA, Schumacher HR, Espinoza LR, Wells AF, MacDonald P, Lloyd E, et al. The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout: the CONFIRMS trial. Arthritis Res Ther 2010;12:R63.
- Kamatani N, Fujimori S, Hada T, Hosoya T, Kohri K, Nakamura T, et al. An allopurinol-controlled, randomized, double-dummy, double-blind, parallel between-group, comparative study of febuxostat (TMX-67), a non-purine-selective inhibitor of xanthine oxidase, in patients with hyperuricemia including those with gout in Japan: phase 3 clinical study. J Clin Rheumatol 2011;17(4 Suppl 2):S13–18.
- Kamatani N, Fujimori S, Hada T, Hosoya T, Kohri K, Nakamura T, et al. Placebo-controlled, double-blind study of the non-purine-selective xanthine oxidase inhibitor Febuxostat (TMX-67) in patients with hyperuricemia including those with gout in Japan: phase 3 clinical study. J Clin Rheumatol 2011;17(4 Suppl 2):S19–26.
- Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yü TF. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum 1977;20:895–900.
- Schumacher HR Jr, Becker MA, Wortmann RL, Macdonald PA, Hunt B, Streit J, et al. Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28-week, phase III, randomized, double-blind, parallel-group trial. Arthritis Rheum 2008;59:1540–8.
- Kamatani N, Fujimori S, Hada T, Hosoya T, Kohri K, Nakamura T, et al. An allopurinol-controlled, multicenter, randomized, open-label, parallel between-group, comparative study of febuxostat (TMX-67), a non-purine-selective inhibitor of xanthine oxidase, in patients with hyperuricemia including those with gout in Japan: phase 2 exploratory clinical study. J Clin Rheumatol 2011;17(4 Suppl 2):S44–9.
- Ye P, Yang S, Zhang W, Lv Q, Cheng Q, Mei M, et al. Efficacy and tolerability of febuxostat in hyperuricemic patients with or without gout: a systematic review and meta-analysis. Clin Ther 2013;35:180–9.
- Roddy E, Doherty M. Epidemiology of gout. Arthritis Res Ther 2010;12:223.
- Chen JH, Chuang SJ, Chen HJ, Yeh WT, Pan WH. Serum uric acid level as an independent risk factor for all-cause, cardiovascular, and ischemic stroke mortality: a Chinese cohort study. Arthritis Rheum 2009;61:225–32.
- Kuo CF, See LC, Yu KH, Chou IJ, Chiou MJ, Luo SF. Significance of serum uric acid levels on the risk of all-cause and cardiovascular mortality. Rheumatology (Oxford) 2013;52:127–34.
- Chen JH, Pan WH, Hsu CC, Yeh WT, Chuang SY, Chen PY, et al. Impact of obesity and hypertriglyceridemia on gout development with or without hyperuricemia: a prospective study. Arthritis Care Res (Hoboken) 2013;65:133–40.
- Hatoum H, Khanna D, Lin SJ, Akhras KS, Shiozawa A, Khanna P. Achieving serum urate goal: a comparative effectiveness study between allopurinol and febuxostat. Postgrad Med 2014;126:65–75.
- Maekawa M, Tomida H, Aoki T, Hishida M, Morinaga T, Tamai H. Successful treatment of refractory gout using combined therapy consisting of febuxostat and allopurinol in a patient with chronic renal failure. Intern Med 2014;53:609–12.
- Huang X, Du H, Gu J, Zhao D, Jiang L, Li X, et al. An allopurinol-controlled, multicenter, randomized, double-blind, parallel between-group, comparative study of febuxostat in Chinese patients with gout and hyperuricemia. Int J Rheum Dis 2014;17:679–86.
- Whelton A, Macdonald PA, Zhao L, Hunt B, Gunawardhana L. Renal function in gout: long-term treatment effects of febuxostat. J Clin Rheumatol 2011;17:7–13.
- Kim HA, Seo YI, Song YW. Four-week effects of allopurinol and febuxostat treatments on blood pressure and serum creatinine level in gouty men. J Korean Med Sci 2014;29:1077–81.