Abstract
Objectives. The main objective of this 1.5-year prospective study was to evaluate the value of T-SPOT®.TB as compared to the tuberculin skin test (TST) for the first-line assessment of uveitis.
Methods. A total of 108 consecutive non-immunocompromised patients with acute or chronic uveitis, including 92/108 (85.2%) with previous BCG vaccination, underwent a general examination, a TST, and a T-SPOT.TB test (Oxford Immunotec; Oxford, UK), blood and serological tests, and chest imaging. Concordance between tests was assessed using kappa coefficients. The performance of binary classification tests was evaluated with sensitivity, specificity, and likelihood ratios.
Results. The results of the TST and the T-SPOT.TB test differed significantly (55.5% versus 29.6% positivity, P < 0.001), with a low concordance between the two tests (κ = 0.362, P = 0.001). The sensitivity of the TST was 100% (diagnosis of suspected tuberculous uveitis included a positive TST), but its specificity was only 53.3%. The sensitivity and the specificity of the T-SPOT.TB test were 94.4% and 83.3%, respectively. The positive and negative likelihood ratios of the T-SPOT.TB test were 5.67 and 0.07, respectively.
Conclusion. In uveitis patients with frequent previous BCG vaccination, the T-SPOT.TB test is more specific than the TST and therefore allows more accurate selection of patients requiring extensive investigations to rule out TB diagnosis.
| Abbreviations | ||
| CFP-10 | = | culture filtrate protein-10 |
| ESAT-6 | = | early secretory antigenic target-6 |
| IFN-γ | = | interferon gamma |
| IGRA | = | in-vitro interferon-gamma release assay |
| PPD | = | purified protein derivative |
| TB | = | tuberculosis |
| TST | = | tuberculin skin test |
Key messages
In a population of non-immunocompromised patients with active or chronic uveitis, the TST and T-SPOT®.TB have similar sensitivities but T-SPOT.TB has a much higher specificity. The TST may remain the first clinical test in this population.
If the TST is positive, a negative T-SPOT.TB makes further invasive investigations unnecessary.
In contrast, a positive T-SPOT.TB should alert clinicians to latent tuberculosis and an antibiotic treatment should be discussed.
Introduction
In patients with severe uveitis, an etiology can be found in 66% of the cases, with infections accounting for 31.8% of the identified causes (Citation1). Tuberculosis (TB), a prevalent and life-threatening infectious disease worldwide (Citation2), is responsible for 4%–5.6% of all uveitis cases (Citation3). Human TB is due to Mycobacterium tuberculosis in most cases, and less frequently to M. bovis, M. africanum, and M. canettii, all these species belonging to the so-called M. tuberculosis complex. Tuberculous ocular lesions are varied but more commonly correspond to granulomatous uveitis, vasculitis, neuroretinitis, and/or choroiditis (Citation4). These lesions occur in patients with active or latent TB, supposedly because of direct spread of mycobacteria to the eyes during bacteremia (Citation3) or more frequently because of delayed immune response to mycobacterial antigens (Citation4,Citation5). Diagnosing TB in a patient with uveitis is important since 1) patient treatment with antituberculosis drugs will reduce ocular inflammation and the rate of recurrence, and it will prevent subsequent development of active TB in patients with latent disease (Citation6); and 2) identifying persons with active TB is crucial to prevent spread of the disease in the general population.
In the majority of cases, a definite diagnosis of ocular TB by detection of bacteria of the M. tuberculosis complex from eye samples using culture or polymerase chain reaction (PCR) is not obtained. Detection of such bacteria from extraocular lesions in patients with concomitant uveitis is usually considered highly significant. In many patients, however, only a presumptive diagnosis can be established, based on suggestive epidemiological, clinical, and paraclinical data, a positive tuberculin skin test (TST), and retrospectively a good response to antituberculosis treatment.
The TST uses a M. tuberculosis purified protein derivative (PPD), which contains antigens shared by all M. tuberculosis complex species. In countries where most persons have been BCG-vaccinated, antigenic cross-reactions between M. bovis BCG strains and other species of the M. tuberculosis complex are responsible for frequent TST false-positive results (Citation7) and thus unnecessary invasive, time-consuming, and expensive investigations to rule out TB diagnosis. In-vitro interferon-gamma (IFN-γ) release assays (IGRAs) are based on the concept that T cells from patients with active or latent TB secrete IFN-γ when exposed to the mycobacterial specific proteins ESAT-6 (early secretory antigenic target-6) and CFP-10 (culture filtrate protein-10) (Citation8). IFN-γ is a major mediator of the immune response in TB patients, promoting macrophage stimulation and lymphocyte Th1 transformation. Genes encoding these proteins are found in natural strains of the M. tuberculosis complex species, but are deleted in the attenuated M. bovis BCG strains. The IGRAs are thus considered much more specific than the TST in detecting active or latent TB in BCG-vaccinated patients. T-SPOT®.TB, with a European regulatory approval as an in-vitro diagnostic test for latent TB, is increasingly being used, especially in the context of immune-mediated inflammatory diseases (Citation9,Citation10).
Our goal was to evaluate the value of the T-SPOT.TB test (Oxford Immunotec; Oxford, UK), an IGRA test based on the ELISPOT (enzyme-linked immunospot) technique (Citation9,Citation10), in the first-line assessment of 108 uveitis patients during a 1.5-year prospective study.
Materials and methods
Patients and inclusion criteria
This prospective study was conducted in 108 consecutive patients newly referred to the Grenoble University Hospital (tertiary center) for the work-up of uveitis during the 2006–2007 period. It complied with the Declaration of Helsinki guidelines for research involving human subjects and was approved by the local Institutional Review Board (#5891). The inclusion criteria were patients older than 18 years, newly referred for active or chronic uveitis. Exclusion criteria were immunosuppression, treatment with systemic steroids or immunosuppressive therapy within the previous 12 months, and patients not affiliated to the national health care system.
General and ocular investigations
All patients filled out a standard questionnaire, including general medical history, previous TB or TB contact, status of BCG vaccination, previous TST results, travels in TB-endemic areas (defined by a tuberculosis incidence greater than 25 per 100,000 person-years), and past and current treatments. Blood tests (complete blood count, erythrocyte sedimentation rate, electrolytes, serum protein electrophoresis, creatinine, liver function tests, angiotensin-converting enzyme, HLA typing), serological tests (TPHA, VDRL, HIV, toxoplasmosis, HSV, VZV, CMV), and a pulmonary digitized radiography were systematically performed. A high-resolution chest computed tomography (CT scan) was done for patients with a positive TST (see definition, ). Chest imaging results were interpreted according to previously defined criteria (Citation11,Citation12).
Table I. Definitions for TST positivity, chest imaging classification, and suspected intraocular tuberculosis.
The ocular examination systematically included best corrected visual acuity and complete slit lamp examination with funduscopy. Fluorescein and indocyanine green angiographies (Topcon IMAGEnet™; Clichy, France) and optical coherence tomography (OCT3 Zeiss™; Oberkochen, Germany) were performed when necessary. Uveitis was classified according to the International Uveitis Study Group (Citation13). The criteria used to define suspected and presumed ocular TB cases are summarized in .
Specific tuberculosis diagnostic tests
Ziehl Neelsen staining and mycobacterial cultures in Lowenstein Jensen medium (bioMérieux; Marcy l'Etoile, France) and in MGIT 960 automated system (Becton Dickinson; Pont-de-Claix, France) were performed on gastric lavage and/or bronchoalveolar washing samples when available.
The strictly intradermal injection of 0.1 mL of tuberculin (Tubertest 50 UI/mL, Sanofi Pasteur MSD; Lyon, France) at the inside of the forearm was performed by trained nurses. The transverse diameter of the skin induration at the TST site was measured 72 h after the injection by physicians blinded to patient history. The different levels of TST positivity () were defined according to the previous BCG vaccination, mandatory in France for all children until July 2007. The TST was performed the same day, immediately following the blood collection for the T-SPOT.TB test.
For each patient, 7 mL of blood was collected in heparinized tubes and processed within 8 h. Peripheral blood mononuclear cells were prepared by density gradient centrifugation over Lymphocyte Separation Medium (Eurobio; Paris, France). The T-SPOT.TB test (Oxford Immunotec; Oxford, UK) was performed according to the manufacturer's instructions: 250,000 cells were seeded in each of the four wells of the plate assay; the cells were stimulated for 16–20 h (under 5% carbon dioxide at 37°C) with medium (nil control), phytohemagglutinin (mitogen-positive control), or the M. tuberculosis complex-specific antigens ESAT-6 (Panel A) or CFP-10 (Panel B). The number of IFN-γ spot-forming T cells was quantified using the ELISPOT Bioreader 4000 ProX plate reader (Biosys; Karben, Germany). The results were visually checked and, if deemed necessary, corrected by manual counting by a technician. The test result was considered positive if either or both panel A (containing antigens derived from ESAT-6) and/or panel B (containing antigens derived from CFP-10) had six or more spots than the negative control and this number was at least twice the number of spots in the negative control. The laboratory technicians were blinded to the subject identifiers.
Statistical analysis
Data are summarized as size and frequency for categorical data and mean ± standard deviation for quantitative data. The Student t test and ANOVA were used to compare continuous data between groups. Independence between qualitative parameters was studied with the chi-square test incorporating Yates’ correction if necessary. The McNemar test was used to compare the distribution of two paired binary parameters. Concordance between tests (TST and T-SPOT.TB test) was assessed using kappa coefficients (excellent agreement if κ > 0.75, fair to good agreement if 0.4 < κ < 0.75, poor agreement if κ < 0.4) (Citation14). The performance of the binary classification tests was evaluated with sensitivity, specificity, and likelihood ratios (LR) (calculated as sensitivity/(1 − specificity)). Accuracy was defined by the ratio: (true positive cases + true negative cases)/(positive cases + negative cases). The statistical analysis was performed using the Statistical Package for the Social Sciences program (SPSS 17.0 for Windows; Chicago, IL, USA). P values under 0.05 were considered statistically significant.
Results
Results of the epidemiological, clinical, and paraclinical investigations for the 108 uveitis patients are summarized in and . The mean age of the population was 49 ±19 years, and most patients were females (male/female ratio of 0.8). The ocular examination revealed anterior uveitis in 21 patients (19.4%), intermediate uveitis in 6 patients (5.6%), posterior uveitis in 30 patients (27.8%), panuveitis in 33 patients (30.6%), choroiditis in 13 patients (12%), and vasculitis in 9 patients (8.3%). Most patients had inflammation of the posterior segment (84.3%). An etiology was found in 38 of 108 (35.1%) cases, including 9 infectious diseases (22.5%: toxoplasmosis n = 6, VZV n = 1, HSV n = 1, CMV n = 1). The retained uveitis causes included sarcoidosis (n = 8), B27 uveitis (n = 6), Behçet disease (n = 3), birdshot chorioretinopathy (n = 7), lupus erythematosus (n = 1), multiple sclerosis (n = 1), Vogt-Koyanagi-Harada syndrome (n = 2), and ocular and central nervous system lymphoma (n = 1).
Table II. Epidemiological, clinical and paraclinical investigations for the 108 uveitis patients according to their TST and T-SPOT®.TB status.
We first compared patients according to the positivity of each test (). Whereas 60 of 108 (55.6%) of the investigated uveitis patients had a positive TST, only 32 of 108 (29.6%) had a positive T-SPOT.TB test. A total of 66% of the patients had concordant TST and T-SPOT.TB test results: both tests were negative in 40.7% of cases, and both tests were positive in 25.9% of cases. TST positivity was significantly associated with male gender and a history of TB contact. T-SPOT.TB test positivity was significantly associated with higher age, a history of TB contact, and a lower rate of BCG vaccination. There was a trend of more frequent TB-typical chest imaging radiological lesions in the group with a positive T-SPOT.TB test.
Patients were then classified into three groups, including uveitis patients with determined non-tuberculous etiologies, patients with undetermined uveitis etiologies, and patients with suspected tuberculous uveitis (). Eighteen patients were classified in the last group. M. tuberculosis was grown in culture from 1 of 18 (5%) gastric lavage samples, but not from the 2 available bronchoalveolar washing samples. The only patient with a positive M. tuberculosis culture had occlusive vasculitis and tuberculous cavitation on CT scan (). This patient had a positive TST and a strongly positive T-SPOT.TB test. The other patients had intermediate uveitis (n = 1), panuveitis (n = 6), posterior uveitis (n = 5), and choroiditis (n = 5: three cases of serpiginous choroidopathy and two of acute multifocal placoid pigment epitheliopathy ()). Of the 18 patients, 13 (72.2%) had a significant reduction of ocular inflammation and uveitis recurrence after specific antituberculosis treatment during a minimum of 6 months’ follow-up (mean 18.9 ± 8.1 months) after antibiotic treatment withdrawal, which could be considered a further criterion in favor of tuberculous uveitis diagnosis. The other patients (n = 5) refused the antibiotic treatment.
Figure 1. Patient with proved tuberculosis. A: Photograph of the fundus showing retinal hemorrhages and occlusive vasculitis of the left eye. B: Fluorescein angiography of the left eye showing vasculitis, with absence of dye filling in some retinal arteries masking retinal hemorrhages. C: Tuberculous cavitation on CT scan. D: Highly positive T-SPOT®.TB test (panel A, ESAT-6 = 177 spots).
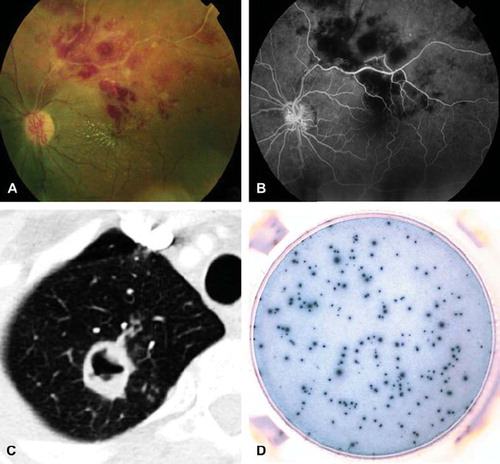
Figure 2. Patient with presumed intraocular tuberculosis and serpiginous choroiditis. A: Fundus photography showing white dots at the choroid, in the posterior pole of the left eye. B: Fluorescein angiography showing hyperfluorescence at the rim of the lesions and hypofluorescence in the center of the lesions. C: Indocyanine angiography showing hypofluorescence of the lesions. D: Highly positive T-SPOT®.TB test (panel B, CFP-10 = 57 spots).
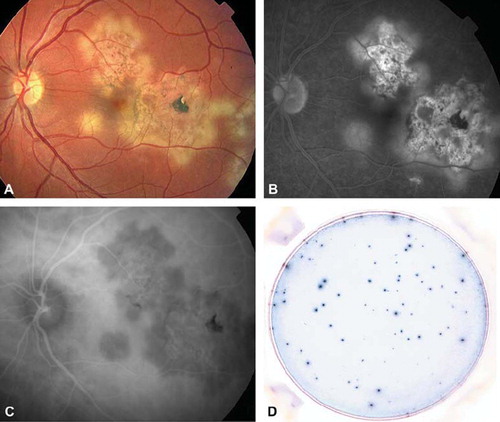
Table III. Epidemiological, clinical, and paraclinical investigations for the 108 uveitis patients included in the study according to their classification as uveitis with determined or undetermined cause, and suspected ocular tuberculosis cases.
The 18 suspected tuberculous uveitis cases were compared to uveitis patients with defined non-tuberculous etiologies and to patients with undetermined etiologies. No significant differences were found between the three groups according to the mean age and the male/female ratio. The suspected tuberculous group was significantly associated with more frequent previous TB or TB contact (61.1% versus 21.1% and 25% in other groups, respectively; P = 0.005). In all groups, the majority of patients had been previously BCG-vaccinated. The TST was positive in all 18 suspected tuberculous uveitis patients since this criterion was used to define this group, but 42.1% of the patients with determined non-tuberculous uveitis etiologies also displayed a positive TST. The T-SPOT.TB test was positive in 94.4% of suspected tuberculous uveitis cases as compared to 13.2% in the determined non-tuberculous uveitis cases (P < 0.001). Finally, patients with suspected tuberculous uveitis displayed more frequent abnormal chest findings (47.2% versus 33.7%; P = 0.02, as compared to the determined non-tuberculous uveitis cases). The group of uveitis patients with undetermined etiologies displayed comparable findings to those with non-tuberculous uveitis etiologies.
Our main objective was to define the relative risk of ocular TB in patients with a positive T-SPOT.TB test, in order to define the potential role of this new test in the primary management of uveitis patients. In the overall population studied, the results of the TST and the T-SPOT.TB test differed significantly (55.5% versus 29.6% positivity; P < 0.001), with a low concordance between the two tests (κ = 0.362; P = 0.001). When considering the 18 suspected ocular TB cases, both the TST and the T-SPOT.TB test were positive in 17 cases (94.4%), whereas in the remaining patient only the TST was positive. None of the four patients with a positive T-SPOT.TB test but a negative TST was considered as a suspected ocular TB case. The calculated sensitivity, specificity, and accuracy of the T-SPOT.TB test () were 94.4% (95% CI: 72.7–99.9), 83.3% (74.0–90.4), and 85.2% (77.1–91.3), respectively. The positive and negative likelihood ratios of the T-SPOT.TB test were 5.67 and 0.07, respectively. Although the prevalence of suspected tuberculous uveitis cases in the studied populations (i.e. 16.7%) may be considered high, the positive and negative predictive values were 53.1% (34.7–70.9) and 98.7% (92.9–99.9), respectively.
Table IV. Test performance of TST, T-SPOT®.TB in the population of 108 uveitis patients (95% confidence interval are noted in brackets).
Discussion
Extensive investigations are often needed in uveitis patients to rule out potentially life-threatening diseases (e.g. inflammatory diseases or lymphoma) and to prevent ocular complications (including blindness) by administration of appropriate therapy. In the present prospective study, a non-tuberculous etiology was found in 35.2% of the 108 consecutive uveitis patients. Infectious causes accounted for 23.6% of all uveitis cases, and TB was the most frequently diagnosed infectious disease. Not surprisingly, only one case of ocular TB was culture-confirmed, whereas the majority of cases were suspected.
The usefulness of the TST for initial screening of patients with suspected latent or active TB has been previously reported (Citation15), including in uveitis patients (Citation16). We therefore considered a positive TST an indication that extensive investigations were required to rule out the TB diagnosis. Since most of our patients had been BCG-vaccinated, intermediate reactions (skin induration less than 10 mm in diameter) were considered negative in order to increase the test's specificity (Citation16–18). Nevertheless, 55% of the patients in the present series had a positive TST and underwent complementary investigations such as gastric lavage sampling and/or bronchoalveolar washing for mycobacterial cultures, and chest CT scan. These expensive and invasive exams were normal in the majority of cases. TST positivity in this population probably reflected previous BCG vaccination rather than latent or active TB. As compared to the TST, the T-SPOT.TB test yielded the same sensitivity but a much higher specificity (83.3% versus 53.3%). The positive and negative likelihood ratios were also much better for the T-SPOT.TB test than for the TST. Interestingly, the T-SPOT.TB test was not contributory to TB diagnosis in the 44.4% of patients with a negative TST. In the four patients (4/48, 8%) with a negative TST and a positive T-SPOT.TB, neither risk factors for TB infection nor specific pulmonary lesions were found. This situation was observed in three non-vaccinated patients and in one vaccinated patient (with 5 mm TST induration). The positivity of ELISPOT in these cases could be related to a contact with environmental mycobacteria such as M. kansasii, M. szulgai, or M. marinum.
In the present BCG-vaccinated population, the use of the T-SPOT.TB test would have allowed a much more specific first-line screening of patients requiring thorough TB investigations. It should be noted, however, that neither the TST nor the T-SPOT.TB test allowed definite exclusion of TB diagnosis when negative. One patient, classified as a suspected ocular TB case, had a positive TST but a negative T-SPOT.TB test. This patient had an acute multifocal placoid pigment epitheliopathy, a history of TB contact, a 13-mm TST, and the CT scan showed one cavitation. Cultures of gastric lavage sampling and bronchoalveolar washing were negative. On the other hand, a negative TST in uveitis patients with a history of previous BCG vaccination may be interpreted as a defect in cellular immunity (type IV delayed hypersensitivity) and may suggest sarcoidosis. In the present study, the rate of false-negative TST tests was limited by the exclusion of patients with immunodeficiency (Citation7) and/or previous steroid therapy and by using highly standardized TST technique, including PPD injection, reading interval, and interpretation (Citation19). Overall, our results suggest that a strategy combining the use of the TST as first-line test and the T-SPOT.TB test only in patients with a positive TST may be optimal in uveitis patients. Using this rule could have prevented further invasive TB diagnostic procedures in 30% of the study's uveitis cases.
Given the above-mentioned limitations of TST, the lack of laboratory confirmation of M. tuberculosis complex infection in most cases, and the absence of associated TB pulmonary lesions in 30%–80% of patients with extrapulmonary manifestations of TB (Citation20,Citation21), IGRAs may be beneficial for the diagnosis of TB-associated uveitis. This is suggested by recent prospective (n = 27, 31 patients) (Citation22,Citation23) and retrospective (n = 157, 21) (Citation24,Citation25) studies using Quantiferon, a whole-blood in-tube ELISA-based test that determines the level of soluble interferon-γ. IGRAs have higher specificities (> 90%) than the TST for the detection of active TB in patients with culture-confirmed TB (Citation26–28) but also for the detection of individuals with latent TB (Citation8). Our prospective study assesses for the first time the usefulness of T-SPOT.TB in a large cohort of uveitis patients.
This study shows that the concordance between TST and T-SPOT.TB is low, similar to that previously reported when comparing the Quantiferon-TB test with TST in a large population with varying risks of TB (κ = 0.41 in vaccinated subjects) (Citation15), in patients with inflammatory diseases (κ = 0.2) (Citation29), and also in patients with chronic posterior uveitis (κ = 0.56) (Citation22). As explained above, one reason for the discrepancies observed between the two tests in our study was the high prevalence of BCG-vaccinated patients (Citation15).
To date, no perfect gold standard exists for detecting latent TB. It is, therefore, not possible to determine accurately sensitivities and specificities of IGRAs for the diagnosis of uveitis associated with latent TB. However, considering the diagnosis of suspected intraocular TB, we found comparable sensitivities for TST and T-SPOT.TB in our series but at a much higher specificity for T-SPOT.TB (79% versus 49%). This higher specificity of T-SPOT.TB was suggested by the association between its positivity and the higher rate of previous TB contact. Consistent with results previously reported using the Quantiferon-TB test in Asian patients with uveitis (Citation24), the probability of positive TST and T-SPOT.TB in patients with a presumed intraocular TB was nearly six times higher than that of patients without TB.
The IGRAs have a few limitations, such as the need for careful handling of blood samples (Citation30) and the cost of the test (100 euros in our center). However, as shown in this study, IGRAs can be performed only in a selected uveitis population with positive TST. This strategy could limit the indication of this test to approximately 50% of the uveitis patients and the cost of IGRAs may be balanced with the cost of expensive and invasive complementary TB investigations, such as the chest CT scan or fibroscopy, since IGRAs should also drastically reduce their indications. The limitations of IGRAs should be balanced by their advantages, such as the objectivity and speed of the blood test, the absence of boosting effects, and no need for a return visit for test reading.
In conclusion, the diagnosis of intraocular TB remains a clinical challenge. Newer in-vitro techniques evaluating cellular immune responses to specific TB antigens, such as the T-SPOT.TB test, are useful to guide the management of uveitis patients. TST could remain the first clinical test in non-immunocompromised patients with uveitis. According to our results, the T-SPOT.TB test mainly improves TB diagnostic specificity, especially in positive TST patients. In a population vaccinated with BCG, since there is no effect of TST on IGRA positivity (Citation31,Citation32), the T-SPOT.TB test may be performed on the day of measurement of skin induration, if TST is positive. The negativity of TST and T-SPOT.TB tests makes further invasive investigations and administration of a toxic and long antituberculosis treatment unnecessary. A positive T-SPOT.TB test should alert the clinicians to active or latent TB.
Declaration of interest: The authors state no conflict of interest and have received no payment in the preparation of this manuscript.
References
- Bodaghi B, Cassoux N, Wechsler B, Hannouche D, Fardeau C, Papo T, . Chronic severe uveitis: etiology and visual outcome in 927 patients from a single center. Medicine (Baltimore). 2001;80:263–70.
- Frieden TR, Sterling TR, Munsiff SS, Watt CJ, Dye C. Tuberculosis. Lancet. 2003;362:887–99.
- Bodaghi B, LeHoang P. Ocular tuberculosis. Curr Opin Ophthalmol. 2000;11:443–8.
- Gupta V, Gupta A, Rao NA. Intraocular tuberculosis—an update. Surv Ophthalmol. 2007;52:561–87.
- Foster S. Tuberculosis. Foster S, Vitale AT. Diagnosis and treatment of uveitis. Philadelphia: WB Saunders Company; 2002. 264–71.
- Bansal R, Gupta A, Gupta V, Dogra MR, Bambery P, Arora SK. Role of anti-tubercular therapy in uveitis with latent/manifest tuberculosis. Am J Ophthalmol. 2008;146:772–9.
- Huebner RE, Schein MF, Bass JB Jr. The tuberculin skin test. Clin Infect Dis. 1993;17:968–75.
- Lalvani A, Pathan AA, Durkan H, Wilkinson KA, Whelan A, Deeks JJ, . Enhanced contact tracing and spatial tracking of Mycobacterium tuberculosis infection by enumeration of antigen-specific T cells. Lancet. 2001;357:2017–21.
- Lalvani A. Diagnosing tuberculosis infection in the 21st century: new tools to tackle an old enemy. Chest. 2007;131: 1898–906.
- Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med. 2008;149:177–84.
- Andreu J, Caceres J, Pallisa E, Martinez-Rodriguez M. Radiological manifestations of pulmonary tuberculosis. Eur J Radiol. 2004;51:139–49.
- Lee JJ, Chong PY, Lin CB, Hsu AH, Lee CC. High resolution chest CT in patients with pulmonary tuberculosis: characteristic findings before and after antituberculous therapy. Eur J Radiol. 2008;67:100–4.
- Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509–16.
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74.
- Mazurek GH, LoBue PA, Daley CL, Bernardo J, Lardizabal AA, Bishai WR, . Comparison of a whole-blood interferon gamma assay with tuberculin skin testing for detecting latent Mycobacterium tuberculosis infection. JAMA. 2001; 286:1740–7.
- Morimura Y, Okada AA, Kawahara S, Miyamoto Y, Kawai S, Hirakata A, . Tuberculin skin testing in uveitis patients and treatment of presumed intraocular tuberculosis in Japan. Ophthalmology. 2002;109:851–7.
- Jones HE, Miller SD, Greenberg JH. Measurement of tuberculin reactions. N Engl J Med. 1972;287:721.
- Sellam J, Hamdi H, Roy C, Baron G, Lemann M, Puechal X, . Comparison of in vitro-specific blood tests with tuberculin skin test for diagnosis of latent tuberculosis before anti-TNF therapy. Ann Rheum Dis. 2007;66:1610–5.
- Targeted tuberculin testing and treatment of latent tuberculosis infection. Am J Respir Crit Care Med. 2000;161: S221–47.
- Alvarez S, McCabe WR. Extrapulmonary tuberculosis revisited: a review of experience at Boston City and other hospitals. Medicine (Baltimore). 1984;63:25–55.
- Rose AM, Watson JM, Graham C, Nunn AJ, Drobniewski F, Ormerod LP, . Tuberculosis at the end of the 20th century in England and Wales: results of a national survey in 1998. Thorax. 2001;56:173–9.
- Cordero-Coma M, Calleja S, Torres HE, Barrio ID, Franco M, Yilmaz T, . The value of an immune response to Mycobacterium tuberculosis in patients with chronic posterior uveitides revisited: utility of the new IGRAs. Eye (Lond). 2010;24:36–43.
- Itty S, Bakri SJ, Pulido JS, Herman DC, Faia LJ, Tufty GT, . Initial results of QuantiFERON-TB Gold testing in patients with uveitis. Eye (Lond). 2009;23:904–9.
- Ang M, Htoon HM, Chee SP. Diagnosis of tuberculous uveitis: clinical application of an interferon-gamma release assay. Ophthalmology. 2009;116:1391–6.
- Mackensen F, Becker MD, Wiehler U, Max R, Dalpke A, Zimmermann S. QuantiFERON TB-Gold—a new test strengthening long-suspected tuberculous involvement in serpiginous-like choroiditis. Am J Ophthalmol. 2008;146: 761–6.
- Kim SH, Song KH, Choi SJ, Kim HB, Kim NJ, Oh MD, . Diagnostic usefulness of a T-cell-based assay for extrapulmonary tuberculosis in immunocompromised patients. Am J Med. 2009;122:189–95.
- Lalvani A, Pathan AA, McShane H, Wilkinson RJ, Latif M, Conlon CP, . Rapid detection of Mycobacterium tuberculosis infection by enumeration of antigen-specific T cells. Am J Respir Crit Care Med. 2001;163:824–8.
- Diel R, Loddenkemper R, Nienhaus A. Evidence-based comparison of commercial interferon-gamma release assays for detecting active TB : a metaanalysis. Chest. 2010;137:952–68.
- Soborg B, Ruhwald M, Hetland ML, Jacobsen S, Andersen AB, Milman N, . Comparison of screening procedures for Mycobacterium tuberculosis infection among patients with inflammatory diseases. J Rheumatol. 2009;36:1876–84.
- Menzies D, Pai M, Comstock G. Meta-analysis: new tests for the diagnosis of latent tuberculosis infection: areas of uncertainty and recommendations for research. Ann Intern Med. 2007;146:340–54.
- Leyten EM, Prins C, Bossink AW, Thijsen S, Ottenhoff TH, van Dissel JT, . Effect of tuberculin skin testing on a Mycobacterium tuberculosis-specific interferon-gamma assay. Eur Respir J. 2007;29:1212–6.
- Richeldi L, Bergamini BM, Vaienti F. Prior tuberculin skin testing does not boost QuantiFERON-TB results in paediatric contacts. Eur Respir J. 2008;32:524–5.
- Al-Mezaine HS, Al-Muammar A, Kangave D, Abu El-Asrar AM. Clinical and optical coherence tomographic findings and outcome of treatment in patients with presumed tuberculous uveitis. Int Ophthalmol. 2008;28:413–23.