Abstract
Context: Bitter taste, as well as dissolve time, presents a significant challenge for the acceptability of formulations for oral transmucosal drug delivery.
Objective: To characterize a novel sublingual tablet formulation of buprenorphine/naloxone with regards to pharmacokinetics, dissolve time and formulation acceptability.
Methods: Dry mixing techniques were employed to produce a small and fast dissolving buprenorphine/naloxone sublingual tablet formulation, OX219 (Zubsolv®), using sucralose and menthol as sweetener and flavor to mask the bitter taste of the active ingredients. Two cross-over studies were performed in healthy volunteers to evaluate pharmacokinetics, dissolve time and acceptability of OX219 5.7/1.4 mg tablets compared to the commercially available buprenorphine/naloxone formulations Suboxone® tablets and films (8/2 mg).
Results: Buprenorphine exposure was equivalent in OX219 and Suboxone tablets. Sublingual dissolve times were significantly shorter for OX219 than for Suboxone tablets and were similar to Suboxone films. The OX219 formulation received significantly higher subjective ratings for taste and overall acceptability than both Suboxone formulations. OX219 was preferred over Suboxone tablet and film formulations by 77.4% and 88.9% of subjects, respectively.
Conclusions: A sublingual tablet formulation with an improved acceptability has been successfully developed.
Introduction
For drugs with a limited oral bioavailability due to degradation in the gastrointestinal tract and/or first pass metabolism in the liver, oral transmucosal drug delivery presents a viable option. Several dosage forms have been developed for oral transmucosal delivery, including tablets, solutions, sprays, buccal and sublingual films, chewing gums, lozenges, patches and hydrogelsCitation1. Tablets prepared by direct compression of a dry mixture have technical advantages over pharmaceutical formulations prepared by wet processes, including the presentation of robust and efficient processes of manufacturing, packaging and distribution, easy drug handling and favorable stability. However, as dissolution in the oral cavity is a prerequisite for absorption, taste acceptability issues resulting from bitterness of the drug substance presents a significant challenge with possible implications on treatment complianceCitation2. Furthermore, as sublingual administration may interfere with common activities, such as eating, drinking and talking, it is desirable to keep sublingual dissolve times to a minimum, especially if the medication is used on a daily basis over extended time periods.
A sublingual tablet formulation comprising buprenorphine and naloxone, Suboxone® (Reckitt Benckiser Pharmaceuticals Inc., Richmond, VA), was approved for maintenance treatment of opioid dependence in the US in 2002Citation3. A sublingual film formulation based on polyethylene oxide, (Suboxone film), was subsequently approved in 2010Citation4. Both active ingredients have a bitter taste/aftertaste. In a survey conducted by the manufacturer, a high proportion of patients reported issues with the taste and sublingual dissolve time of the Suboxone tablet formulation.Citation5
A novel tablet formulation, OX219 (Zubsolv®, Orexo US Inc., New York, NY), has been developed, which demonstrates an improved dissolve time and palatability compared to other buprenorphine/naloxone combinations when administered sublingually. The purpose of this work was to characterize this novel formulation pharmacokinetically as well as to compare sublingual dissolve times and formulation acceptability to Suboxone tablets and films.
Methods
Materials
The OX219 5.7/1.4 mg buprenorphine/naloxone composition consists of buprenorphine HCL, naloxone HCL dihydrate, mannitol, microcrystalline cellulose, croscarmellose sodium, sodium stearyl fumarate, tri-sodium citrate dihydrate, citric acid, silicon dioxide, sucralose and menthol. Suboxone tablets and films (both 8/2 mg; Reckitt Benckiser Pharmaceuticals Inc., Richmond, VA) and naltrexone hydrochloride tablets (50 mg) were sourced from commercial stock.
Formulation
OX219 tablets were prepared by dry mixing and direct compression resulting in tablets with a weight of 110 mg, approximately 1/4 of the tablet weight of Suboxone tablet. Sucralose and menthol were included in the formulation as sweetener and flavor to mask the bitter taste of the active ingredients. Sucralose of a granular grade was added in the dry mixing without previous processing. Menthol, crystalline, was first milled by a screen-mill to powder form and then mixed and co-milled (screen-mill) with silicon dioxide before adding to the dry mixing. The 30% lower buprenorphine dose was selected based on a previous pharmacokinetic study, indicating approximately 40% higher bioavailability of OX219 compared to Suboxone tablet (unpublished data). The naloxone:buprenorphine dose ratio was kept at 1:4 to achieve the same deterrent effect on intravenous abuse as marketed formulations.
Comparative bioavailability study, OX219-003
A fasting, open-label, two-period, randomized sequence, cross-over, comparative bioavailability study was performed in 60 male and female healthy volunteers to compare buprenorphine and naloxone exposure from OX219 to Suboxone tabletCitation3. The study consisted of a screening visit and two treatment periods where a single dose of the test or reference product was administered at each period. Subjects were fasting at least 10 h prior to dosing until 4 h after dosing. The opioid antagonist naltrexone 50 mg was administered at three occasions, 12 h and 1 h prior to dosing and 12 h after dosing to block the effects of buprenorphine. Plasma samples were collected for 72 h after dosing and were analyzed for levels of buprenorphine and naloxone using LC-MS/MS methods. Subjects indicated when they perceived that the medication was completely dissolved. Study personnel then checked the oral cavity for tablet remnants, with repeated checks as necessary. The confirmed dissolve time was recorded at each dosing, with a precision of whole minutes. Taste and overall formulation experience (disregarding any perceived effects and side effects) was rated on a numeric rating scale (NRS) (1 = “extremely unpleasant”; 10 = “extremely pleasant”). Overall preference of formulation was assessed after the last dosing.
Fully validated LC-MS/MS methods were employed for the analysis of buprenorphine and naloxone in K2-EDTA plasma. The methods were validated for the range 0.025 to 10.0 ng/mL for buprenorphine, based on the analysis of 0.500 mL of plasma, and for the range 1.00–250 pg/mL for naloxone, based on the analysis of 1.00 mL plasma. Incurred sample reproducibility (ISR) was evaluated for both analytes, by selecting at least 10% of the samples near Cmax or in the elimination phase for reanalysis. ISR fulfilled pre-specified criteria for both analytes (at least 2/3 of the samples being within ±20% of the originally reported value).
The study was performed by Novum Pharmaceutical Research Services (Las Vegas, NV) and was approved by Novum Independent Institutional Review Board. Bioanalysis was performed by Worldwide Clinical Trials, Austin, TX.
Acceptability/preference study, OX219-005
An open-label, two-period, randomized sequence, cross-over study was performed in 28 healthy volunteers to compare the acceptability and preference of OX219 to Suboxone filmCitation4. The study consisted of one screening visit and one inpatient treatment period in which subjects received both OX219 and Suboxone film in a randomized sequence with a washout period of 36 h between treatments. The opioid antagonist naltrexone 50 mg was administered at seven occasions, 12 and 1 h prior to each dosing and 12 h after each dosing +24 h after the last dose, to block the effects of buprenorphine. Subjects recorded the time when they first perceived that the medication (tablet/film) was completely dissolved with a precision of whole seconds.
Visual analogue scale (VAS) ratings were collected for overall formulation acceptability (disregarding any perceived effects and side effects; 0 mm = “extremely unpleasant” and 100 mm = “extremely pleasant”), taste (0 mm = “extremely unpleasant” and 100 mm = “extremely pleasant”), mouthfeel (0 mm = “extremely unpleasant” and 100 mm = “extremely pleasant”) and ease of drug administration (0 mm = “extremely easy” and 100 mm = “extremely difficult”). Unpleasant aftertaste was rated on a categorical scale (“None”, “Mild”, “Moderate” or “Strong”). Preference of formulation was assessed after the last dosing (overall preference as well as preference considering the individual formulation properties “taste”, “mouthfeel” and “ease of drug administration”).
The study was performed by Quintiles Phase I Services, Overland Park, KS and was approved by Midland Independent Review Board.
Statistical methods
Pharmacokinetic parameters were determined by non-compartmental methods. Maximum plasma concentration (Cmax) and time to maximum plasma concentration (tmax) were determined for the individual plasma profiles. The area under the plasma concentration versus time curve to the last quantifiable concentration (AUC0–t) was determined by the linear trapezoidal method. The elimination constant (λz) was estimated from the slope of the regression line for the terminal ln-linear concentration–time values when possible. The area under the plasma concentration versus time curve extended to infinity, was calculated as AUC0–t + Ct/λz, where Ct represented the last measurable plasma concentration. OX219:Suboxone tablet geometric mean ratios (GMRs) with 90% confidence intervals (CI) were calculated for AUC0–t, AUC0–∞ and Cmax using an ANOVA model with main effects of sequence, subject within sequence, treatment and period. Standard bioequivalence criteria, i.e. GMRs within 0.8000–1.2500, were pre-specified for declaring equivalent exposure. Dissolve times were compared between treatments using non-parametric statistics on paired data (Hodge–Lehmann estimate of median difference between treatments with 95% CI according to Hahn and Meeker, Wilcoxon’s signed rank tests). NRS ratings were compared by a paired student’s t-test. VAS ratings were compared between treatments using an ANOVA model with fixed effect for treatment, sequence and period and random effect for subjects within sequence. Preference assessments were evaluated by a χ2 test.
Determination of sample size
The sample size of the OX219-003 study was determined from a previous dose-finding pharmacokinetic study (unpublished data). For the selected power of 80% and α of 0.05, a mean within subject coefficient of variation for buprenorphine and naloxone AUC and Cmax of 0.26 and a safety margin of 10% (to account for potential errors in assumptions of dose proportionality) gave a sample size of 53 subjects. Sixty subjects were included to account for a drop-out rate of about 10%.
The sample size of study OX219-005 was selected to achieve an 80% power of detecting a difference between treatments of 15 mm. With an estimated VAS standard deviation of 25 mm, the number of subjects needed was 24. Twenty-eight subjects were included to account for a dropout rate of 10%.
Results
Study conduct
In the comparative bioavailability study, OX219-003, sixty healthy volunteers were randomized and treated with at least one dose of study medication and 53 subjects received both treatments. Four subjects experienced emesis within 2 times median tmax of buprenorphine and were excluded from buprenorphine pharmacokinetic analyses (as per study protocol). In the acceptability and preference study, OX219-005, 28 subjects were randomized and treated with at least one dose of study medication and 27 subjects received both treatments. ( and )
Table 1. Subject disposition.
Table 2. Demographic data (randomized subjects).
Pharmacokinetics
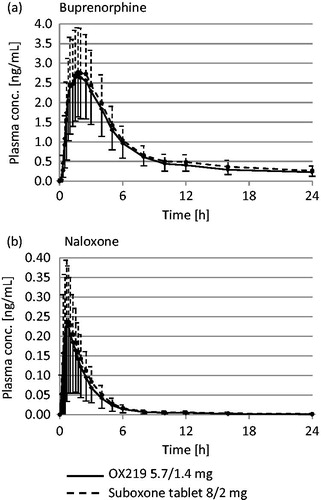
In the pharmacokinetic comparison between OX219 5.7/1.4 mg and Suboxone tablet 8/2 mg, buprenorphine met standard, pre-specified equivalence criteria for AUC0–t, AUC0–∞ and Cmax (90% CI of GMRs were within 0.8000 to 1.2500) and demonstrated a similar tmax (median of 1.75 h for both treatments). Naloxone exposure from OX219 was not higher than from Suboxone tablet (90% CI of AUC0–t, AUC0–∞ and Cmax GMRs were <1.2500) and tmax was similar (median of 0.83 h for both treatments). ()
Sublingual dissolve time
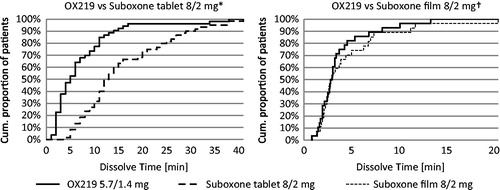
The observer confirmed sublingual dissolve time of OX219 was significantly shorter than that of Suboxone tablet (median of 5 min versus 12.5 min; median OX219 to Suboxone difference: −8 min; 95% CI: −10 to −5 min; p < 0.0001). The subjectively reported sublingual dissolve time of OX219 was similar to that of Suboxone film (median of 2.88 min for both formulations; median OX219 to Suboxone difference: −0.25 min; 95% CI: −1.13 to 0.51 min; p = 0.232). (, )
Table 3. Sublingual dissolve time, acceptability and preference results.
Formulation acceptability and preference
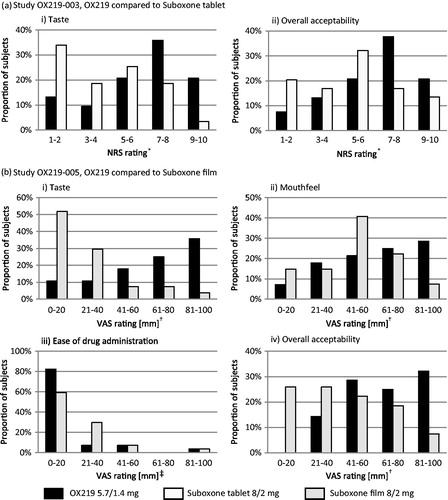
Compared to Suboxone tablet, OX219 received significantly higher mean NRS ratings for taste (6.4 versus 4.2, p < 0.0001) and overall acceptability (6.5 versus 5.2, p = 0.0003). OX219 was preferred over Suboxone tablet by 77.4% of subjects.
Compared to Suboxone film, OX219 received significantly higher mean VAS ratings for taste (62 mm versus 26 mm, p < 0.0001) and a lower proportion of subjects experienced unpleasant aftertaste from the OX219 formulation (42.9% versus 92.6%). The taste of OX219 was preferred by 96.3% of subjects. The mean VAS rating for mouthfeel was also significantly higher than for Suboxone film (59 mm versus 48 mm, p = 0.0384) and 81.5% subjects preferred the mouthfeel of OX219. There was no significant difference in “ease of drug administration” VAS ratings (16 mm versus 20 mm, p = 0.0873); however, the OX219 formulation was preferred by 88.9% of subjects considering ease of drug administration. OX219 received a higher mean overall formulation acceptability VAS rating (65 mm versus 40 mm, p = 0.0002) and overall, 88.9% preferred the OX219 formulation over Suboxone film. (, )
Discussion
The more efficient sublingual absorption from OX219 enabled a 30% lower buprenorphine dose compared to the Suboxone tablet, while maintaining equivalent systemic exposure. One benefit of the lower dose is that less buprenorphine is available for parenteral abuse. Furthermore, the lower dose leads to a lower local exposure of the gut, which could be beneficial for gastro-intestinal buprenorphine side effects such as constipation.
Both Suboxone formulations received low ratings for taste, which is well aligned with data previously reported by Lintzeris et al., where Suboxone tablet and film received mean VAS taste ratings of 32.1 and 34.4 mm respectivelyCitation6. More than 50% and 80% of subjects rated the taste on the unpleasant side of the rating scales for the Suboxone tablet (<5 on the NRS) and Suboxone film (≤40 mm), respectively (). The improved taste of OX219 may be attributed at least in part to the use of sucralose and menthol for taste masking of the bitter active ingredients in the OX219 formulation. For example, sucralose has a higher sweetness potency and a lower degree of bitter aftertaste than acesulfame K present in the Suboxone formulationsCitation7. Furthermore, menthol is volatile, quickly filling the oral cavity creating a slight cooling/numbing effect. It has previously been described as an efficient masker for the bitter taste of valdecoxibCitation8 and diclofenacCitation9 in orally dissolving film formulations.
Although the volatile nature of menthol may contribute to an efficient taste masking, it also adds challenges to a pharmaceutical formulation. As it has the potential to sublime at room temperature and atmospheric pressure, menthol is known to present a risk for the creation of “whiskers” (rod-like, re-crystallized menthol) in the primary packaging during storageCitation10. In the OX219 formulation the menthol was stabilized by co-processing with silicon dioxide and the formulation presented excellent stability.
With only a small volume of sublingual saliva available for tablet disintegration, a small tablet size is an important attribute for a sublingual formulation to possess, and may provide for a short sublingual dissolve time. Furthermore, although super disintegrants have been shown to be beneficial for shortening dissolve timesCitation11, a too high amount of water absorbing excipients could potentially inhibit disintegration by binding up water. The OX219 formulation had a tablet weight of approximately 1/4 of the Suboxone tablet, consisted predominantly of freely soluble excipients and included a small amount of the super disintegrant croscarmellose sodium, which might, at least partly, explain the reduction in sublingual dissolve time compared to Suboxone tablet.
A range of salivary flow rate between 0.1 and 2 mL/min has been demonstrated in healthy volunteersCitation12. This variability is consistent with, and could likely be a reason for the large range in dissolve times seen for all treatments in both clinical studies (, ). The apparent difference in sublingual dissolve time between studies may at least partly be due to the differences in study design. The primary focus of the OX219-003 study was to evaluate the pharmacokinetics of the two formulations, and in that context, it was logical to focus on complete tablet dissolution to minimize variability in absorption. However, this required an extra step before the time was recorded potentially contributing to longer dissolve times. The focus of the OX219-005 study, which did not include pharmacokinetic measurements, was fully on the perception of the two formulations, and in that context it was more logical to measure the subjectively perceived dissolve times, which could be recorded directly when the subject perceived that the tablet was dissolved.
In addition to taste and dissolve time, a film and a tablet formulation are likely to differ in how they feel in the mouth (e.g. texture, adhesive properties, etc.). While the sublingual tablet disintegrates directly into small, comparably dry units before dissolving, the film first hydrates to a gel. To capture this aspect of the formulation, assessment of “mouthfeel” acceptability and preference was included in the OX219-005 study and the tablet formulation was perceived as more convenient than the film.
Blinding in a traditional sense was not possible in these studies since the formulations were different in appearance and taste, and evaluation of these different properties was part of the study objectives. To minimize potential influence on subjective assessments, questions were phrased in a neutral way, not mentioning product names. As the study was conducted in naltrexone blocked healthy volunteers, screened for previous drug abuse, study subjects were unlikely to have previous experience of the medication, and compared to patient studies the risk of bias due to preconceptions is likely to be low.
Conclusions
A sublingual tablet formulation with an improved acceptability not only compared to the original tablet formulation but also compared to a more recently developed sublingual film, has been successfully developed. This research highlights the importance of taste and dissolve time for acceptability of sublingual formulations and demonstrates the potential of the directly compressed sublingual tablet as an efficient and highly acceptable option for oral transmucosal drug delivery. Improved acceptability might be important for treatment compliance and adherence and future clinical studies in patients are needed to address this topic.
Declaration of interest
The authors are employees of Orexo AB and the research was fully funded by Orexo AB. The authors declare no conflicts of interest.
References
- Zhang H, Zhang J, Streisand JB. Oral mucosal drug delivery: clinical pharmacokinetics and therapeutic applications. Clin Pharmacokinet 2002;41:661–80
- Shahiwala A. Formulation approaches in enhancement of patient compliance to oral drug therapy. Expert Opin Drug Deliv 2011;8:1521–9
- Suboxone sublingual tablet, Prescribing Information. Reckitt-Benckiser Pharmaceuticals Inc.; 2012
- Suboxone sublingual film, Prescribing Information. Reckitt-Benckiser Pharmaceuticals Inc.; 2012
- Suboxone commercial webpage. Reckitt-Benckiser Inc.; 2013 Available from: http://www.suboxone.com/; http://www.suboxone.com/hcp/about_suboxone/treatment_evolution.aspx [updated 2013; last accessed 28 Jun 2013]
- Lintzeris N, Leung SY, Dunlop AJ, et al. A randomised controlled trial of sublingual buprenorphine-naloxone film versus tablets in the management of opioid dependence. Drug Alcohol Depend 2013;131:119–26
- Wiet SG, Beyts PK. Sensory characteristics of sucralose and other high intensity sweeteners. J Food Sci 1992;57:1014–19
- Sharma R, Soniwala M, Parikh R, Gohel M. Development of taste masked film of valdecoxib for oral use. Indian J Pharm Sci 2007;69:320–3
- Cilurzo F, Cupone IE, Minghetti P, et al. Diclofenac fast-dissolving film: suppression of bitterness by a taste-sensing system. Drug Dev Ind Pharm 2011;37:252–9
- Soottitantawat A, Takayama K, Okamura K, et al. Microencapsulation of l-menthol by spray drying and its release characteristics. Innov Food Sci Emerg Technol 2005;6:163–70
- Battu SK, Repka MA, Majumdar S, Madhusudan RY. Formulation and evaluation of rapidly disintegrating fenoverine tablets: effect of superdisintegrants. Drug Dev Ind Pharm 2007;33:1225–32
- Fenoll-Palomares C, Munoz Montagud JV, et al. Unstimulated salivary flow rate, pH and buffer capacity of saliva in healthy volunteers. Rev Esp Enferm Dig 2004;96:773–83