Abstract
The tumor microenvironment plays an important role in the progression of cancer. This study focused on carcinoma-associated fibroblasts (CAFs) and stromal–epithelial interaction between CAFs and epithelial ovarian carcinoma (EOC) cells. We isolated and established primary cultures of CAFs and co-cultured CAFs and EOC cells in vitro. The co-culture conditioned medium (CC-CM) was harvested and its influence on EOC cells was examined. Cytokine, chemokine, and growth factor levels were screened using a biotin label-based human antibody array system. We found that the stromal–epithelial crosstalk provided a suitable microenvironment for the progression of ovarian cancer cells in vitro.
INTRODUCTION
Epithelial ovarian cancer (EOC) accounts for more than 90% of all ovarian malignancies and is the second most frequent invasive malignancy of the female genital tract after cancer of the uterine corpus (Citation1). The high mortality rate of ovarian carcinoma stems primarily from the lack of effective screening and early detection strategies. There has been little change to the 5-year cumulative survival rate for EOC since platinum-based treatment was universally introduced more than 30 years ago (Citation2). Therefore, there is an urgent need for an in-depth understanding of the mechanisms involved in the progression of ovarian cancer in order to develop new diagnostic or therapeutic agents.
The tumor microenvironment has been suggested to play an active role in cancer initiation and progression (Citation3, 4). Clinical and experimental evidence indicates that tumor development is intimately related to the complex tumor microenvironment (Citation5, 6). Neoplastic epithelia engage in reciprocal molecular crosstalk with surrounding cells, including pro-inflammatory cells, vascular cells, and fibroblast cells (Citation7–9), resulting in the production of growth factors, proteases, chemokines, and cytokines. These cytokines promote angiogenesis and allow malignant tumor cells to escape host immunity and resist apoptosis (Citation10–12). Carcinoma-associated fibroblasts (CAFs), a particular type of stromal fibroblast that exists within carcinomas, have been linked to multifaceted tumor-promoting effects, including cytokine and protease secretion, regulation of tumor motility and metabolism, reorganization of the tumor extracellular matrix (ECM), and preparation of the metastatic niche (Citation13–17). Therefore, identification of the specific factors derived from the stromal–epithelial biochemical interaction needs to be a primary focus of research.
Recently, two studies have provided evidence that CAFs in EOC can contribute to tumor progression, disease stage, lymph node metastases, and omentum metastases and may be a poor prognostic indicator (Citation18, 19). However, detailed characterization of the tumor microenvironment and specific factors secreted by the synergistic interaction between CAFs and EOC cells have not been carried out. Thus, a thorough understanding of the biochemical interactions between stromal cells and epithelial cells is critical.
To better characterize the role of this stromal cell and the biochemical interactions in tumorigenesis, we isolated CAFs from resected specimens, co-cultured CAFs, and EOC cells in vitro, and examined their influence on ovarian cancer cells in vitro. We found that CAFs and the tumor microenvironment could supply contextual signals that serve to promote cancer progression in vitro.
MATERIALS AND METHODS
Cell culture
OVCAR-3 and SKOV-3 ovarian tumor cells were obtained from the Shanghai Institute of Biochemistry and Cell Biology (Shanghai, China). The cells were cultured in Dulbecco's modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS), 2 mM glutamine, and 1% penicillin-streptomycin solution (HyClone, Rockford, IL, USA) at 37°C in a humidified incubator containing 5% CO2.
CAFs were isolated according to a modified version of the protocol described by Zhang et al. (Citation20). Twenty-five tumor tissue specimens were obtained from residual ovarian adenocarcinoma specimens collected from adult patients undergoing primary surgical resection at the First Affiliated Hospital of Nanjing Medical University, Nanjing, China. Each patient provided informed consent to participate in the study and the Committee for Ethical Review of Research at Nanjing Medical University approved all samples used.
Briefly, tumor tissues were minced in sterile phosphate-buffered saline solution, followed by collagenase digestion (0.1% collagenase type IV; Sigma-Aldrich, St. Louis, MO, USA) at 37°C for 30 min. The suspension was filtered through a 20-μm stainless steel wire mesh to collect a single-cell suspension. The filtrate was centrifuged at 1,500 rpm for 5 min and washed twice with DMEM before being plated on 6-cm tissue culture dishes in 5 mL DMEM supplemented with 10% FBS (DMEM/10% FBS). After culturing for 30 min at 37°C, nonadherent cells (mainly tumor cells) were removed to obtain pure fibroblasts; the adhesion time required for fibroblasts is much shorter (∼20–30 min) than that for tumor cells (usually >1 hr). The adherent fibroblasts were subcultured for further study. When the fibroblasts grew to confluence, cells were trypsinized and passaged 1:3. All fibroblasts used for this study were between passages 3 and 5.
Cell purity was determined by immunohistochemical staining for various antibodies as well as by morphological assessment (spindle-shaped cells with cytoplasmic extensions). Antibodies to the following were used: myofibroblast marker α-smooth muscle actin (α-SMA; mouse monoclonal, 1:100; LabVision, Kalamazoo, MI, USA), mesenchymal marker vimentin (mouse monoclonal, 1:10; LabVision), fibroblast-specific protein-1 (FSP1)/S100A4 (rabbit polyclonal, 1:50; Abcam, Cambridge, MA, USA), endothelial marker CD31 (mouse monoclonal, 1:100; BD Biosciences, San Jose, CA, USA), and ovarian epithelial marker cytokeratin 7 (mouse monoclonal, 1:100; LabVision). Fibroblasts were verified by cytokeratin 7- and CD31-negative and α-SMA–, vimentin-, and FSP1/S100A4-positive status. All experiments were performed using cells in the exponential growth phase.
Preparation of co-culture conditioned medium
To understand the stromal–epithelial crosstalk better, in vitro co-cultures of fibroblasts (CAFs) and EOC cells (OVCAR-3 or SKOV-3) were established. CAFs mixed with EOC cells in a 1:1 ratio (1 × 105 cells) were seeded in 20-cm2 dishes in DMEM/10% FBS. When cells reached 70% to 80% confluence, the medium was changed to serum-free DMEM and cells were cultured for another 48 hr. The co-culture conditioned medium (CC-CM) was collected, centrifuged at 1,000 ×g for 10 min, and the supernatant was concentrated with Centricon YM-3 filters (Millipore, Bedford, MA, USA). The protein content of the CC-CM was determined using the Bradford assay (Bio-Rad Laboratories, Hercules, CA, USA). All aliquots were stored at −80°C until used.
Proliferation assays
To examine the effect of CC-CM on the growth of EOC cells, OVCAR-3 cells were seeded at 3,000 cells per well in 96-well plates and SKOV-3 cells were seeded at 1,000 cells per well in 96-well plates; both were cultured in DMEM/10% FBS. The medium was changed to serum-free DMEM for overnight incubation. Concentrated CC-CM (1 μg/μL) was added to the experimental wells and serum-free DMEM was added to the control wells. Cell growth was analyzed every 24 hr; 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reagent (Sigma-Aldrich) was added 1 hr before obtaining the spectrophotometric reading according to the manufacturer's directions. Independent experiments were performed in triplicate.
Migration and invasion assays
To examine the effect of CC-CM on EOC migration, Transwell inserts (Corning, Lowell, MA, USA) were used. Briefly, 1 × 105 OVCAR-3 or SKOV-3 cells in 500 μL serum-free medium were added to the top 8-μm pore chamber. Medium containing 10% FBS or concentrated CC-CM (1 μg/μL) was added into the bottom chamber. Serum-free medium was added to the bottom chamber of the control wells. The number of cells that migrated through the pores to the undersurface within 48 hr indicated cell motility.
The effect of CC-CM on tumor cell invasiveness was investigated in a similar fashion using BioCoat Matrigel-coated invasion chambers (BD Biosciences). The cells were allowed to invade the Matrigel for 48 hr at 37°C in a 5% CO2 atmosphere.
After 48 hr, cells that did not migrate or invade through the pores were removed by scraping the membrane with a cotton swab. Cells that had invaded through the pores and migrated to the underside of the membrane were fixed in 95% ethanol and stained with hematoxylin and eosin. Five random regions on the inserts and membranes were then viewed with a microscope by two independent observers. Independent experiments were performed in triplicate.
Serum deprivation–induced apoptosis assays
An important feature of tumor cells is their ability to regulate their survival. We therefore tested whether CC-CM could rescue EOC cells from serum deprivation-induced apoptosis. OVCAR-3 or SKOV-3 cells were seeded in eight-well chamber slides (1 × 105 cells per well) in DMEM/10% FBS. After overnight incubation, cells were washed and the medium was changed to DMEM/10% FBS with or without concentrated CC-CM (1 μg/μL) for 24 hr, then cells were washed and incubated for 48 hr in serum-free DMEM. After 48 hr, apoptotic cells were identified using the conjugated human annexin V and propidium iodide double staining method (BD Biosciences). In total, 10,000 cells were analyzed by flow cytometry using CellQuest software (Becton Dickinson, Mountain View, CA, USA). Independent experiments were performed in triplicate.
Protein detection assays
The aforementioned observations indicate that CAFs supply locally acting paracrine cues that induce EOC cells to progress in vitro. To understand this crosstalk better, the CM was harvested from CAFs, OVCAR-3 cells, or CAF+OVCAR-3 cell co-cultures. The levels of various factors in the cell-free culture supernatants were measured with the RayBio® Biotin Label-based Human Antibody Array system (RayBiotech, Kangchen, China) and were normalized to the levels observed in the medium of OVCAR-3 cells cultured alone. Data are expressed as fold induction of triplicates.
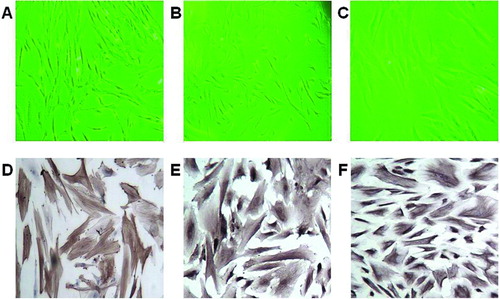
Figure 1 Typical morphology and characterization of CAFs harvested from human EOC tissues. Typical CAF morphology: (A) spindle-shaped, (B) intermediate, and (C) flattened. Positive immunohistochemical staining for (D) α-SMA, (E) vimentin, and (F) FSP1, original magnification ×200.
According to the manufacturer's protocol, there are 507 proteins in the RayBio® system (Supplementary Table 1, available online), including cytokines, chemokines, growth and differentiation factors, angiogenic factors, adipokines, adhesion molecules, and matrix metalloproteinases (MMPs), as well as binding proteins, inhibitors, and soluble receptors.
Briefly, the protein chips were blocked and incubated with 400 μL biotin-labeled samples at room temperature for 2 hr, and then washed to remove unbound components. Streptavidin-conjugated fluorescent dye, HiLyte Plus™ 555 (Cy3 equivalent; AnaSpec, Fremont, CA, USA), was incubated with the chips at room temperature for 1 hr. The excess streptavidin was removed and the signals were scanned by a GenePix™ 4000B laser scanner (Axon Instruments, Sunnyvale, CA, USA). The data were normalized based on the positive control signals, and then compared to a common reference array. After subtraction of local background signals, the fluorescent signal intensity for each spot was multiplied by a normalization factor. The results were expressed as relative densities compared to the positive control in each membrane, and the relative ratios were calculated as fold induction. A series of diluted antistreptavidin and biotin-conjugated IgG were used as positive controls, while capture antibody diluent was used as the negative control.
Statistical analyses
All statistical tests were performed using SPSS Statistical Software, Release 16 (Chicago, IL, USA). Graphs were generated by GraphPad Prism 4.0 software (San Diego, CA, USA). Comparisons were made using two-tailed Student's t-test and significant difference was defined as p < .05. Data are expressed as the mean ± standard deviation of at least three independent experiments.
RESULTS
Successful isolation of CAFs
Twenty-two CAFs were successfully isolated and cultured. The CAFs typically displayed a spindle-like, intermediate, or flattened shape (, , and ) and their identity was confirmed by positive immunohistochemical staining for α-SMA, vimentin, FSP1 (, , and ) and negative immunohistochemical staining for cytokeratin 7 and CD31 (data not shown). The CAFs could be maintained for over 10 passages.
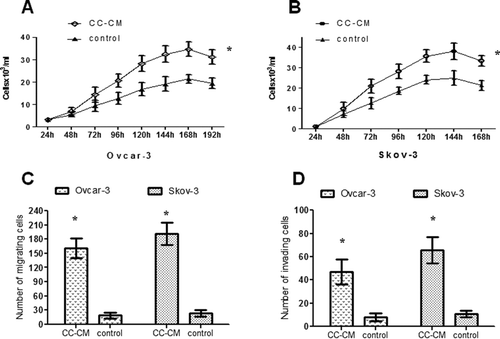
Figure 2 Effect of CC-CM on the proliferation, migration, and invasion of EOC cell lines. A: Proliferation of OVCAR-3 cells cultured in CC-CM or control medium. B: Proliferation of SKOV-3 cells cultured in CC-CM or control medium. C: Migration of OVCAR-3 and SKOV-3 cells cultured in CC-CM or control medium. D: Invasion of OVCAR-3 and SKOV-3 cells cultured in CC-CM or control medium. *p < .05.
CC-CM stimulated EOC cell proliferation, migration, and invasion
Comparing CC-CM to control medium, the cell proliferation assays showed that CC-CM significantly increased the growth rate of OVCAR-3 and SKOV-3 cells (p < .05, and ). Interestingly, the SKOV-3 cells grew so fast that they could be tested at a lower concentration (1,000 cells per well); otherwise, it was difficult to observe the obvious difference between the CC-CM group and the control group.
Next, we tested the effect of CC-CM on the mobility of OVCAR-3 and SKOV-3 cells using Transwell migration assays. As shown in , the number of OVCAR-3 cells cultured in CC-CM that migrated to the undersurface of the membrane was significantly greater than that of cells cultured in the control medium (191.3 ± 40.2 versus 22.7 ± 12.0, p < .05). A similar effect was observed for the SKOV-3 cells. The number of SKOV-3 cells cultured in CC-CM that migrated to the undersurface of the membrane was also significantly greater than that of cells cultured in the control medium (190.7 ± 36.1 versus 16.3 ± 7.0, p < .05; ).
Subsequently, we measured the capacity of OVCAR-3 and SKOV-3 cells to invade through the Matrigel, an artificial ECM. OVCAR-3 cells incubated with CC-CM displayed increased invasive ability compared to OVCAR-3 cells incubated with control medium (65.0 ± 19.9 versus 8.3 ± 3.2, p < .05; ). SKOV-3 cells incubated with CC-CM also displayed increased invasive ability compared to the control group (47.0 ± 18.5 versus 5.0 ± 3.2, p < .05; ).
CC-CM inhibited serum deprivation–induced apoptosis
In this assay, culture in serum-free conditions strongly induced the apoptosis of EOC cells. However, the percentage of apoptotic OVCAR-3 cells was reduced by 23.9% when cells were cultured in CC-CM as compared to control medium (26.2% ± 14.7% versus 2.3% ± 1.6%, p < .05; ), and that of SKOV-3 cells was reduced by 25.7% when cells were cultured in CC-CM as compared to control medium (27.9% ± 17.4% versus 2.2% ± 1.7%, p < .05; ).
Figure 3 Effect of CC-CM on serum deprivation–induced apoptosis of EOC cell lines. A and B: Flow cytometric analyses and quantification of serum deprivation–induced apoptosis in OVCAR-3 cells cultured in CC-CM or control medium. C and D: Flow cytometric analyses and quantification of serum deprivation–induced apoptosis in SKOV-3 cells cultured in CC-CM or control medium. *p < .05.
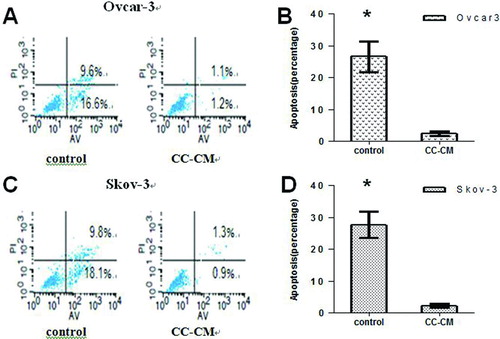
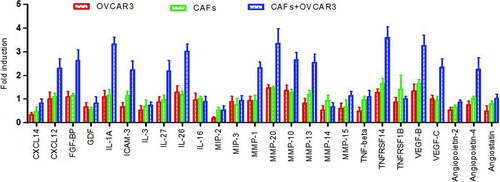
Figure 4 The interaction of CAFs with OVCAR-3 cells leads to increased levels of various cytokines (representative results, not of all 507 cytokines). The data are expressed as the fold induction ± s.d. of triplicates. There were significant expression of some cytokines, while the expression of large number other cytokines remained unchanged. Red column, OVCAR-3 cells only; green column, CAFs only; blue column, CAF+OVCAR-3 cells.
Stromal–epithelial crosstalk caused an increase in the levels of various cytokines
In total, nine diagrams were generated: three of CC-CM from CAFs and OVCAR-3 cell co-culture, three of CM from CAFs, and three of CM from OVCAR-3 cells. Representative diagrams depicting a typical image are presented in Supplementary Figure 1 (available online). The overall sensitivity, specificity, and variability of the arrays were tested according to the protocol of Huang et al. (Citation21). The coefficient of variation (defined as the standard deviation divided by the mean) was generally less than 20%, suggesting reasonably good reliability of the system.
The levels of various factors in the cell-free culture supernatants were measured with the RayBio® Biotin Label-based Human Antibody Array system and normalized to the levels observed in the CM of OVCAR-3 cells cultured alone. Some factors were upregulated, others remained unchanged, and the levels of some factors could not be detected. In some cases, the resulting levels of certain released factors reflected the additive contributions of the two cell types when cultured individually. Notably, the levels of some cytokines reflected a synergistic interaction between the CAFs and OVCAR-3, as these accumulated to levels more than 2-fold higher than those produced by individual CAF or OVCAR-3 cultures. We defined an arbitrary cutoff signal ratio of ≥2.0- or ≤2.0-fold changes as significant expression. That is, we compared the CC-CM from CAF+OVCAR-3 cells to the CM from CAFs only, and compared the CC-CM from CAF+OVCAR-3 cells to the CM from OVCAR-3 cells only, and only ratios of ≥2.0- or ≤2.0-fold changes were considered significant expression. depicts partial representative results, not of all 507 cytokines. shows that there were significant expression of some cytokines, such as IL-1A and MMP-20, while the expression of large number other cytokines remained unchanged. Of the 507 cytokines, there was significant expression of 57 in the CC-CM from CAF+OVCAR-3 cells, and these significantly upregulated cytokines are listed in .
Of these 57 cytokines, 44 were upregulated and 13 were downregulated. The first group of cytokines included tumor necrosis factor (TNF) superfamily members, insulin-like growth factor (IGF)-binding protein, hepatocyte growth factor (HGF), and fibroblast growth factor (FGF). These cytokines can promote the proliferation of cancer stem cells and are associated with cell growth. The second group of cytokines included MMPs, intercellular adhesion molecule-3, and connective tissue growth factor and is associated with ECM remodeling and decreased cell adhesion that contribute to tumor cell migration and invasion. The third group of cytokines comprised some interleukins (ILs) and chemokine (C-X-C motif) ligands that are responsible for immune escape by tumors and can affect inflammation in cancer. The fourth group of cytokines included vascular endothelial growth factor and angiopoietins. These cytokines are associated with cancer cell proliferation and tumor angiogenesis. The fifth group comprised downregulated cytokines. These cytokines are involved in cell proliferation, organ development, signal transduction, immunoregulatory and inflammatory processes, and angiogenesis and blood vessel stability.
DISCUSSION
The importance of the local tumor microenvironment in tumor progression has been recognized for many years and highlighted in several reviews (Citation3, Citation22, Citation23). Two recent studies on ovarian cancer showed that tumor CAFs could induce cancer cell invasion, migration, and progression (Citation18, 19). However, in contrast, little is known about the possible contribution of stromal–epithelial interaction in ovarian cancer. Using ovarian CAFs, we found that CC-CM from CAF and EOC cell co-cultures stimulated ovarian tumor cell proliferation, migration, and invasion. Moreover, the CC-CM inhibited the apoptosis of tumor cell induced by serum deprivation. These observations indicate that soluble factors are produced by crosstalk between CAFs and EOC cells that stimulate the proliferation and survival of ovarian cancer cells. We also screened some significantly expressed cytokines in the CC-CM produced by biochemical interactions between CAFs and EOC cells. These cytokines contributed to cell proliferation, ECM remodeling, inflammation, and angiogenesis. Taken together, these studies indicate that the stromal–epithelial interaction of ovarian cancer plays an important role in the aggressiveness of this disease.
The tumor microenvironment is composed of both cellular (fibroblasts, endothelial, and immune cells) and noncellular components (proteins, proteases, and cytokines). Proliferation is the first step in tumor cell invasion and metastasis to other organs. In the present study, high levels of a variety of growth-promoting factors were identified in the CC-CM from CAF and EOC cell co-cultures, including transforming growth factor (TGF)-β, HGF, and IGF. TGF-β is secreted by a number of malignant tumors, including those in colon cancer (Citation24) and breast cancer (Citation25), and can induce the differentiation of fibroblasts into CAFs. HGF is secreted by many types of cancer cells and stromal cells, playing an important role in the tumor stromal microenvironment (Citation26). HGF can stimulate the migration of SKOV-3 cells (Citation19). In this study, we demonstrated that synergistic interaction in the stromal–epithelial microenvironment led to the expression of higher levels of HGF and TGF-β as well as epidermal growth factor (EGF) and basic FGF. Each of these factors may be potentially responsible for cancer cell proliferation and metastasis.
Table 1 Significantly Expressed* Cytokines in CAF and OVCAR-3 Cell CC-CM as Compared to OVCAR-3 cell CM
Remodeling of the ECM by MMPs is a crucial step for cancer progression as well as formation of the cancer microenvironment (Citation27). Crosstalk between cancer cells and CAFs may direct both cell types to modify the adjacent ECM and basement membrane (Citation3, Citation15). It is widely recognized that breakdown of the basement membrane is the first step required for cancer cell intravasation into the circulation. MMP-13 secreted by CAFs promotes tumor angiogenesis and can lead to increased cancer cell invasion (Citation28). MMP-11 expression has a significant relationship with the inflammatory molecular profile of breast carcinomas and distant metastasis (Citation29). Recently, it was shown that overexpression of MMP-20 may be useful as a prognostic factor for lymph node metastases in laryngeal squamous cell carcinoma (Citation30). These cytokines may play an important role in promoting tumor cell invasion and metastasis.
Inflammation has recently been proposed as the seventh hallmark of cancer (Citation31). Pro-inflammatory cytokines secreted by CAFs and cancer cells attract excessive numbers of immune cells to the tumor, allowing cancer cells to be exposed to growth factors while avoiding immune surveillance (Citation32). In this study, a set of pro-inflammatory mediators were upregulated and associated with immune response and tumor-promoting behavior. These findings suggest that an inflammatory microenvironment plays a crucial role in malignant cell progression.
Angiogenesis is required for a tumor to grow, and the microenvironment plays an important role in regulating the angiogenic switch (Citation33). In this study, pro-angiogenic factors, including vascular endothelial growth factor β, angiopoietin-4, and angiopoietin-like 4 were identified. These factors are essential for tumor growth and metastasis and essential for controlling tumor-associated angiogenesis (Citation34). Angiopoietin 4 is a protein that plays important roles in vascular development and angiogenesis (Citation35), while angiopoietin-like 4 has been shown to prevent the metastatic process by inhibiting vascular activity and tumor cell motility and invasiveness (Citation36).
The upregulated promoter cytokines exert promoting effects in crosstalk interaction. The downregulated suppressor cytokines may also exert promoting effects, and occasionally require feedback effects to maintain microenvironment homeostasis. Thus, there is another group of downregulated cytokines. These cytokines are involved in cell proliferation (FGF-8, EGF), organ development (FGF-20, FGF-4), signal transduction (TNFSF10), immunoregulatory and inflammatory processes (CCL18, CXCL6, RANTES, IL-13, ENA-78), and angiogenesis and blood vessel stability (TIE-1, angiopoietin-like 4). Some; such as TIE-1, IL-10, IL-13; and angiopoietin-like 4, are tumor suppressor cytokines. TIE-1 is a member of the tyrosine protein kinase family and plays a critical role in angiogenesis and blood vessel stability by inhibiting angiopoietin 1 signaling through the endothelial receptor tyrosine kinase TIE-2 (Citation37). IL-13 downregulates macrophage activity and inhibits the production of pro-inflammatory cytokines and chemokines, some of which play an important role in cell proliferation, organ development, signal transduction, and immune regulation (Citation38). The stromal–epithelial crosstalk downregulation of some promoter cytokines may aid in preventing hyperreaction and in maintaining homeostatic control. For example, TNFSF10 belongs to the TNF ligand family and preferentially induces apoptosis in transformed and tumor cells. The binding of this protein to its receptors has been shown to trigger the activation of MAPK8/JNK, caspase-8, and caspase-3. Downregulation of this cytokine may prevent TNFα-mediated apoptosis in some primary tumors (Citation39).
In fact, the soluble factors produced in vitro by stromal cells do not always stimulate proliferation; some may inhibit cancer cell proliferation and induce apoptosis (Citation40), suggesting that these fibroblast-secreted factors interfere in the cell growth and death of neoplastic cells.
One limitation in this study is that we evaluated CAFs as the sole stromal element interacting with tumor cells. In reality, many other cell types in the stroma, such as endothelial cells, immune cells, adipocytes, and pericytes are known to interact with tumor cells to mediate malignant behavior. Another limitation is that we did not verify the observation in vivo. Thus, a more physiologically relevant design would be one that includes all stromal cell types along with tumor cells and that is performed in vivo.
In conclusion, our data provide evidence that stromal fibroblasts and stromal tumor cells interact and promote tumor progression in ovarian cancer. These findings improved our understanding of the role and effects of the synergistic interactions between the tumor and stromal cells in the tumor microenvironment during ovarian cancer progression. With this information, targeting the stroma in ovarian cancer may not only be effective in suppressing tumor development, but may also prevent tumor metastasis. Further investigation of the role of these factors may be a high priority for future work.
DECLARATION OF INTEREST
All author declared no any conflict of interest. The authors alone are responsible for the content and writing of the paper.
This study was supported in part by the Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (JX10231081).
ACKNOWLEDGMENTS
We would like to thank Ms Tingting Liao for her assistance in gathering the pictures and the KangChen Bio-Tech Company (Shanghai, China) for assistance with microarray data analysis.
Supplementary Table 1: Cytokines in the RayBio® Biotin Label-based Human Antibody Array system (507).
Download JPEG Image (61.8 KB)Supplementary Material
Download MS Word (103 KB)REFERENCES
- Siegel R, Naishadham D, Jemal A. Cancer statistics 2013. CA Cancer J Clin 2013;63:11–30.
- Jelovac D, Armstrong DK. Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J Clin 2011;61: 183–203.
- Allen M, Louise Jones J. Jekyll and Hyde: the role of the microenvironment on the progression of cancer. J Pathol 2011;223: 162–176.
- Balkwill FR, Capasso M, Hagemann T. The tumor microenvironment at a glance. J Cell Sci 2012;125:5591–5596.
- McMillin DW, Negri JM, Mitsiades CS. The role of tumour-stromal interactions in modifying drug response: challenges and opportunities. Nat Rev Drug Discov 2013;12:217–228.
- Pietras K, Ostman A. Hallmarks of cancer: interactions with the tumor stroma. Exp Cell Res 2010;316:1324–1331.
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature 2008;454:436–444.
- Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med 2011;17:1359–1370.
- Polanska UM, Orimo A. Carcinoma-associated fibroblasts: Non-neoplastic tumour-promoting mesenchymal cells. J Cell Physiol 2013;228:1651–1657.
- Viola A, Sarukhan A, Bronte V, Molon B. The pros and cons of chemokines in tumor immunology. Trends Immunol 2012;33:496–504.
- Seton-Rogers S. Cytokine cues. Nat Rev Cancer 2011;11:690.
- Mukaida N, Baba T. Chemokines in tumor development and progression. Ex Cell Res 2012;318:95–102.
- Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer 2006;6:392–401.
- Hu C, Wang Z, Zhai L, Yang M, Shan L, Chai C, Liu M, Wang L. Effects of cancer-associated fibroblasts on the migration and invasion abilities of SGC-7901 gastric cancer cells. Oncol Lett 2013;5:609–612.
- Cirri P, Chiarugi P. Cancer-associated-fibroblasts and tumour cells: a diabolic liaison driving cancer progression. Cancer Metastasis Rev 2012;31:195–208.
- Liu M, Xu J, Deng H. Tangled fibroblasts in tumor-stroma interactions. Int J Cancer 2011;129:1798–1805.
- Brentnall TA, Lai LA, Coleman J, Bronner MP, Pan S, Chen R. Arousal of cancer-associated stroma: overexpression of palladin activates fibroblasts to promote tumor invasion. PLoS One 2012;7:e30219.
- Zhang Y, Tang H, Cai J, Zhang T, Guo J, Feng D, Wang Z. Ovarian cancer-associated fibroblasts contribute to epithelial ovarian carcinoma metastasis by promoting angiogenesis, lymphangiogenesis and tumor cell invasion. Cancer Lett 2011;303:47–55.
- Cai J, Tang H, Xu L, Wang X, Yang C, Ruan S, Guo J, Hu S, Wang Z. Fibroblasts in omentum activated by tumor cells promote ovarian cancer growth, adhesion and invasiveness. Carcinogenesis 2012;33:20–29.
- Zhang C, Fu L, Fu J, Hu L, Yang H, Rong TH, Li Y, Liu H, Fu SB, Zeng YX, Guan XY. Fibroblast growth factor receptor 2-positive fibroblasts provide a suitable microenvironment for tumor development and progression in esophageal carcinoma. Clin Cancer Res 2009;15:4017–4027.
- Huang R, Jiang W, Yang J, Mao YQ, Zhang Y, Yang W, Yang D, Burkholder B, Huang RF, Huang RP. A biotin label-based antibody array for high-content profiling of protein expression. Cancer Genomics Proteomics 2010;7:129–141.
- Swartz MA, Iida N, Roberts EW, Sangaletti S, Wong MH, Yull FE, Coussens LM, DeClerck YA. Tumor microenvironment complexity: emerging roles in cancer therapy. Cancer Res 2012;72:2473–2480
- Finley SD, Popel AS. Effect of Tumor Microenvironment on Tumor VEGF During Anti-VEGF Treatment: Systems Biology Predictions. J Natl Cancer Inst 2013;105:802–811.
- Lampropoulos P, Zizi-Sermpetzoglou A, Rizos S, Kostakis A, Nikiteas N, Papavassiliou AG. TGF-beta signalling in colon carcinogenesis. Cancer Lett 2012;314:1–7.
- Taylor MA, Lee YH, Schiemann WP. Role of TGF-β and the tumor microenvironment during mammary tumorigenesis. Gene Expr 2011;15:117–132.
- Steffan JJ, Coleman DT, Cardelli JA. The HGF-met signaling axis: emerging themes and targets of inhibition. Curr Protein Pept Sci 2011;12:12–22.
- Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 2010;141: 52–67.
- Lederle W, Hartenstein B, Meides A, Kunzelmann H, Werb Z, Angel P, Mueller MM. MMP13 as a stromal mediator in controlling persistent angiogenesis in skin carcinoma. Carcinogenesis 2010;31:1175–1184.
- Eiró N, González L, González LO, Fernandez-Garcia B, Lamelas ML, Marín L, González-Reyes S, del Casar JM, Vizoso FJ. Relationship between the inflammatory molecular profile of breast carcinomas and distant metastasis development. PLoS One 2012;7:e49047.
- Liu Y, Li Y, Liu Z, Zhang L, Anniko M, Duan M. Prognostic significance of matrix metalloproteinase-20 overexpression in laryngeal squamous cell carcinoma. Acta Otolaryngol 2011;131:769–773.
- Candido J, Hagemann T. Cancer-related inflammation. J Clin Immunol 2013;33:S79–S84.
- Franks AL, Slansky JE. Multiple associations between a broad spectrum of autoimmune diseases, chronic inflammatory diseases and cancer. Anticancer Res 2012;32:1119–1136.
- Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med 2011;17:1359–1370.
- Smith NR, Baker D, James NH, Ratcliffe K, Jenkins M, Ashton SE, Sproat G, Swann R, Gray N, Ryan A, Jürgensmeier JM, Womack C: Vascular endothelial growth factor receptors VEGFR-2 and VEGFR-3 are localized primarily to the vasculature in human primary solid cancers. Clin Cancer Res 2010;16: 3548–3561.
- Brunckhorst MK, Wang H, Lu R, Yu Q. Angiopoietin-4 promotes glioblastoma progression by enhancing tumor cell viability and angiogenesis. Cancer Res 2010;70:7283–7293.
- Grootaert C, Van de Wiele T, Verstraete W, Bracke M, Vanhoecke B. Angiopoietin-like protein 4: health effects, modulating agents and structure-function relationships. Expert Rev Proteomics 2012;9:181–199.
- Singh H, Tahir TA, Alawo DO, Issa E, Brindle NP. Molecular control of angiopoietin signalling. Biochem Soc Trans 2011;39: 1592–1596.
- Deepak P, Kumar S Jr, Kishore D, Acharya A. IL-13 from Th2-type cells suppresses induction of antigen-specific Th1 immunity in a T-cell lymphoma. Int Immunol 2010;22:53–63.
- Stagg HW, Bowen KA, Sawant DA, Rodriguez M, Tharakan B, Childs EW. Tumor necrosis factor-related apoptosis-inducing ligand promotes microvascular endothelial cell hyperpermeability through phosphatidylinositol 3-kinase pathway. Am J Surg 2013;205:419–425.
- Rodrigues-Lisoni FC, Peitl P Jr, Vidotto A, Polachini GM, Maniglia JV, Carmona-Raphe J, Cunha BR, Henrique T, Souza CF, Teixeira RA, Fukuyama EE, Michaluart P Jr, de Carvalho MB, Oliani SM; Head and Neck Genome Project GENCAPO, Tajara EH, Cury PM, de Carvalho MB, Dias-Neto E, Figueiredo DL, Fukuyama EE, Góis-Filho JF, Leopoldino AM, Mamede RC, Michaluart-Junior P, Moyses RA, Nóbrega FG, Nóbrega MP, Nunes FD, Ojopi EF, Serafini LN, Severino P, Silva AM, Silva WA Jr, Silveira NJ, Souza SC, Tajara EH, Wünsch-Filho V, Amar A, Bandeira CM, Braconi MA, Brandão LG, Brandão RM, Canto AL, Cerione M, Cicco R, Chagas MJ, Chedid H, Costa A, Cunha BR, Curioni OA, Fortes CS, Franzi SA, Frizzera AP, Gazito D, Guimarães PE, Kaneto CM, López RV, Macarenco R, Magalhães MR, Meneses C, Mercante AM, Pinheiro DG, Polachini GM, Rapoport A, Rodini CO, Rodrigues-Lisoni FC, Rodrigues RV, Rossi L, Santos AR, Santos M, Settani F, Silva FA, Silva IT, Souza TB, Stabenow E, Takamori JT, Valentim PJ, Vidotto A, Xavier FC, Yamagushi F, Cominato ML, Correa PM, Mendes GS, Paiva R, Ramos O, Silva C, Silva MJ, Tarl´ MV. Genomics and proteomics approaches to the study of cancer-stroma interactions. BMC Med Genomics 2010;3:14.