Abstract
Clinical studies have documented morning-evening, administration-time differences of several different classes of hypertension medications in blood pressure (BP)-lowering efficacy, duration of action, safety profile, and/or effects on the circadian BP pattern. In spite of these published findings, most hypertensive subjects, including those under combination therapy, are instructed by their physicians and pharmacists to ingest all of their BP-lowering medications in the morning. The potential differential reduction of cardiovascular (CVD) morbidity and mortality risk by a bedtime versus upon-awakening treatment schedule has never been evaluated prospectively. The prospective MAPEC study was specifically designed to test the hypothesis that bedtime chronotherapy with ≥1 hypertension medications exerts better BP control and CVD risk reduction than conventional therapy, i.e., all medications ingested in the morning. A total of 2156 hypertensive subjects, 1044 men/1112 women, 55.6 ± 13.6 (mean ± SD) yrs of age, were randomized to ingest all their prescribed hypertension medications upon awakening or ≥1 of them at bedtime. At baseline, BP was measured at 20-min intervals from 07:00 to 23:00 h and at 30-min intervals at night for 48 h. Physical activity was simultaneously monitored every min by wrist actigraphy to accurately determine the beginning and end of daytime activity and nocturnal sleep. Identical assessment was scheduled annually and more frequently (quarterly) if treatment adjustment was required. Despite lack of differences in ambulatory BP between groups at baseline, subjects ingesting medication at bedtime showed at their last available evaluation significantly lower mean sleep-time BP, higher sleep-time relative BP decline, reduced prevalence of non-dipping (34% versus 62%; p < .001), and higher prevalence of controlled ambulatory BP (62% versus 53%; p < .001). After a median follow-up of 5.6 yrs, subjects ingesting ≥1 BP-lowering medications at bedtime exhibited a significantly lower relative risk of total CVD events than those ingesting all medications upon awakening (0.39 [0.29–0.51]; number of events 187 versus 68; p < .001). The difference between the treatment-time groups in the relative risk of major events (including CVD death, myocardial infarction, ischemic stroke, and hemorrhagic stroke) was also highly statistically significant (0.33 [0.19–0.55]; number of events: 55 versus 18; p < .001). The progressive decrease in asleep BP and increase in sleep-time relative BP decline towards a more normal dipping pattern, two novel therapeutic targets requiring proper patient evaluation by ambulatory BP, were best achieved with bedtime therapy, and they were the most significant predictors of event-free survival. Bedtime chronotherapy with ≥1 BP-lowering medications, compared to conventional upon-waking treatment with all medications, more effectively improved BP control, better decreased the prevalence of non-dipping, and, most importantly, significantly reduced CVD morbidity and mortality. (Author correspondence: [email protected])
INTRODUCTION
Several attributes of the cardiovascular system, including blood pressure (BP) and heart rate (HR), are characterized by predictable changes during the 24 h, for the most part in synchrony with the rest-activity cycle (Hermida et al., Citation2007a; Portaluppi & Smolensky, Citation2000). Because the body's circadian rhythms contribute to the 24-h variation in BP as well as affect the absorption, distribution, metabolism, and elimination of medications (Labrecque & Beauchamp, Citation2003; Portaluppi & Smolensky, Citation2000), it is not surprising that hypertension medications may display ingestion-time differences in their pharmacokinetics and pharmacodynamics (Hermida et al., Citation2007b; Lemmer, Citation2000). A number of published prospective trials reviewed elsewhere (Hermida et al., Citation2007b; Smolensky et al., Citation2010) have reported clinically meaningful morning-evening, treatment-time differences in BP-lowering efficacy, duration of action, safety profile, and/or effects on the circadian BP pattern for hypertension medications of six different classes. For instance, a once-daily evening, in comparison to morning, ingestion schedule of angiotensin receptor blockers (ARBs) and angiotensin-converting enzyme inhibitors (ACEIs) results in greater therapeutic effect on asleep BP, significant increase in the sleep-time relative BP decline (an index of BP dipping), and thus normalization of the circadian BP profile towards more of a dipping pattern, independent of the terminal half-life of each individual medication (Hermida & Ayala, Citation2009; Hermida et al., Citation2003, Citation2007c, Citation2009, Citation2010b; Tofé & García, Citation2009). Additionally, bedtime administration of the ARBs valsartan (Hermida et al., Citation2005) and candesartan (Kario et al., Citation2010) has been also shown to be more effective than morning administration in reducing microalbuminuria, a measure of renal function status. Moreover, bedtime ingestion of dihydropyridine calcium channel blockers (CCBs) significantly reduces their adverse effects, mainly edema (Hermida et al., Citation2007b, Citation2007d, Citation2008a). Despite these documented advantages of bedtime hypertension chronotherapy, most clinicians and pharmacists continue to advise patients, including those requiring combination therapies, to ingest their once-a-day BP-lowering medications in the morning (de la Sierra et al., Citation2009; Salles et al., Citation2008).
The impact of bedtime chronotherapy on sleep-time BP regulation, now documented for a large number of investigated hypertension medications (Hermida et al., Citation2007b; Smolensky et al., Citation2010), is of great clinical importance. This perspective is based on the growing number of studies (Boggia et al., Citation2007; Dolan et al., Citation2005; Ohkubo et al., Citation2002; Salles et al., Citation2008; Staessen et al., Citation1999; Verdecchia et al., Citation1994), all based on ambulatory BP monitoring (ABPM), that have consistently shown an association between blunted asleep BP decline and increased incidence of fatal and nonfatal cardiovascular disease (CVD) events. Moreover, independent prospective studies have also found that the asleep BP mean is a better predictor of CVD risk than the awake or 24-h BP means (Dolan et al., Citation2005; Kikuya et al., Citation2005; Staessen et al., Citation1999). However, a limitation of all of the previous studies is their reliance on a single baseline ABPM profile from each participant at the time of inclusion, without accounting for possible changes in the BP pattern or level during the many years of follow-up. Thus, the potential reduction in CVD risk associated with either increasing the sleep-time relative BP decline towards a more normal dipping pattern or with specifically reducing asleep BP, which has been found to be much more feasible by bedtime than upon-waking dosing of conventional hypertension medications (Hermida et al., Citation2007b), is still a matter of debate.
Despite all the impressive collective findings relating to the influence of the time of hypertension treatment on BP control (Hermida et al., Citation2007b; Smolensky et al., Citation2010), the potential differential effect of bedtime chronotherapy versus conventionally timed (morning) therapy on CVD risk reduction has never been properly evaluated. The MAPEC (Monitorización Ambulatoria para Predicción de Eventos Cardiovasculares, i.e., Ambulatory Blood Pressure Monitoring for Prediction of Cardiovascular Events) study was specifically designed to investigate prospectively whether bedtime chronotherapy with ≥1 hypertension medications exerts significantly better BP control and CVD risk reduction than conventional therapy—all medications ingested upon-waking (Hermida, Citation2007).
METHODS
Inclusion and Exclusion Criteria
The full details of the rationale and design of the MAPEC study are described in a previous publication (Hermida, Citation2007). Briefly, the sample represents a population of diurnally active Spanish subjects of both sexes ≥18 yrs of age evaluated by ABPM, either to confirm the diagnosis of hypertension made by daytime clinic cuff BP measurement in untreated subjects or to evaluate treatment efficacy in already treated patients. Inclusion criteria for participants of the aspect of the MAPEC study reported here required that subjects be hypertensive and be either untreated or resistant to treatment (uncontrolled BP according to the ABPM threshold values outlined below while compliant to ≥3 optimally dosed hypertension medications of different classes, including a diuretic unless contraindicated or intolerant [Calhoun et al., Citation2008]), when ingesting all their prescribed BP-lowering medications in the morning upon awakening. Hypertensive subjects who were under treatment with ≤2 medications were also included after washed-out for ≥2 wks before study. Exclusion criteria were pregnancy, history of drug/alcohol abuse, night/shiftwork employment, diagnosis of acquired immunodeficiency syndrome (AIDS), type 1 diabetes, secondary hypertension, CVD disorders (primarily unstable angina pectoris, heart failure, life-threatening arrhythmia, nephropathy, and grade III–IV retinopathy), intolerance to ABPM measurement, and inability to communicate and comply with all of the study requirements. Participants in the MAPEC study represent a consecutive series of hypertensive subjects fulfilling the exclusion/inclusion criteria. This prospective single-center study (registered at www.clinicaltrials.gov, with identifier code NCT00295542) was approved by the state Ethics Committee of Clinical Research and adhered to the ethical standards outlined in the Helsinki Declaration (Portaluppi et al., Citation2008). All subjects gave written informed consent.
Subjects and Diagnostic Criteria
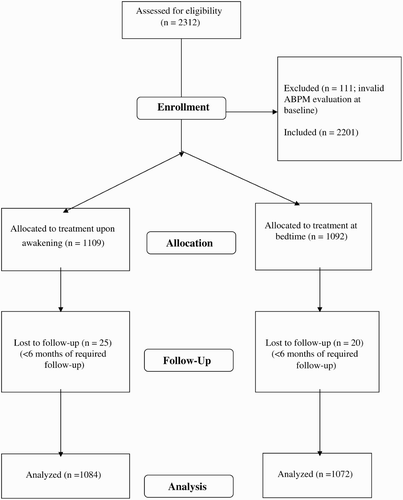
For the specific hypothesis tested here (influence of time of hypertension treatment on CVD risk), we assessed 2312 subjects for eligibility. Among these, 2156 (1044 men/1112 women, 55.6 ± 13.6 [mean ± SD] yrs of age) provided all required information for the study. The remaining 156 subjects were eliminated due to inadequate ABPM sampling at baseline and/or lack of the required ≥6-month minimal follow-up (). Diagnosis of hypertension was based on accepted ABPM criteria—an awake BP mean of ≥135/85 mm Hg for systolic (SBP)/diastolic BP (DBP), or an asleep BP mean ≥120/70 mm Hg (Mancia et al., Citation2007). At baseline, 1380 subjects were untreated hypertensive patients, and 776 were resistant hypertension patients.
Study Design
Subjects with untreated hypertension were randomly assigned to one of two monotherapy treatment-time groups, either upon awakening in the morning or at bedtime at night. The MAPEC study did not specify or require a unique investigational BP-lowering medication; rather, participating physicians were given the choice of prescribing, as first-line therapy, at least one medication of the recommended (Mancia et al., Citation2007) therapeutic classes, i.e., ARBs, ACEIs, CCBs, β-blockers, and diuretics. Thus, during the years of recruitment, the allowed choices for ARBs were valsartan, telmisartan, and olmesartan; for ACEIs ramipril and spirapril; for CCBs amlodipine and nifedipine; for β-blockers nebivolol; and for diuretics torasemide. Results of studies pertaining to the treatment-time-dependent effects on ambulatory BP of monotherapies have been reported previously (Hermida & Ayala, Citation2009; Hermida et al., Citation2003, Citation2007c, Citation2008a, Citation2008b, Citation2009, Citation2010b). Short-term treatment (∼3 months) efficacy trials with selected medications of the same class (e.g., the ACEIs ramipril and spirapril) were performed sequentially during the years of patient recruitment for the MAPEC study. Accordingly, the randomization of subjects to treatment-time (awakening or bedtime) was done separately for each allowed individual hypertension medication. This ensured that the proportion of subjects treated with each medication was similar across the two treatment-times. If subjects were uncontrolled based on ABPM criteria after 3 months of monotherapy, additional medications could be added in keeping with current clinical practice. The diuretic hydrochlorothiazide (up to 25 mg/day) or a dihydropyridine CCB were the primary choices as second-line therapy, and either one of these medications or the α-blocker doxazosin were the choices as third-line therapy.
Subjects with resistant hypertension were first randomized to either (i) modify the nature of their treatment, exchanging one of their medications with a new one but retaining the upon-waking ingestion time for all medications, or (ii) shift of the ingestion-time of one BP-lowering medication to bedtime (Hermida et al., Citation2008c). After this first randomization, physicians were allowed to prescribe additional medications and/or progressively shift additional medications to bedtime.
Lifestyle modification instructions, i.e., recommendations for weight loss, sodium restriction, limitation of alcohol intake, cessation of cigarette smoking, engagement in regular aerobic exercise, and adoption of the Dietary Approach to Stop Hypertension diet, were given to all the participants at every follow-up visit. The prescription and administration time of medications other than hypertension ones, such as statins, aspirin, and/or diabetes medications, were not part of the randomized protocol and were prescribed as needed in keeping with current clinical practice.
Among the 2156 participants who completed the study, 1084 were randomized to ingest all their hypertension medication upon awakening, and 1072 were randomized to ingest ≥1 medication at bedtime (). Among the later, 502 (46.8%) were ingesting all their medication at bedtime at the time of the last available evaluation. Blood samples were obtained the same week when each 48-h ABPM session was initiated. Subjects reported to the hospital between 08:00 and 09:00 h, after overnight fasting, for blood withdrawal from the antecubital vein. The samples were analyzed using routine automatic techniques in the hospital laboratory. Just before commencing ABPM, six clinic BP measurements were obtained with a validated automatic oscillometric device (HEM-705IT; Omron Health Care, Vernon Hills, IL) after the subject had rested in a seated position for ≥10 min.
ABPM Assessment
At inclusion, the SBP and DBP of each subject were automatically measured every 20 min between 07:00 and 23:00 h and every 30 min during the night for 48 consecutive hours with a calibrated SpaceLabs 90207 BP monitor (SpaceLabs, Issaquah, WA). Participants were instructed to do their usual activities with minimal restrictions but to adhere to a similar schedule during the 2 days of ABPM and avoid daytime napping. During monitoring, subjects maintained a diary listing the times of retiring to bed at night and awakening in the morning. BP series were considered invalid for analysis if ≥30% of the measurements were missing, if data were lacking for an interval of >2 h, if data were obtained while subjects had an irregular rest-activity schedule during the 2 days of monitoring, or if the nighttime sleep period was <6 or >12 h during ABPM.
All ABPM profiles obtained at baseline, and also during follow-up, were analyzed by comparison to circadian (with reference to rest-activity cycle) time–specified tolerance intervals previously constructed for each sex based on databases derived from previous assessments of Spanish normotensive individuals (400 men/343 women) who also had been evaluated by 48-h ABPM (Hermida et al., Citation2002, Citation2004). This procedure, as previously described (Hermida, Citation2007; Hermida et al., Citation2002), enabled the calculation of the so-called hyperbaric index, i.e., area of BP excess above the upper limit of the time-of-day–specified reference threshold values, and also the hypobaric index, i.e., area of BP deficit below the lower limit of the time-of-day–specified tolerance intervals, of each ABPM profile. We incorporated the hyperbaric index because it has been shown to be more reproducible than BP mean values in the diagnosis of hypertension (Hermida et al., Citation2000, Citation2002). During the course of the MAPEC study, a hyperbaric index ≥150 mm Hg·h was utilized as the basis to up-titrate treatment, whereas a hypobaric index ≥50 mm Hg·h was used as the basis to reduce treatment, mainly as a means of avoiding nocturnal hypotension.
Actigraphy
All subjects wore an actigraph (Mini-Motion-Logger; Ambulatory Monitoring, Ardsley, NY) on the dominant wrist to monitor physical activity every minute during ABPM. We synchronized the internal clocks of the activity and ABPM devices through their respective interfaces using the same computer. The actigraphy data, combined with patient diaries, were used to corroborate the absence of daytime napping and to accurately define the commencement and termination of the diurnal awake and nocturnal asleep spans so the respective BP means for each subject could be accurately determined.
Follow-up
The same evaluation procedure described above, including conventional clinic BP measurement, 48-h ABPM and wrist activity monitoring, blood sampling, plus other complementary tests as ordered by the physicians (e.g., electrocardiogram, funduscopic evaluation, and echocardiogram), was scheduled annually, or more frequently (every 3 months) if adjustment of treatment was required to improve ambulatory BP control.
Investigators blinded to the timed-treatment scheme of each participant (thus excluding those performing clinic evaluation, BP measurement, and/or statistical analyses) reviewed at least annually the complete clinical records of all enrolled subjects to assess CVD morbidity and mortality. Verification and categorization of CVD events listed in the patient records were accomplished following customary medical practice by the corresponding hospital services, including cardiology, neurology, and nephrology, by personnel not participating in the MAPEC study and who were thus unaware of the randomization and treatment of the patients.
Registered events for the primary outcome included death from all causes, myocardial infarction, angina pectoris, coronary revascularization, heart failure, acute arterial occlusion of the lower extremities, rupture of aortic aneurisms, thrombotic occlusion of the retinal artery, hemorrhagic stroke, ischemic stroke, and transient ischemic attack. The diagnostic criteria for each of these events were previously published (Hermida, Citation2007). Briefly, myocardial infarction was diagnosed on the basis of at least two of the standard three criteria (typical chest pain, electrocardiographic QRS changes, elevation of myocardial enzymes by twice the upper normal laboratory limit value). Angina pectoris was defined as chest pain accompanied by typical ischemic indicators in the electrocardiogram. Stroke was diagnosed on the basis of rapid onset of localizing neurological deficit lasting >24 h and in the absence of any other disease process. Transient ischemic attack was defined by any sudden focal neurological deficit that completely cleared in <24 h. Standard clinical and ultrasonographic criteria were used to assess heart failure and acute arterial occlusions and ruptures, as appropriate.
Statistical Methods
To correct for measurement errors and outliers, ABPM profiles were edited according to conventional criteria (Staessen et al., Citation1991). Thus, SBP readings >250 or <70 mm Hg, DBP >150 or <40 mm Hg, and pulse pressure (PP, difference between SBP and DBP) >150 or <20 mm Hg were automatically discarded. The “48-h BP mean” was calculated as the average of all valid readings obtained during the 48 h of ABPM sampling. The sleep-time relative BP decline (an index of BP dipping) was calculated as using all the data sampled by 48-h ABPM. For comparative purposes, a subject was defined as dipper if the sleep-time relative SBP decline was ≥10%, and as non-dipper otherwise.
[(Awake BP mean − Asleep BP mean)/Awake BP mean] × 100
The primary outcomes study endpoint was total CVD morbidity and mortality, which included all the events listed in “Follow-up.” Demographic and clinical characteristics were compared on an intention-to-treat basis among groups of subjects randomized to the two treatment-time groups—(i) all hypertension medications ingested upon awakening or (ii) ≥1 BP-lowering medication ingested at bedtime—by t test (quantitative variables) or nonparametric chi-square test (proportions). The Cox proportional-hazard model was used to estimate relative risks (with 95% confidence intervals) for events associated with time of treatment, with adjustment for significant confounding variables. Event rates for fatal and nonfatal CVD events during follow-up were also expressed as the number/1000 patient-yrs, i.e., ratio of the observed number of events to the total number of patient-yrs of exposure. For survival analysis, follow-up was established as either the time to the documented event or the time to the last evaluation in event-free subjects. Survival curves were generated using the Kaplan-Meier product-limit method (Kaplan & Meier, Citation1958) and compared by the Mantel log-rank test (Mantel, Citation1966). Statistical analyses were performed using SPSS, version 13.0 (SPSS, Chicago, IL) and KaleidaGraph version 3.6.4 (Synergy Software, Reading, PA).
RESULTS
Demographic Characteristics, Laboratory Variables, and Ambulatory BP
At baseline, the two treatment-time groups were comparable for the prevalence of type 2 diabetes, obstructive sleep apnea, metabolic syndrome (ATP-III revised definition [Grundy et al., Citation2005]), and obesity (body mass index ≥25 kg/m2), plus the majority of anthropometric variables and clinical laboratory test values (). Age, waist perimeter, and serum creatinine, which were slightly higher among subjects of the upon-awakening treatment-time group, were no longer significantly different after correcting the p values for multiple testing. The clinic BP, mean ambulatory BP values, and prevalence of non-dipping at baseline were mostly comparable between the groups (). In keeping with the study design, there were no differences in the classes and number of hypertension medications used for therapy between the treatment-time groups, except for the anticipated lower percentage of subjects ingesting diuretics at bedtime ().
TABLE 1 Baseline characteristics of patients investigated according to treatment-time (either all hypertension medications upon awakening or ≥1 medications at bedtime)
TABLE 2 Final characteristics of patients investigated according to treatment-time (either all hypertension medications upon awakening or ≥1 medications at bedtime)
The data of the last evaluation revealed differences between the two treatment-time groups. The group of subjects ingesting ≥1 medications at bedtime showed significantly lower mean sleep-time BP than the group of subjects ingesting all their medications upon awakening (p < .001; ). Treatment-time differences were greater for SBP than DBP, thus reflecting a stronger impact of bedtime treatment in reducing ambulatory PP. Generally, differences between groups in the mean awake BP were small and nonsignificant (as for SBP, p = .546). The sleep-time relative BP decline was also significantly greater among subjects ingesting ≥1 medications at bedtime; accordingly, the proportion of subjects in this treatment-time group with a non-dipper BP profile was significantly lower than that in the upon-awakening treatment-time group (34% versus 62%; p < .001). Finally, the proportion of subjects with controlled BP, with reference to established ABPM criteria, was significantly greater among subjects ingesting ≥1 medications at bedtime than in those ingesting all medications upon awakening (62% versus 53%; p < .001; ).
CVD Risk According to Time-of-Day of Hypertension Treatment
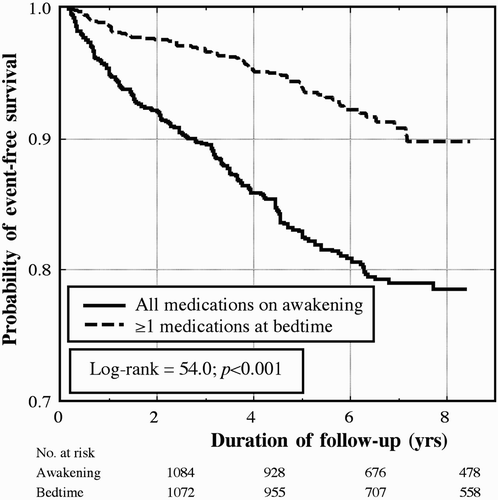
The median time of follow-up was 5.6 yrs (range, 0.5 to 8.6 yrs). During this time, we documented 255 events (40 deaths, 35 myocardial infarctions, 43 angina pectoris, 26 coronary revascularizations, 31 cerebrovascular events, 41 heart failures, 17 cases of aortoiliac occlusive disease, and 22 thrombotic occlusions of the retinal artery). presents, for the total of all the described and documented events, the Kaplan-Meier survival curves for the subjects of the two treatment-time groups; a highly significant difference was detected in event-free survival (log-rank 54.0, p < .001).
FIGURE 2 Kaplan-Meier survival curves as a function of time-of-day of hypertension treatment, i.e., for subjects ingesting either all their medication upon awakening or ≥1 medications at bedtime.
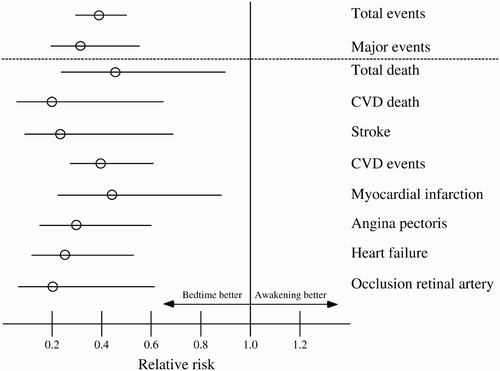
provides further information on the distribution of the CVD events in both treatment-time groups. The bedtime group consistently showed a significantly lower incidence of each of the study endpoint events. Particularly relevant is the finding that total deaths were significantly more prevalent among subjects who took all their hypertension medications upon awakening, explained mainly by the significantly (p = .008) higher incidence of CVD deaths in this group.
shows the relative risks of CVD events estimated by the Cox proportional-hazard model for the subjects of the respective treatment-time groups. Adjustments were applied for sex, age, and diabetes in all comparisons, as these influential factors were the only ones, among all the demographic and laboratory variables shown in , that were consistently significant in all tested Cox regression models. Subjects ingesting ≥1 BP-lowering medications at bedtime evidenced a significantly lower relative risk of total events than subjects ingesting all of their medications upon awakening (relative risk [95% confidence interval]: 0.39 [0.29–0.51]; p < .001). Particularly relevant is the difference between the two treatment-time groups in the adjusted relative risk of major CVD events, i.e., CVD deaths, myocardial infarction, ischemic stroke, and hemorrhagic stroke (0.33 [0.19–0.55]; p < .001).
FIGURE 3 Relative risks (with 95% confidence intervals) of CVD events (adjusted by age, sex, and diabetes) as a function of time-of-day of hypertension treatment, i.e., for subjects ingesting either all their medication upon awakening or ≥1 medications at bedtime. Total events include death (from all causes), CVD events, cerebrovascular events (stroke and transient ischemic attack), heart failure, acute arterial occlusion of the lower extremities, rupture of aortic aneurisms, and thrombotic occlusion of the retinal artery. Major events include CVD deaths, myocardial infarction, ischemic stroke, and hemorrhagic stroke. CVD events include myocardial infarction, angina pectoris, and coronary revascularization.
Changes in Clinic and Ambulatory BP During Follow-up as Predictors of CVD Risk
The results shown in indicate that those subjects randomized to ingest ≥1 medications at bedtime experienced significantly better BP control during sleep as expressed by the enhanced reduction of the asleep BP mean and increased sleep-time relative BP decline towards a more prominent dipping BP pattern. Accordingly, we further evaluated the influence on CVD risk of changes in ambulatory BP during follow-up.
shows the adjusted relative risk for each 5 mm Hg decrease in conventional clinic or ambulatory BP means and for each 5% increase in the sleep-time relative BP decline occurring between the first and last available ABPM profile of each participant. The Cox regression models were adjusted for the significant influential parameters of sex, age, diabetes, and number of hypertension medications (left column of ). The increase in the sleep-time relative BP decline and decrease in asleep BP mean during follow-up were most significantly associated with event-free survival. Moreover, the decrease in mean asleep BP during follow-up was the only parameter significantly associated with survival when two or more of the mean values in were simultaneously included in the Cox regression models. In a complementary analysis, we further adjusted the Cox regression models for changes in the 48-h mean BP during follow-up. The decrease in the asleep BP mean and the increase in the sleep-time relative BP decline were still strong independent predictors of event-free survival, even after correcting the results for reduction in the 48-h mean BP occurring during follow-up (right column in ). Results further indicate increased survival for increased awake BP when the 48-h mean is decreased, a statistical result further verifying the significant relation between increased dipping of the circadian BP pattern and event-free survival.
TABLE 3 Relative risk of total CVD events associated with changes in clinic and ambulatory (ABPM) blood pressure (BP) during follow-up
DISCUSSION
The MAPEC study prospectively investigated the hypothesis that bedtime chronotherapy with ≥1 hypertension medications exerts better BP control and CVD risk reduction than conventional therapy—when all medications are ingested upon-waking. The results document, first, greater ambulatory BP control in subjects ingesting ≥1 hypertension medications at bedtime than in subjects ingesting all their medications upon awakening. The main differences between the bedtime versus morning-time treatment groups in terms of BP control were achievement in the former of (i) significantly lower mean asleep BP and (ii) greater sleep-time relative BP decline, with just a minor loss of awake BP-lowering efficacy (). These administration-time-dependent effects on asleep BP control and circadian BP pattern regulation were strongly associated with lower CVD risk and increased event-free survival. Indeed, the progressive increase in the sleep-time relative BP decline towards a more dipping circadian pattern plus the progressive reduction in the asleep BP mean from baseline were the most significant predictors of survival (). As documented in a series of prospective controlled trials reviewed elsewhere (Hermida et al., Citation2007b; Smolensky et al., Citation2010), and also corroborated in the long-term evaluation provided here, treatment at bedtime is the most cost-effective and simplest strategy of successfully achieving the therapeutic goals of adequate asleep BP reduction and preserving or reestablishing the normal 24-h BP dipping pattern. One could thus conclude, as corroborated by the results shown in , that the greater reduction in CVD risk and increased survival associated with bedtime chronotherapy with ≥1 BP-lowering medications, compared to upon-waking treatment of all medications, are linked to better achievement of these novel hypertension therapeutic goals.
Therapeutic intervention in hypertension consists of adequate BP control, the ultimate clinical goal being to reduce/avert CVD morbidity and mortality. Commonly, the therapeutic strategies used to improve BP control, mainly defined solely by the lowering of the conventional daytime clinic BP level, include (i) increase of therapeutic dose, (ii) sequential change of medications, and/or (iii) application of a combination of medications that exert synergic effects (Mancia et al., Citation2007). In practice, all of these therapeutic strategies involve a common element: morning ingestion of therapy, either upon awakening or, more commonly, with breakfast (de la Sierra et al., Citation2009). We previously documented that increasing the number of morning-time hypertension medications results in progressive reduction in the sleep-time relative BP decline towards a more non-dipper BP pattern, and that bedtime ingestion of ≥1 hypertension medications significantly reduces the prevalence of non-dipping and improves overall BP control (Hermida et al., Citation2008c, Citation2010a). However, these results were not corroborated by a recent study also conducted on Spanish hypertensive subjects (De la Sierra et al., Citation2009) but based on different methodology, as time of treatment was not randomized and the percentage of subjects ingesting medication at night involved <20% of the total sample.
As the time of treatment of traditional hypertension medications has never been prospectively assessed beforehand in terms of a potential variable influencing CVD risk, there is no apparent robust scientific reason for the morning-time bias for the dosing of hypertension medications. Predictable changes during the 24 h in a number of environmental and biological variables, beyond the rest-activity cycle, give rise to the circadian BP pattern (Hermida et al., Citation2007a; Portaluppi & Smolensky, Citation2000). In persons with normal BP and uncomplicated essential hypertension, ABPM shows that BP declines to lowest levels during nighttime sleep, rises abruptly with morning awakening, and attains near peak or peak values during the initial hours of diurnal activity, which to some degree could be taken as justification for recommending morning-time hypertension treatment. This normal dipping pattern, where asleep BP is at least 10% lower than awake BP, however, does not characterize all or even a majority of patients. The non-dipping BP pattern is not only common, but also highly predominant (>70%) in elderly, diabetic, secondary, and resistant hypertensive patients, among others (Hermida et al., Citation2007a; Salles et al., Citation2008). Taking into account that (i) most marketed medications fail to provide homogeneous long-lasting efficacy throughout the entire 24 h and (ii) there exists a high prevalence of nocturnal hypertension and non-dipper BP pattern, we believe it is inappropriate, based on medical perspectives, to treat all hypertensive subjects by the same morning once-a-day dosing strategy.
Current international guidelines recommend the prescription of long-acting, once-daily medications that provide 24-h efficacy, as “they improve adherence to therapy and minimize BP variability, providing smoother and more consistent BP control” (Mancia et al., Citation2007). However, in our opinion, these recommendations are valid only for medications ingested in the morning. With morning-time treatment, medications with high smoothness index—a measure of the homogeneity of the BP reduction throughout the 24 h—and sustained duration of action are likely to exert only minimal effect on the sleep-time relative BP decline, as they are expected to exert a comparable BP-lowering effect on the awake and asleep BP. Accordingly, the morning ingestion of a medication with high 24-h homogeneous efficacy could exemplify, if any, a potential treatment choice for dipper hypertensive patients. However, this therapeutic scheme does not seem appropriate for non-dippers, since it is important to avoid the higher CVD risk associated with nocturnal hypertension (Dolan et al., Citation2005; Kikuya et al., Citation2005; Staessen et al., Citation1999), which can be better controlled by bedtime treatment as demonstrated by the results presented in .
The available scientific evidence thus far indicates that non-dipper hypertensives derive more benefit from a bedtime as opposed to morning-dosing schedule of most BP-lowering medications (Hermida & Ayala, Citation2009; Hermida et al., Citation2003, Citation2007b, Citation2007c, Citation2008c, Citation2009; Lemmer, Citation2000; Smolensky et al., Citation2010; Tofé & García, Citation2009); this treatment schedule best reduces abnormally high sleep-time BP and converts an abnormal non-dipping circadian BP profile toward a normal dipper one, which is known to be associated with reduced CVD risk (Boggia et al., Citation2007; Dolan et al., Citation2005; Ohkubo et al., Citation2002; Salles et al., Citation2008; Staessen et al., Citation1999; Verdecchia et al., Citation1994). Most importantly, the pharmacodynamic characteristics of most hypertension medications have been shown to be highly dependent on treatment-time (Hermida & Ayala, Citation2009; Hermida et al., Citation2003, Citation2007b, Citation2007c, Citation2009; Lemmer, Citation2000; Smolensky et al., Citation2010). Several authors have proposed that the differential administration-time-dependent effects on asleep BP regulation of some hypertension medications and their ability to restore a normal dipping BP pattern may be just a direct consequence of their short half-life and limited duration of action; thus, they argue that treatment timing might be relevant only for short half-life ACEI and ARB medications (Morgan, Citation2009). Recent studies, however, have shown those relevant therapeutic advantages of bedtime treatment apply also to long-acting medications, including ramipril (Hermida & Ayala, Citation2009), spirapril (Hermida et al., Citation2010b), telmisartan (Hermida et al., Citation2007c), and olmesartan (Hermida et al., Citation2009; Tofé & García, Citation2009), all showing an dose-response curve and duration of action markedly dependent on time of treatment.
At least two previous morbidity trials have reported the yet uncommon use of evening dosing of hypertension medications. The first one, the Syst-Eur trial (Staessen et al., Citation1997), investigated whether active treatment could reduce CVD complications of isolated systolic hypertension in elderly subjects. Participants were randomized at inclusion to an evening schedule of either placebo or the dihydropyridine CCB nitrendipine. In the Heart Outcomes Prevention Evaluation (HOPE) study (Yusuf et al., Citation2000), patients in the active treatment group ingested ramipril at bedtime, a critical piece of information withheld from the original publication. Results of a small subgroup of only 38 patients evaluated with 24-h ABPM at inclusion and 1 yr after randomization showed significant nighttime BP reduction in subjects taking ramipril, but not in those on placebo (Svensson et al., Citation2001). The authors concluded that the beneficial effects on CVD morbidity and mortality seen with ramipril in the HOPE study might be related to the 8% increase in the sleep-time relative BP decline obtained when ramipril was ingested at bedtime (Svensson et al., Citation2001), a finding now fully corroborated by the results of the present much larger sample-sized prospective MAPEC study. The tested hypertension medications (nitrendipine and ramipril) in these previous trials reporting on bedtime treatment were not randomized according to treatment-time (morning or evening), and thus these studies cannot be considered as proper chronotherapy trials.
There are several potential limitations of the MAPEC study. First, compared to other larger multicenter clinical hypertension trials entailing only clinic BP during follow-up (Staessen et al., Citation1997; Yusuf et al., Citation2000), the sample size of the single-center MAPEC study might seem a limitation. However, the number of subjects participating in our study was considerably greater than that of most other published trials on the prognostic value of ABPM (Ohkubo et al., Citation2002; Salles et al., Citation2008; Staessen et al., Citation1999; Verdecchia et al., Citation1994), and sufficient according to a priori sample size calculations and the statistical significance of the reported results. Second, in keeping with usual clinical practice, the design of the MAPEC study allowed treatment with hypertension medications of different classes. Although the sample size was not specifically calculated for the conduct of comparison between classes of medications on the benefits, in terms of CVD risk reduction, of bedtime versus upon-awakening scheduling of treatment, further analyses would, nonetheless, allow for exploring such. Finally, the use of a prospective, randomized, open-label, blinded endpoint (PROBE) design might also be considered a limitation. However, the PROBE design was specifically developed for the conduct of long-term morbidity and mortality trials, even when it is also frequently used today for the conduct of short-term efficacy clinical trials in patients evaluated in a blinded manner by ABPM (Smith et al., Citation2003).
The design of the MAPEC study also incorporates several strengths. Whereas all previous trials on the prognostic value of ABPM relied only on a single baseline profile from each subject, the MAPEC study is the first to provide results that are based on systematic and periodic multiple evaluations by ABPM throughout the median 5.6 yrs of follow-up. This so-far unique approach allowed for the first time determination of the influence of specific changes in awake, asleep, and 48-h BP means, as well as in the sleep-time relative BP decline during follow-up on CVD risk. This extensive evaluation by ABPM will thus allow exploration of additional hypotheses beyond that of the advantages of a bedtime treatment strategy reported here. Further strengths of the MAPEC study are the use of (i) 48-h, instead of the most common 24-h, ABPM sampling to increase the reproducibility of the BP findings (Hermida et al., Citation2007e); (ii) wrist actigraphy to precisely and individually determine the beginning and end of the activity and sleep spans for each subject to enable the accurate calculation of the awake and asleep BP means, sleep-time relative BP decline, and type of dipping pattern; and (iii) the highly reproducible hyperbaric and hypobaric indices to confirm the diagnosis of hypertension, modify treatment, and avoid nocturnal hypotension.
In conclusion, we recommend the pharmacotherapy of hypertension take into account the seldom considered, yet critically important, variable of treatment-time with respect to the 24-h rest-activity and BP pattern of each patient. The MAPEC study documents, for the first time, that a bedtime schedule with ≥1 hypertension medications, in comparison to a schedule in which all such medications are ingested upon awakening, not only significantly and cost-effectively improves BP control and decreases the prevalence of non-dipping, but that it significantly reduces CVD risk. The MAPEC study also documents for the first time that reducing the asleep BP mean, while avoiding nocturnal hypotension, and increasing the sleep-time relative BP decline towards a more normal dipping pattern—two novel therapeutic targets requiring proper patient evaluation by ABPM—significantly decrease CVD morbidity and mortality.
ACKNOWLEDGMENTS
This independent investigator-promoted research was supported by unrestricted grants from Dirección General de Investigación, Ministerio de Educación y Ciencia (SAF2006-6254-FEDER); Consellería de Presidencia, Relacións Institucionais e Administración Pública, Secretaría Xeral de Investigación e Desenvolvemento, Xunta de Galicia (PGIDIT03-PXIB-32201PR); Consellería de Educación e Ordenación Universitaria, Dirección Xeral de Promoción Científica e Tecnolóxica do Sistema Universitario de Galicia, Xunta de Galicia (RCH); Consellería de Economía e Industria, Dirección Xeral de Investigación e Desenvolvemento, Xunta de Galicia (INCITE07-PXI-322003ES, INCITE08-E1R-322063ES, INCITE09-E2R-322099ES); and Vicerrectorado de Investigación, University of Vigo.
Declaration of Interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Boggia J, Li Y, Thijs L, Hansen TW, Kikuya M, Björklund-Bodegård K, Richart T, Ohkubo T, Kuznetsova T, Torp-Pedersen CH, Lind L, Ibsen H, Imai Y, Wang J, Sandoya E, O'Brien E, Staessen JA. (2007). Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet 370:1219–1229.
- Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica D, Ferdinand K, Giles TD, Falkner B, Carey RM. (2008). Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension 51:1403–1419.
- de la Sierra A, Redon J, Banegas JR, Segura J, Parati G, Gorostidi M, de la Cruz JJ, Sobrino J, Llisterri JL, Alonso J, Vinyoles E, Pallarés V, Sarría A, Aranda P, Ruilope LM, Spanish Society of Hypertension Ambulatory Blood Pressure Monitoring Registry Investigators. (2009). Prevalence and factors associated with circadian blood pressure patterns in hypertensive patients. Hypertension 53:466–472.
- Dolan E, Stanton A, Thijs L, Hinedi K, Atkins N, McClory S, Den Hond E, McCormack P, Staessen JA, O'Brien E. (2005). Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertension 46:156–161.
- Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Frauss RM, Savage PJ, Smith SC, Spertus JA, Costa F. (2005). Diagnosis and management of the metabolic syndrome. An American Hearty Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 112:2735–2752.
- Hermida RC. (2007). Ambulatory blood pressure monitoring in the prediction of cardiovascular events and effects of chronotherapy: rationale and design of the MAPEC study. Chronobiol. Int. 24:749–775.
- Hermida RC, Ayala DE. (2009). Chronotherapy with the angiotensin-converting-enzyme inhibitor ramipril in essential hypertension: improved blood pressure control with bedtime dosing. Hypertension 54:40–46.
- Hermida RC, Fernández JR, Mojón A, Ayala DE. (2000). Reproducibility of the hyperbaric index as a measure of blood pressure excess. Hypertension 35:118–125.
- Hermida RC, Mojón A, Fernández JR, Alonso I, Ayala DE. (2002). The tolerance-hyperbaric test: a chronobiologic approach for improved diagnosis of hypertension. Chronobiol. Int. 19:1183–1211.
- Hermida RC, Calvo C, Ayala DE, Domínguez MJ, Covelo M, Fernández JR, Mojón A, López JE. (2003). Administration-time-dependent effects of valsartan on ambulatory blood pressure in hypertensive subjects. Hypertension 42:283–290.
- Hermida RC, Fernández JR, Ayala DE, Mojón A, Alonso I, Calvo C. (2004). Circadian time-qualified tolerance intervals for ambulatory blood pressure monitoring in the diagnosis of hypertension. Chronobiol. Int. 21:149–170.
- Hermida RC, Calvo C, Ayala DE, López JE. (2005). Decrease in urinary albumin excretion associated to the normalization of nocturnal blood pressure in hypertensive subjects. Hypertension 46:960–968.
- Hermida RC, Ayala DE, Portaluppi F. (2007a). Circadian variation of blood pressure: the basis for the chronotherapy of hypertension. Adv. Drug. Deliv. Rev. 59:904–922.
- Hermida RC, Ayala DE, Calvo C, Portaluppi F, Smolensky MH. (2007b). Chronotherapy of hypertension: administration-time dependent effects of treatment on the circadian pattern of blood pressure. Adv. Drug. Deliv. Rev. 59:923–939.
- Hermida RC, Ayala DE, Fernández JR, Calvo C. (2007c). Comparison of the efficacy of morning versus evening administration of telmisartan in essential hypertension. Hypertension 50:715–722.
- Hermida RC, Calvo C, Ayala DE, López JE, Rodríguez M, Chayán L, Mojón A, Fontao MJ, Fernández JR. (2007d). Dose and administration-time-dependent effects of nifedipine GITS on ambulatory blood pressure in hypertensive subjects. Chronobiol. Int. 24:471–493.
- Hermida RC, Ayala DE, Fernandez JR, Mojón A, Calvo C. (2007e). Influence of measurement duration and frequency on ambulatory blood pressure monitoring. Rev. Esp. Cardiol. 60:131–138.
- Hermida RC, Ayala DE, Mojón A, Fernández JR. (2008a). Chronotherapy with nifedipine GITS in hypertensive patients: improved efficacy and safety with bedtime dosing. Am. J. Hypertens. 21:948–954.
- Hermida RC, Ayala DE, Mojón A, Chayán L, Domínguez MJ, Fontao MJ, Soler R, Alonso I, Fernández JR. (2008b). Comparison of the effects on ambulatory blood pressure of awakening versus bedtime administration of torasemide in essential hypertension. Chronobiol. Int. 25:950–970.
- Hermida RC, Ayala DE, Fernández JR, Calvo C. (2008c). Chronotherapy improves blood pressure control and reverts the nondipper pattern in patients with resistant hypertension. Hypertension 51:69–76.
- Hermida RC, Ayala DE, Chayán L, Mojón A, Fernández JR. (2009). Administration-time-dependent effects of olmesartan on the ambulatory blood pressure of essential hypertension patients. Chronobiol. Int. 26:61–79.
- Hermida RC, Ayala DE, Mojón A, Fernández JR. (2010a). Effects of time of antihypertensive treatment on ambulatory blood pressure and clinical characteristics of subjects with resistant hypertension. Am. J. Hypertens. 23:432–439.
- Hermida RC, Ayala DE, Fontao MJ, Mojón A, Alonso I, Fernández JR. (2010b). Administration-time-dependent effects of spirapril on ambulatory blood pressure in uncomplicated essential hypertension. Chronobiol. Int. 27:560–574.
- Kaplan ER, Meier P. (1958). Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 53:457–481.
- Kario K, Hoshide S, Shimizu M, Yano Y, Eguchi K, Ishikawa J, Ishikawa S, Shimada K. (2010). Effect of dosing time of angiotensin II receptor blockade titrated by self-measured blood pressure recordings on cardiorenal protection in hypertensives: the Japan Morning Surge-Target Organ Protection (J-TOP) study. J. Hypertens. 28:1574–1583.
- Kikuya M, Ohkubo T, Asayama K, Metoki H, Obara T, Saito S, Hashimoto J, Totsune K, Hoshi H, Satoh H, Imai Y. (2005). Ambulatory blood pressure and 10-year risk of cardiovascular and noncardiovascular mortality. The Ohasama Study. Hypertension 45:240–245.
- Labrecque G, Beauchamp D. (2003). Rhythms and pharmacokinetics. In Redfern P (ed.). Chronotherapeutics. London: Pharmaceutical Press, pp. 75–110.
- Lemmer B. (2000). Cardiovascular chronobiology and chronopharmacology. Importance of timing of dosing. In White WB (ed.). Blood pressure monitoring in cardiovascular medicine and therapeutics. Totowa, NJ: Humana Press, pp. 255–271.
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, for the Modification of Diet in Renal Disease Study Group. (1999). A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann. Int. Med. 130:461–470.
- Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Struijker HAJ, Zanchetti A. (2007). 2007 guidelines for the management of arterial hypertension. The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J. Hypertens. 25:1105–1187.
- Mantel N. (1966). Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother. Rep. 50:163–170.
- Morgan TO. (2009). Does it matter when drugs are taken? Hypertension 54:23–24.
- Ohkubo T, Hozawa A, Yamaguchi J, Kikuya M, Ohmori K, Michimata M, Matsubara M, Hashimoto J, Hoshi H, Araki T, Tsuji I, Satoh H, Hisamichi S, Imai Y. (2002). Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. J. Hypertens. 20:2183–2189.
- Portaluppi F, Smolensky MH. (2000). Circadian rhythm and environmental determinants of blood pressure regulation in normal and hypertensive conditions. In White WB (ed.). Blood pressure monitoring in cardiovascular medicine and therapeutics. Totowa, NJ: Humana Press, pp. 79–118.
- Portaluppi F, Touitou Y. Smolensky MH. (2008). Ethical and methodological standards for laboratory and medical biological rhythm research. Chronobiol. Int. 25:999–1016.
- Salles GF, Cardoso CR, Muxfeldt ES. (2008). Prognostic influence of office and ambulatory blood pressures in resistant hypertension. Arch. Intern. Med. 168:2340–2346.
- Smith DH, Neutel JM, Lacourciere Y, Kempthorne-Rawson J. (2003). Prospective, randomized, open-label, blinded-endpoint (PROBE) designed trials yield the same results as double-blind, placebo-controlled trials with respect to ABPM measurements. J. Hypertens. 21:1291–1298.
- Smolensky MH, Hermida RC, Ayala DE, Tiseo R, Portaluppi F. (2010). Administration-time-dependent effect of blood pressure-lowering medications: basis for the chronotherapy of hypertension. Blood Press. Monit. 15:173–180.
- Staessen J, Fagard R, Lijnen P, Thijs L, Vaa Hoof R, Amery A. (1991). Ambulatory blood pressure monitoring in clinical trials. J. Hypertens. 9( Suppl 1):s13–s19.
- Staessen JA, Fagard R, Thijs L, Celis H, Arabidze GG, Birkenhager WH, Bulpitt CJ, de Leeuw PW, Dollery CT, Fletcher AE, Forette F, Leonetti G, Nachev C, ÓBrien ET, Rosenfeld J, Rodicio JL, Tuomilehto J, Zanchetti A. (1997). Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. Lancet 350:757–764.
- Staessen JA, Thijs L, Fagard R, ÓBrien ET, Clement D, de Leeuw PW, Mancia G, Nachev C, Palatini P, Parati G, Tuomilehto J, Webster J, for the Systolic Hypertension in Europe (Syst-Eur) trial investigators. (1999). Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. JAMA 282:539–546.
- Svensson P, de Faire U, Sleight P, Yusuf S, Östergren J. (2001). Comparative effects of ramipril on ambulatory and office blood pressures. A HOPE substudy. Hypertension 38:e28–e32.
- Tofé S, García B. (2009). 24-Hour and nighttime blood pressures in type 2 diabetic hypertensive patients following morning or evening administration of olmesartan. J. Clin. Hypertens. (Greenwhich) 11:426–431.
- Verdecchia P, Porcellati C, Schillaci G, Borgioni C, Ciucci A, Battistell M, Guerrieri M, Gatteschi C, Zampi I, Santucci A, Santucci C, Reboldi G. (1994). Ambulatory blood pressure: an independent predictor of prognosis in essential hypertension. Hypertension 24:793–801.
- Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. (2000). Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients: the Heart Outcomes Prevention Evaluation Study Investigators. N. Engl. J. Med. 342:145–153.