Abstract
Background. NAFLD ranges from simple steatosis (SS) to non-alcoholic steatohepatitis (NASH). The natural history of NAFLD and the optimal strategy to identify subjects with progressive liver disease are unclear.
Objectives. To assess the evidence in: (1) natural history of NAFLD; and (2) non-invasive methods to differentiate NAFLD histological subtypes.
Design and setting. Among 4185 articles published on MEDLINE, Cochrane Library, EMBASE, Pubmed, national and International meeting abstracts through July 2010, 40 articles assessing the natural history of NAFLD and 32 articles evaluating the diagnostic accuracy of non-invasive tests against liver biopsy (LB) were included.
Measurements. Two reviewers retrieved articles and evaluated study quality by appropriate scores. Main outcomes were pooled using random- or fixed-effects models.
Results. NAFLD has an increased overall mortality (OR: 1.57, 95% CI: 1.18–2.10), deriving from liver-related and cardiovascular disease, and a 2-fold risk of diabetes. Compared to SS, NASH has a higher liver-related (OR for NASH: 5.71, 2.31–14.13; OR for NASH with advanced fibrosis: 10.06, 4.35–23.25), but not cardiovascular mortality (OR: 0.91, 0.42–1.98). Three non-invasive methods received independent validation: pooled AUROC, sensitivity and specificity of cytokeratin-18 for NASH are 0.82 (0.78–0.88), 0.78 (0.64–0.92), 0.87 (0.77–0.98). For NASH with advanced fibrosis, pooled AUROC, sensitivity and specificity of NAFLD fibrosis score and Fibroscan are 0.85 (0.80–0.93), 0.90 (0.82–0.99), 0.97 (0.94–0.99) and 0.94 (0.90–0.99), 0.94 (0.88–0.99) and 0.95 (0.89–0.99).
Conclusions. NAFLD warrants screening for cardio-metabolic risk and for progressive liver disease. The combination of three noninvasive tests with LB may optimally individuate patients with NASH, with or without advanced fibrosis.
| Abbreviations | ||
| AASLD | = | American Association for the Study of Liver Disease |
| ADA | = | American Diabetes Association |
| AGA | = | American Gastroenterological Association |
| ALT | = | alanine aminotransferase |
| AST | = | aspartate aminotransferase |
| AUROC | = | area under the receiver operator curve |
| BMI | = | body mass index |
| CK-18 | = | cytokeratin-18 fragments |
| CVD | = | cardiovascular disease |
| DDW | = | Digestive Disease Week |
| EASL | = | European Association for the Study of Liver Disease |
| ELF | = | Enhanced Liver Fibrosis panel |
| GGT | = | gamma-glutamyltyranspeptidase |
| IMT | = | carotid intima-media thickening |
| LB | = | liver biopsy |
| LR | = | likelihood ratio |
| MOOSE | = | Meta-Analyses of Observational Studies in Epidemiology |
| MRS | = | magnetic resonance spectroscopy |
| NAFLD | = | non-alcoholic fatty liver disease |
| NASH | = | non-alcoholic steatohepatitis |
| NHANES-III | = | National Institutes of Health Survey-III |
| OGTT | = | oral glucose tolerance test |
| OR | = | odds ratio |
| RCT | = | Randomized Controlled Trial |
| SHIP | = | Study of Health in Pomerania |
| SS | = | simple steatosis |
| STARD | = | Standards for Reporting of Diagnostic Accuracy |
| STROBE | = | Strengthening the Reporting of Observational Studies in Epidemiology |
Key messages
NAFLD is associated with an increased mortality than in the general population, deriving from liver-related and cardiovascular disease, and carries also a 2-fold increased risk of type 2 diabetes.
Different histological subtypes have different prognoses: while liver-related mortality is confined to NASH (and particularly to NASH with advanced fibrosis), cardiovascular mortality does not differ between simple steatosis and NASH.
Liver biopsy, the gold standard for staging and monitoring NAFLD, is not feasible in all patients; three non-invasive methods received independent validation for individuating NASH (plasma cytokeratin-18 fragments) and advanced fibrosis (NAFLD fibrosis score and Fibroscan). While biopsy remains the gold standard for staging and monitoring NAFLD, a combination of these non-invasive tests with liver biopsy may be used to individuate patients with progressive liver disease, who should be referred to a gastroenterologist for experimental therapies and tight follow-up.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is defined by the presence of liver fat accumulation exceeding 5% of hepatocytes, in the absence of significant alcohol intake (20 grams/day for men and 10 g/d for women), viral infection, or any other specific etiology of liver disease (Citation1). NAFLD encompasses a histological spectrum ranging from simple steatosis (SS) to non-alcoholic steatohepatitis (NASH), the latter characterized by steatosis plus necroinflammation; NASH can have different stages of fibrosis ranging from absent (stage F0) to cirrhosis (stage F4). SS, NASH, and different fibrosis stages can only be differentiated by liver biopsy (LB).
The prevalence of NAFLD in the general adult population depends heavily on the sensitivity of the method used, ranging from 33% (by magnetic resonance spectroscopy (MRS)), to 25% (by ultrasonography), or 3%–12% (by liver enzymes) (Citation2,Citation3). The prevalence of ultrasonographic NAFLD reaches 59% in subjects with the metabolic syndrome, 70% in diabetic and obese, and 90% in morbidly obese patients (Citation4,Citation5), while NASH affects 3% of the general adult population, 25%–30% of obese and diabetic, and 35% of morbidly obese individuals (Citation6,Citation7).
NAFLD carries a significant burden for the public health: in a 5-year population-based follow-up, the presence of NAFLD increased by 26% the overall health care costs, after controlling for co-morbidities. Furthermore, NASH is projected to be the leading cause of liver transplantation by 2020 (Citation8,Citation9).
There is no agreement on how to manage NAFLD patients, because the prognosis of NAFLD and its histological subtypes (SS and NASH with different fibrosis severity) is unclear. Furthermore, liver biopsy, the gold standard for staging liver disease, is not feasible in all NAFLD patients, and an alternative strategy to screen subjects with progressive liver disease remains to be defined.
We reviewed the evidence regarding two issues:
What is the prognosis (in terms of overall and cause-specific mortality and morbidity) of NAFLD and its histological subtypes (simple steatosis, NASH with different fibrosis stages)? To this aim, fibrosis was defined as significant (histological stage ≥ F2) or advanced (histological stage ≥ F3) in accordance with current guidelines (Citation1).
What is the diagnostic accuracy of non-invasive tests for differentiating NAFLD histological subtypes?
Methods
Data sources and searches
We searched English and non-English language publications on MEDLINE, Ovid MEDLINE In-Process, Cochrane Library, EMBASE, PubMed, and abstracts from annual AASLD (American Association for the Study of Liver Disease), AGA (American Gastroenterological Association), EASL (European Association for the Study of Liver Disease), and DDW (Digestive Disease Week) meetings through July 2010. Relevant meta-analyses of observational studies were also included if following MOOSE (Meta-Analyses of Observational Studies in Epidemiology) Guidelines (Citation10).
We also contacted authors to get information about published studies (see Acknowledgment). Search terms were: NASH, NAFLD, non-alcoholic steatohepatitis, non-alcoholic fatty liver disease, fatty liver, liver fat, steatosis, liver enzymes, transaminase, alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyltyranspeptidase (GGT), diagnosis, prognosis, natural history, non-invasive methods, severity of liver disease, fibrosis.
Study selection
Inclusion criteria: Participant adult population of any sex or ethnicity with NAFLD, including NASH-related cirrhosis, diagnosed on the basis of biochemical, radiological, or histological evidence of fatty liver and exclusion of other competing causes of steatosis (Citation11). For issue 2, studies reporting at least sensitivity, specificity, and area under the receiver operator curve (AUROC) of non-invasive methods against liver biopsy (LB) were included.
Exclusion criteria: non-human studies, letters/case reports, studies enrolling < 10 subjects or subjects aged < 12 years, articles not reporting outcomes of interest or primary data (editorials, reviews), or using inadequate case definition. Non-invasive methods that were not internally (i.e. in the study presenting the method for the first time) or externally validated (i.e. by other studies) against LB were excluded.
Outcome measures
Issue 1: overall and cause-specific morbidity and mortality, including liver-related disease, cardiovascular disease (CVD) (defined as cardiac or cerebrovascular fatal and non-fatal events), and malignancy. Additionally, the risk of developing type 2 diabetes was evaluated.
Issue 2: sensitivity, specificity, negative and positive likelihood ratios (LR+, LR-), and AUROC curves of non-invasive methods against liver biopsy (LB).
Data extraction and quality assessment
Data were extracted from each study independently and in duplicate by two authors (G.M., G.P.); discrepancies were resolved by mutual discussion. The agreement between the two reviewers for selection and validity assessment of trials was decided by kappa coefficient. For issue 1, the analysis was carried out in concordance with the MOOSE Guidelines (Citation10).
Methodological quality of observational and diagnostic accuracy studies was assessed by the 22-item STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) and the 25-item STARD (Standards for Reporting of Diagnostic Accuracy) check-lists, respectively (Citation12,Citation13). The following items were specifically incorporated into the STARD check-list: time elapsed between LB and non-invasive test (≤ 6 months versus > 6 months), sample representative of the typical NAFLD population, blinding of LB-reading pathologist and of non-invasive test researcher, LB processed and scored according to standard criteria, adequate biopsy specimen (fragment length ≥ 1.5 cm, with > 6 portal tracts), compliance with pre-analytical/analytical procedures, interquartile range/median ratio measures < 30% (for Fibroscan).
Data synthesis and analysis
We used WinBUGS 1.4 (WinBUGS 1996–2003, Imperial College of Science & MRC, UK). The analysis was carried out in concordance with the Cochrane Handbook of Systematic Reviews.
Issue 1: We presented dichotomous variables as odds ratios (OR) with 95% confidence interval (CI). Data were stratified by sex whenever possible. The fixed-effect model was used, with significance set at P = 0.05. Statistical heterogeneity was assessed using the I2 statistic: when I2 values ≥ 50%, we used a random effects model and planned to explore characteristics of individual studies and those of subgroups of the main body of evidence. Sensitivity analyses were performed by removing one study at a time, and the meta-analysis was repeated to assess whether any one study significantly affected pooled estimates. The effect of duration of follow-up on study results was assessed by meta-regression. Publication bias was examined using funnel plots. Additionally, for issue 1 subgroup analysis was planned to assess the impact of metabolic syndrome on the association between NAFLD and cardio-metabolic outcomes (studies adjusting versus studies not adjusting for the presence of metabolic syndrome, overall or each component).
Issue 2: The diagnostic accuracy of non-invasive tests was analyzed by a bivariate random effects model to provide mean (95% CI) summary estimate of AUROC, sensitivity, specificity, and positive and negative likelihood ratio (LR+, LR-) (Citation14). The AUROCs of different non-invasive tests were compared with the non-parametric method by DeLong et al. (Citation15). Subgroup analysis was planned to assess the effect of each quality item of the STARD check-list, of different ethnic origin of study population (Caucasian versus non-Caucasian), of different degrees of obesity (non-obese versus obese versus morbidly obese subjects), on pooled estimates of diagnostic accuracy.
Strength of recommendation was rated according to the GRADE system: for each outcome four key domains (risk of bias, consistency, directness, and precision) were assessed, and the level of evidence was ranked as high, moderate, low, insufficient (Citation16). On this basis recommendations were rated as Level I (strong) or Level II (weak).
Results
The agreement between two reviewers for study selection was 0.89 and for quality assessment of trials was 0.90. The flow of study selection is reported in .
Figure 1. Evidence acquisition flow diagram. Quality scores of included studies are provided as median (range).
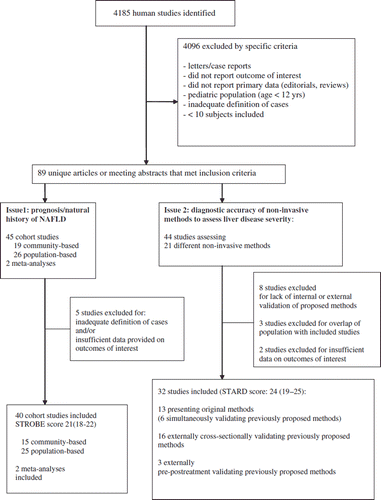
For issue 1, 45 cohort studies and 2 meta-analyses were retrieved: 5 studies were excluded for inadequate definition of cases and/or outcomes; eventually, 40 cohort studies (median, range STROBE score: 21, 18–22) and 2 meta-analyses were included.
For issue 2, 43 studies assessing 21 different non-invasive methods for assessing liver disease severity were retrieved: 8 studies presented methods that did not receive internal or external validation and were excluded, 2 studies overlapped for population with previously published studies, and 2 studies provided insufficient data on outcomes of interest. At the end of selection, 31 studies were included in the analysis for issue 2 (median STARD score 24, range 19–25).
Issue 1: what is the prognosis of NAFLD and its histological subtypes?
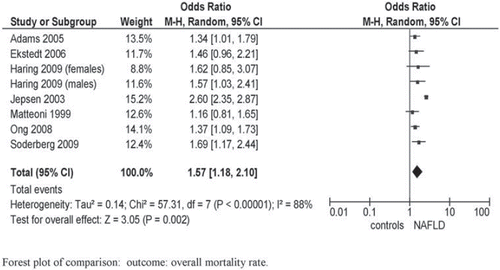
Overall and cause-specific mortality in NAFLD. We retrieved three population-based studies (the Third National Health and Nutrition Examination Survey (NHANES-III), the Danish National Registry of Patients, and the Study of Health in Pomerania (SHIP)) and four community-based studies, with median follow-up ranging from 7.3 to 24 years. The NHANES-III used biochemical definition of NAFLD, the others using ultrasonographic or histological definitions. Pooled overall mortality was higher in NAFLD compared to the general population: OR 1.57; 95% CI 1.18–2.10; I2 = 88%; P = 0.002 () (Citation17–24). Heterogeneity was high: after excluding the Danish study (Citation23), which less extensively adjusted for confounding variables, heterogeneity dropped down while pooled mortality ratio remained similar in magnitude and direction to overall effect: OR 1.40; 95% CI 1.23–1.60; I2 = 0%; P < 0.00001.
In population-based studies, liver disease was the third leading cause of death (13% of all deaths) in NAFLD after malignancy (28% of all deaths) and ischemic heart disease (25% of cases), compared to the eleventh in the general population (Citation21,Citation23). In the NHANES-III the burden of NAFLD was particularly relevant among the 45–54-years age group, with a standardized mortality ratio of 4.40 (95% CI 1.27–13.23) for all-causes and of 8.15 (95% CI 2.00–33.20) for cardiovascular disease, after adjusting for conventional risk factors (Citation22).
In synthesis, the presence of NAFLD as assessed by different methods carried an increased overall mortality.
NAFLD and incident CVD. A total of 14 population-based and 9 community-based prospective studies (STROBE score ranging 19–22), using a biochemical or ultrasonographic/histological definition, assessed NAFLD as a predictor of CVD (mean duration of follow-up ranging 3.3–24 years) () (Citation4,Citation17,Citation25–60).
Table I. Prospective studies assessing the natural history of NAFLD, grouped according to definition of NAFLD (biochemical, radiological, or histological).
Pooled OR for incident CVD of highest versus lowest ALT and GGT quantiles and of ultrasonographic/histological NAFLD were 1.10 (95% CI 0.85–1.41; I2 = 52%; n comparisons = 6), 1.57 (95% CI 1.42–1.74; I2 = 12%; n comparisons = 10), and 2.05 (95% CI 1.81–2.31; I2 = 31%; n comparisons = 8), respectively (). Heterogeneity for ALT was high: after excluding the Hoorn study (Citation49), assessing exclusively coronary heart disease events, heterogeneity disappeared (I2 = 0%) while the magnitude and direction of the effect were unchanged (not shown).
Figure 3. NAFLD as a risk factor for incident CVD events. NAFLD was defined biochemically (increase in ALT and GGT levels, panel A) or by ultrasound or histology (panel B). Forest plot of comparison: Meta-analysis of multiple-adjusted results (OR of top versus bottom quantile of ALT and GGT, panel A, or ultrasound-histologically diagnosed NAFLD, panel B) as determinants of incident CVD, outcome: incident CVD. NAFLD was defined by biochemical criteria (ALT or GGT elevation, panel A) or by ultrasonographic-histological criteria (panel B). OR adjusted for multiple variables from different community-based or population-based prospective studies () were pooled and analyzed by random or fixed effect models. *Studies assessing fatal CVD events.
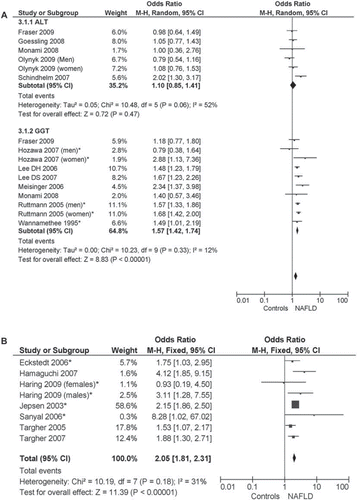
Restricting the analysis to studies adjusting for metabolic syndrome did not affect the magnitude and direction of overall effect: in studies with biochemical definition of NAFLD: in studies using GGT elevation: OR 1.30 (95% CI 1.03–1.83; I2 = 34%; n = 7); in ultrasonographic/histological studies: OR 1.75 (95% CI 1.38–2.23; I2 = 0%; n = 6).
Considering only studies assessing fatal CVD events (CVD mortality), the pooled OR of GGT and ultrasonographic/histological NAFLD were 1.59 (95% CI 1.42–1.78; I2 = 29%; P < 0.00001; n comparisons = 5), and 2.16 (95% CI 1.88–2.49; I2 = 0%; P < 0.00001; n comparisons = 5), respectively.
Funnel plots analysis found no strong publication bias (see online Supplementary Figures). There was also no evidence of an association between duration of follow-up and study results (all P values > 0.51).
In synthesis, the presence of NAFLD as assessed by GGT elevation or by ultrasonographic/histological methods, but not by ALT elevation, carried an increased CVD mortality.
NAFLD and incident type 2 diabetes. A total of 15 population-based and 9 community-based prospective studies assessed biochemical (defined as ALT or GGT elevation) or ultrasonographic-histological NAFLD as a predictor of incident diabetes (). Follow-up ranged 2–20 years, and STROBE score ranged 18–22.
The multiple-adjusted OR for incident diabetes of highest versus lowest ALT and GGT quantiles and of ultrasonographic/histological NAFLD were 1.95 (95% CI 1.63–2.33; I2 = 48%; n comparisons = 17), 2.71 (95% CI 2.30–3.20; I2 = 23%; n comparisons = 13), and 3.51 (95% CI 2.28–5.41; I2 = 70%; n comparisons = 3), respectively (). The heterogeneity of studies using ALT and ultrasonographic/histological definition was entirely due to the studies by Jiamjarasrangsi and by Okamoto, respectively (Citation33,Citation41): the first enrolled a selected community of employees of a University Hospital in Bangkok, and its findings may not be generalizable; the second assessed hyperglycemia (defined by fasting plasma glucose ≥ 110 mg/dL or HbA1c > 6.4%) as outcome, rather than diabetes. The exclusion of these two studies yielded the following results: OR for incident diabetes of ALT elevation: 1.92 (1.72–2.12; I2 = 9%; n comparisons = 16); OR for incident diabetes of ultrasonographic NAFLD: 4.25 (3.39–5.32; I2 = 0%; n comparisons = 2).
Figure 4. NAFLD as a risk factor for incident type 2 diabetes. NAFLD was defined biochemically (increase in ALT and GGT levels, panel A) or by ultrasound or histology (panel B). Forest plot of comparison: Meta-analysis of multiple-adjusted results (OR of top versus bottom quantile of ALT and GGT, panel A, or ultrasonographic NAFLD, panel B) as determinants of incident type 2 diabetes, outcome: incident type 2 diabetes. NAFLD was defined by biochemical criteria (ALT or GGT elevation, panel A) or by ultrasonographic criteria (panel B). OR adjusted for multiple variables from different community-based or population-based prospective studies () were pooled and analyzed by random or fixed effect models. *Studies assessing fatal CVD events.
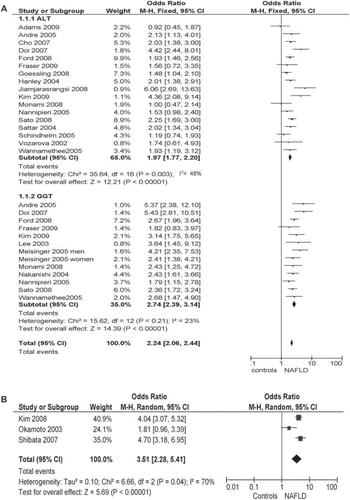
Restricting the analysis to studies adjusting for metabolic syndrome did not affect the magnitude and direction of overall effect: OR 2.08 (95% CI 1.76–2.45; I2 = 17%; n comparisons = 9) in studies with biochemical definition; among ultrasonographic/histological studies only that by Shibata adjusted for metabolic syndrome (Citation34).
No strong evidence was found for the presence of publication bias, or for an association between duration of follow-up and study results (all P values > 0.34).
In synthesis, the presence of NAFLD as assessed by biochemical or by ultrasonographic/histological methods carried an increased risk of developing type 2 diabetes mellitus.
Meta-analyses assessing CVD risk in NAFLD. A meta-analysis of five hospital-based and two population-based case-control studies (total 3,497 subjects) found a 3.13-fold (95% CI 1.75–5.58; P < 0.0002) increased risk of ultrasonography-detected carotid plaques in NAFLD (Citation61).
Other causes of death. Mortality from extrahepatic malignancy was not increased in NAFLD compared to the general population: OR 0.97 (95% CI 0.66–1.26; I2 = 43%; n comparisons = 8; not shown in detail). However, in a cohort of 337 diabetic patients the presence of NAFLD carried a 2.3-fold increased risk (95% CI 0.9–5.9) of dying from malignancy, after adjusting for age, smoking habits, obesity, diabetes duration, hyperlipidemia, earlier malignancy, and cardiovascular disease (Citation62). There were insufficient prospective data to assess the effect of NAFLD on mortality from hepatic malignancy.
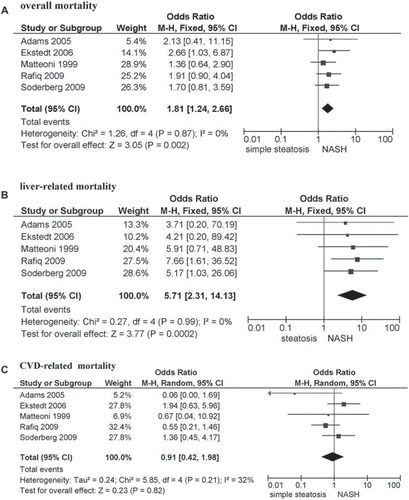
Prognosis of NAFLD histological subtypes (SS, NASH with different fibrosis stages). Five community-based studies (total 559 participants) assessed the prognosis of different histological subtypes of NAFLD (median follow-up ranging 7.6–24 years, STROBE score ranging 19–21) (). While the survival of patients with SS approached that of the general population, NASH patients had a higher overall mortality than those with SS (pooled OR 1.81; 95% CI 1.24–2.66; P = 0.002; I2 = 0%).
Liver disease was the main cause of death excess in NASH, with a liver-related mortality rate of 11%–17.5% compared to 1.7%–2.7% of SS (pooled OR of NASH compared to SS: 5.71; 95% CI 2.31–14.13; P = 0.0002; I2 = 0%) ().
Figure 5. Outcomes of NASH compared to simple steatosis for overall mortality (panel A), liver-related mortality (panel B), or cardiovascular disease (CVD) mortality (panel C). Forest plot of comparison of NASH versus simple steatosis, outcomes: overall mortality (panel A), liver-related mortality (panel B), CVD mortality (panel C). OR adjusted for variables from different community-based or population-based prospective studies () were pooled and analyzed by random or fixed effect models.
The rate of progression of liver disease was slow: NASH developed progressive fibrosis in 25%–30% of cases over 4 years and in 50% of cases over 6 years (Citation19,Citation20,Citation23,Citation63,Citation70,Citation71). In the Swedish cohort, 13% of patients with mild-to-moderate fibrosis (stage F1–2) developed cirrhosis, while 25% of patients with fibrosis stage F3 developed cirrhosis and end-stage liver disease (Citation20). Once cirrhosis developed, median survival approached 6 years; compared to viral cirrhosis, NASH cirrhosis had a similar liver-related mortality but a significantly higher CVD-related death (see below).
Beside NASH, the other factor affecting survival was the presence of advanced (stage F3–4) fibrosis. While NASH with milder (stage F0–2) fibrosis still had a higher liver-related mortality than SS (pooled OR 2.11; 95% CI 1.15–6.36; I2 = 0%; P = 0.02), the presence of advanced fibrosis further increased liver-related mortality compared to NASH with fibrosis stage F0–2: OR 10.06 (95% CI 4.35–23.25; I2 = 0%; n comparisons = 5; P = 0.00001; not shown in detail).
Cardiovascular disease accounted for the remaining mortality excess in NAFLD, but the difference between histological subtypes was non-significant: pooled OR for CVD mortality 0.91 (95% CI 0.42–1.98; P = 0.82; I2 = 32%). CVD remained an important cause of death even in advanced liver disease: in a 10-year follow-up of NASH-related cirrhoses CVD accounted for 28% of deaths (second leading cause of death after liver disease) versus the 2% of CVD-related deaths (sixth leading cause) in HCV-related cirrhosis (Citation64).
Only one study assessed the risk of diabetes of different NAFLD subtypes, finding an OR of 2.98 (95% CI 1.23–7.22; P = 0.01) in NASH compared to SS over 13 years (Citation20).
Sensitivity analysis. There was little or no heterogeneity in the meta-analysis of overall, liver-related, and CVD events, suggesting a consistent disease effect. No strong evidence was found for publication bias or for an association between duration of follow-up and study results (all P values > 0.21).
In synthesis NASH confers a higher liver-related mortality than SS, while mortality from CVD did not differ between NASH and SS. Only one study assessed the risk of diabetes of different NAFLD subtypes, finding an OR of 2.98 in NASH compared to SS.
Issue 2: how to assess the severity of liver disease in NAFLD?
The evidence presented above suggests that different NAFLD histological subtypes (SS, NASH, NASH with advanced fibrosis) have different prognoses, and the two NAFLD stages that should be identified early are NASH and NAFLD with advanced (stage ≥ F3) fibrosis. LB remains the gold standard to stage NAFLD, but it does have limitations that make it an unfeasible approach in all NAFLD subjects: it is costly, invasive, and has complications requiring hospitalization in 1%–3% cases and a procedural mortality of 0.01% (Citation65). Furthermore, LB suffers from intra- and inter-observer reading variability, and from sample inadequacy or variability due to uneven disease distribution, which leads to missing a diagnosis of NASH in up to 27% of cases (Citation66): paired liver biopsies showed a single biopsy has an AUROC, sensitivity, specificity, LR+, and LR− of at best 0.81 (95% CI 0.67–0.89), 0.73, 0.92, 8.62, and 0.30 for the diagnosis of NASH, and of 0.87 (95% CI 0.70–0.95), 0.85, 0.89, 7.73, and 0.16 for advanced (stage 3–4) fibrosis, with a discordance of one or more stages of 41% (Citation67,Citation68).
When taken alone, clinical factors (age ≥ 45 years, obesity, diabetes, metabolic syndrome, insulin resistance, triglycerides ≥ 150 mg/dL (1.7 mmol/L)) lack enough predictive value for advanced liver disease to be clinically useful (Citation69–73). Routine imaging techniques can assess liver fat but not necroinflammation and fibrosis. To overcome these limitations, several non-invasive approaches to assess liver disease severity in NAFLD have been proposed in an attempt to fulfill the requisites of the ideal non-invasive method: cost-effectiveness, ease-of-measurement, liver-specificity, reproducibility within patients and among different populations, and prognostic value. These approaches use radiological techniques or combine clinical and biochemical parameters into quantitative panels.
Among imaging techniques, liver stiffness measurement (LSM) by ultrasonography (Fibroscan) or magnetic resonance (MR) have been used to estimate the presence and severity of hepatic fibrosis. While MR elastography is not broadly available, is expensive, and has not been internally or externally validated yet (Citation17,Citation20,Citation54), ultrasonographic LSM by transient elastography (Fibroscan) is becoming increasingly used. The diagnostic accuracy of Fibroscan for significant (stage ≥ F2) or advanced (stage ≥ F3) fibrosis has been assessed in six studies in NAFLD (median, range STARD score: 24, 22–25) () (Citation74–79). The diagnostic accuracy and reproducibility of Fibroscan for advanced fibrosis were good and were unaffected by the severity of steatosis or inflammation (), but were hampered by the presence of obesity: in all studies obesity was an independent predictor of failure to measure liver stiffness (5%–13% of cases) and, consistently, in all studies mean patients’ BMI was < 30 kg/m2.
Table II. Panel A: Studies assessing biomarker panels validated for non-invasive assessment of the presence of NASH in patients with NAFLD. When > 1 study assessed the panel, summary estimates of diagnostic accuracy were calculated; when the panel was assessed in only 1 study, the AUROC is the summary estimate of the training and validation groups.
Table II. Panel B: Studies assessing biomarker panels validated for non-invasive assessment of fibrosis in patients with NAFLD. When > 1 study assessed the panel, summary estimates of diagnostic accuracy were calculated.
The other group of non-invasive methods to assess liver disease severity in NAFLD includes biomarker panels. Biomarker panels combine routinely assessed clinical variables with different biochemical parameters: routine biochemical tests, markers of hepatocyte apoptosis or of hepatic collagen matrix remodeling, and adipose tissue-released cytokines. Multiple regression analysis of these variables yielded equations predictive of the probability of a single patient having the target condition (i.e. NASH, advanced fibrosis) (Citation80–106).
A total of 21 non-invasive panels were identified; 13 were internally and/or externally validated in 32 studies and included in our meta-analysis (median, range STARD score of included studies 24, 19–25). Each study's characteristics and summary estimates of each test's diagnostic performance are reported in .
Five panels identified the presence of advanced fibrosis among patients with NAFLD. The mean AUROC of these panels ranged 0.80–0.90. The Fibrotest, enhanced liver fibrosis (ELF) panel, combined panel, and NAFLD fibrosis score had better diagnostic accuracy than the BARD (BMI, AST/ALT Ratio, Diabetes) score (P < 0.02 for each of the four panels versus BARD score), while the AUROC of these panels did not differ among each other and among each of these panels and Fibroscan.
Fibrotest, ELF, and combined panel received only internal validation in the original study, so their reproducibility across different populations is unknown. The NAFLD fibrosis score has been most extensively validated for detecting advanced fibrosis. This score combines seven routinely measured clinical-biochemical parameters into a score; two cut-offs with distinct (high and low) probability of advanced fibrosis are provided. This panel has been validated in 13 studies, including 2 multicenter trials from America and Japan, enrolling a total of 3,064 patients of different ethnicities (including American Caucasians, blacks, and Hispanics, Europeans, and Asians), ages, and obesity and diabetes status. The median STARD score of these studies is 24 (range 22–25). Summary estimates of AUROC, sensitivity, specificity, LR+, and LR− of NAFLD fibrosis score for advanced fibrosis are reported in and .
Figure 6. Summary ROC curve and summary point of plasma CK-18 for diagnosing NASH (panel A), and of NAFLD fibrosis score (low cut-off, panel B), NAFLD (high cut-off, panel C), and Fibroscan (panel D) for advanced fibrosis.
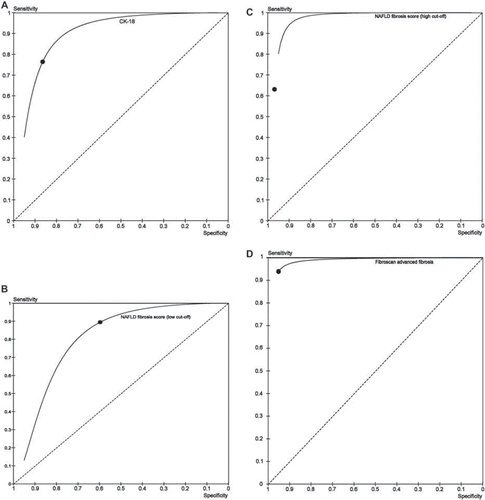
A shortcoming of this panel is that in different studies 20%–58% of all patients fell in-between the two cut-offs and could therefore not be classified as having a high or low probability of advanced fibrosis (indeterminate probability of advanced fibrosis).
Six biomarker panels for detecting NASH met inclusion criteria; their summary AUROC ranged from 0.79 for NASHtest to 0.86 for NASH Predictive Index (P = NS among AUROC of different tests). Five of these panels (NASHtest, NASH Predictive Index, Obesity-related NASH Diagnostics, NASH Clinical Score, NAFIC score) have currently been validated exclusively in the original studies, and two of them (Obesity-related NASH Diagnostics and NASH Clinical Score) were tested exclusively in morbidly obese subject candidates for bariatric surgery: their reproducibility and generalizability remain therefore unknown. Plasma ELISA-detected cytokeratin-18, a marker of hepatocyte apoptosis, is the only marker for NASH that has been externally validated in nine independent studies (median STARD score 25, range 23–25) enrolling a total of 856 NAFLD patients of different ethnicities, BMI, and diabetes status). Summary AUROC, sensitivity, specificity, LR+, and LR− are 0.82 (95% CI 0.76–0.88), 0.78 (0.65–0.91), 0.86 (0.75–0.97), 6.13 (5.09–7.17), and 0.28 (0.26–0.30) (; ). Even after excluding two studies enrolling morbidly obese subjects, not representative of the general NAFLD population, pooled estimates remained similar in magnitude to overall diagnostic accuracy (not shown). Notably, the multicenter NASH Clinical Research Network (CRN) study, the largest study so far on cytokeratin-18 fragments (CK-18), demonstrated that the addition of routinely available clinical-laboratory parameters to CK-18 measurement did not significantly improve its diagnostic performance.
An important limitation of these non-invasive methods is that they were all validated in a cross-sectional fashion, but their accuracy to monitor disease progression and treatment response remains largely unknown: only serum CK-18 and NASHtest/Fibrotest correlated accurately with post-treatment histological changes in three small trials (Citation93,Citation107,Citation108).
In synthesis, three non-invasive methods were independently validated against LB for detecting NASH (serum CK-18) and advanced fibrosis (Fibroscan and NAFLD fibrosis score).
Other diagnostic issues
Oral glucose tolerance test (OGTT). Some authors suggested an OGTT is indicated in all NAFLD patients without known diabetes, based on the association between NAFLD and postprandial hyperglycemia. Current American Diabetes Association (ADA) guidelines recommend fasting plasma glucose (FPG) as the initial screening test for diabetes and OGTT only in patients with impaired fasting plasma glucose (IFG) (i.e. FPG of 100–125 mg/dL, 5.6–6.9 mmol/L) or when diabetes is strongly suspected on the basis of associated clinical risk factors (Citation109). Five observational studies (total 472 NAFLD patients), all from Asian populations, with a median STROBE score of 19 (range 17–21), and one randomized controlled trial (RCT) (Citation110–115) assessed this issue: 16%–63% of newly diagnosed cases of diabetes were based on isolated 2-h glucose elevation, with non-diabetic FPG, and 29%–52% of patients had impaired glucose tolerance (IGT). Most of these newly diagnosed cases of diabetes had IFG or at least one clinical risk factor for diabetes, indicating the need for OGTT. Further well designed studies are needed to identify subgroups of patients who might benefit most from an OGTT before this test can be routinely recommended to all NAFLD patients.
Carotid ultrasound. A systematic review of cross-sectional studies suggested that routine measurement of carotid intima-media thickening (IMT) might be implemented in all NAFLD patients, as they have a 13% higher risk of carotid IMT and an OR for carotid plaques of 3.13 (95% CI 1.75–5.58) (Citation61). Awaiting further prospective confirmation, recent guidelines suggest a B-mode carotid ultrasonography is mostly cost-effective in patients without diabetes or established cardiovascular disease, at intermediate CVD risk (Framingham risk score 6%–20%) (Citation116).
Discussion
Principal findings
Our analysis found that NAFLD confers an increased overall mortality, deriving from CVD and liver disease, and an increased risk of developing diabetes. These associations hold even when adopting different definitions of hepatic steatosis and after adjusting for the presence of metabolic syndrome, a cluster of cardio-metabolic risk factors of which NAFLD is considered the hepatic manifestation.
An important finding is that CVD mortality does not significantly differ between SS and NASH: however, histologically studies assessed CVD mortality, not incident CVD, and patients with NASH may die from liver-related disease before they can develop cardiovascular complications. Therefore, whether cardiovascular risk differs between SS and NASH requires further investigation. Another important finding of our analysis is the different hepatological prognoses of histological subtypes of NAFLD: compared to SS, NASH has a higher liver-related mortality, further increased by the coexistence of advanced fibrosis.
Included studies were heterogeneous with respect to the definition of NAFLD (biochemical, radiological, histological). We addressed this issue by subgrouping studies according to different definitions. The low sensitivity of liver enzyme elevation (particularly ALT) for NAFLD may have attenuated the strength of the association between fatty liver and assessed outcomes. Consistently, in both community and population-based studies, radiological/histological definitions of NAFLD were more strongly associated with outcomes than biochemical definitions. Among liver enzymes, GGT was associated with increased cardiovascular risk, while ALT was not. This may be explained by the fact that ALT is more liver-specific but less sensitive for steatosis, while GGT is more sensitive but less specific for steatosis, and serum GGT levels may be a marker of increased systemic or vascular oxidative stress rather than simply of increased liver fat content. Whether the combination of imaging and biochemical markers of NAFLD may enhance outcome prediction in NAFLD needs further investigation: in the SHIP study, the addition of serum GGT to ultrasonographic steatosis enhanced prediction of future all-cause and cardiovascular mortality compared to either method alone (Citation24).
The different prognosis of NAFLD histological subtypes prompts screening all NAFLD patients for the presence of NASH, with or without advanced fibrosis. Despite its limitations, LB remains the gold standard for staging and monitoring NAFLD, but it is not feasible in all NAFLD patients.
Three non-invasive methods were independently validated to detect respectively NASH (ELISA-detected cytokeratin-18 fragments) and advanced fibrosis (NAFLD fibrosis score and Fibroscan).
NAFLD fibrosis score can be easily calculated with routine clinical parameters, has good diagnostic performance, is cheap, easy-to-calculate from routine parameters, and has been independently validated in populations of various ethnicities, BMI, and diabetic status. A shortcoming of this panel is that 20%–58% of all patients fall in-between the two cut-offs (indeterminate probability of advanced fibrosis) and could not therefore be classified.
Transient elastography (Fibroscan), the other externally validated method for detecting advanced fibrosis, is based on the principle than the stiffer the liver tissue, the faster the vibrations propagate through the tissue underlying the probe. Fibroscan measures liver stiffness in a cylindric volume of 1 per 4 cm, between 25 and 65 mm below the skin surface. Fibroscan accurately individuates advanced fibrosis in non-obese subjects, but cannot measure liver stiffness or may yield unreliable measures in obese patients due to the attenuation of ultrasound and elastic wave by the thick subcutaneous thoracic fat belt: in current studies mean BMI was < 30 kg/m2, and obesity was the strongest predictor of Fibroscan failure. Consistently, in a 5-year prospective study of 13,369 examinations, a BMI > 30 kg/m2 was associated with failed or unreliable LSM in 3.1%–15.8% of cases (Citation117). Therefore, the diagnostic performance of Fibroscan in obesity, which is commonly present in patients with NAFLD, warrants improvement: in this context, a pilot experience with newly developed Fibroscan probes for obese patients showed encouraging results (Citation118).
ELISA-detected serum CK-18 fragment is the only non-invasive marker differentiating NASH from SS that has been currently externally validated: its fair-to-good diagnostic accuracy (AUROC 0.82, sensitivity 0.78, specificity 0.87) precludes its use as the sole screening method, but it can be combined with other non-invasive methods or with LB to enhance prediction of progressive liver disease.
Implications for practice
The results of our analysis have important clinical implications: newly diagnosed NAFLD patients should be thoroughly assessed for their cardiovascular, metabolic, and liver-related risk (). Concerning the last-mentioned, the early individuation of different histological subtypes is mandatory for the management of NAFLD patients: while SS has a benign hepatological course and may be managed with the same measures targeting associated cardio-metabolic risk factors, progressive NASH has an increased liver-related mortality, especially if advanced fibrosis coexists, and requires hepatological referral, with different clinical purposes. NASH without advanced fibrosis requires earlier treatment with experimental therapies to reverse necroinflammatory changes (Citation119), while the coexistence of advanced fibrosis mandates tight monitoring for complications of cirrhosis (hepatocellular carcinoma, esophageal varices, liver failure), pending the discovery of effective anti-fibrotic therapies.
Table III. Suggested assessment of patients with NAFLD for general physicians.
Currently, no non-invasive panel alone is accurate enough to replace LB (still the gold standard despite its limitations), and a clinically feasible approach could combine non-invasive panels with LB to biopsy only patients with a high probability of progressive disease after non-invasive assessment, thus reducing the need for LB. Our analysis suggests two non-invasive methods can be used to individuate patients with a high probability of advanced fibrosis, while serum CK-18 has fair accuracy to detect NASH.
The optimal time interval for monitoring non-biopsied patients is unknown: mean (± SD) rate of fibrosis progression in untreated subjects has been found to be 0.03 ± 0.40 stages/year in overall NASH and 0.41 ± 0.40 stages/year in those NASH patients who progressed (38% of overall cases of NASH) (Citation119). Assuming these figures can be extrapolated to NASH patients under treatment, non-invasive monitoring for advanced fibrosis may be repeated every 1–2 years.
Implications for research
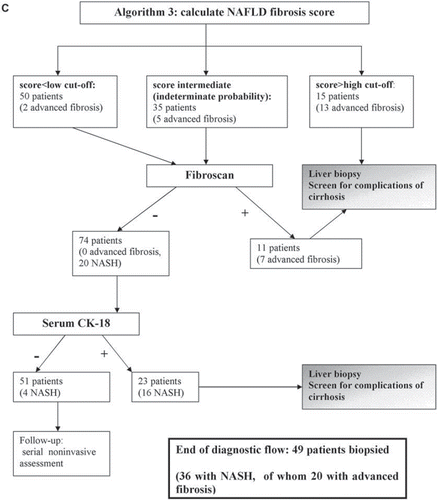
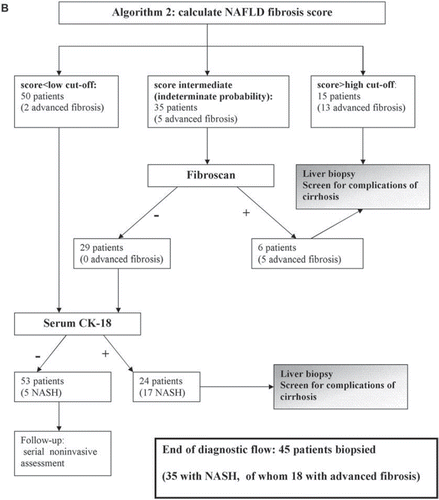
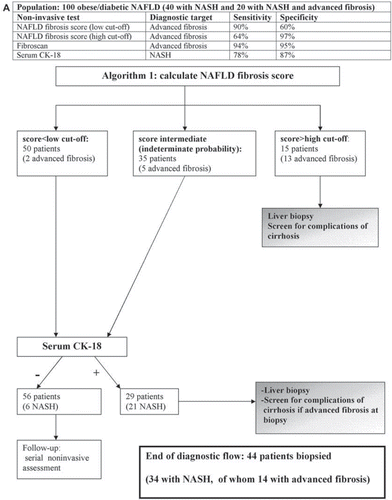
Since no non-invasive panel alone can replace biopsy, a strategy combining non-invasive methods with LB may optimally individuate patients with NASH, with or without fibrosis, and reduce the need for LB: three sequential diagnostic algorithms are presented in . The initial step is the calculation of NAFLD fibrosis score, which is feasible in all patients and, if yielding a high risk of advanced fibrosis, can reliably direct patients to a gastroenterologist for evaluation of cirrhosis. The subsequent step is the assessment of the probability of having NASH by serum CK-18. Fibroscan can be added to NAFLD fibrosis score to further screen for advanced fibrosis in non-obese subjects. Adopting one of these three algorithms, 85%, 88%, and 90%, respectively, of patients with NASH could be individuated, while 22%, 23%, and 26% of patients with SS would (unnecessarily) be biopsied.
Figure 7. Proposed diagnostic algorithms combining non-invasive methods and liver biopsy. Algorithm 1 can be applied to any patient with NAFLD; algorithm 2 and 3 can be applied only to non-obese patients with NAFLD, since Fibroscan has limitations when BMI > 30 kg/m2. In the third algorithm Fibroscan is applied to patients with a low and intermediate score for fibrosis, which implies a wider availability of Fibroscan, has the advantage of individuating all patients with advanced fibrosis, but it biopsies more patients with simple steatosis than the first model (13 versus 10 patients).
All models are conservative estimates of diagnostic performance of NAFLD fibrosis score and Fibroscan, i.e. they assume that all biopsied patients without advanced fibrosis in the first 2 steps have simple steatosis, not NASH, since the diagnostic performance of NAFLD fibrosis score and Fibroscan for detecting NASH is currently unknown.
Explanation: Based on our meta-analysis, three sequential diagnostic algorithms are proposed to individuate NASH, with and without advanced fibrosis; such algorithms require further prospective evaluation in large-scale multicenter cohort studies.
If a sample of 100 NAFLD subjects underwent a single LB, NASH would be found in 40 subjects, of which 20 would have advanced fibrosis (LB-to-all approach). In combined approaches, the first step should be the identification of patients with NASH and advanced fibrosis, requiring prompt gastroenterological referral. To this aim, NAFLD fibrosis score and Fibroscan may be combined with LB.
If we apply NAFLD fibrosis score to our NAFLD sample, this would direct 15 patients (13 with advanced fibrosis) to biopsy and leave 50 patients with a low probability and 35 patients with an indeterminate probability. If patients are obese, then the next step would target NASH with CK-18 measurement. At the end of diagnostic flow, 44 patients would be referred for LB (algorithm 1).
In non-obese subjects Fibroscan is reliable and accurate and may be applied after NAFLD fibrosis score to screen for advanced fibrosis patients with an indeterminate probability (algorithm 2) or with both an indeterminate and low probability (algorithm 3), depending on the local availability and experience.
At the end of the diagnostic flow, algorithm 2 would direct 45 patients (25 NASH, of whom 18 with advanced fibrosis) to LB; algorithm 3 would direct 49 patients (35 NASH, of whom 20 with advanced fibrosis) to LB.
Future research should also evaluate non-invasive or combined approaches not only for screening NASH with or without advanced fibrosis, but also for monitoring the course of disease and treatment response. Follow-up studies should also assess the prognostic value of non-invasive methods for predicting clinical outcomes, including overall and liver-specific morbidity and mortality: promising data have currently come from the ELF panel and Fibrotest, which were able to predict long-term prognosis more accurately than LB in chronic hepatitis C and primary biliary cirrhosis (Citation120–122).
Funnel plots of studies assessing the natural history of NAFLD
Download PDF (302.7 KB)Acknowledgements
This work would have never been possible without the help of the following authors, whom we thank for their courtesy in providing details about their published studies. None of these persons received compensation for the work performed.
Peter Jepsen (Department of Clinical Epidemiology, Aarhus University Hospital, Denmark), Zobair Younossi (Center for Liver Diseases, Inova Fairfax Hospital, Falls Church, Virginia 22042, USA), Nila Rafiq (Center for Liver Diseases, Inova Fairfax Hospital Falls Church, VA 22042, USA), Leon Adams (School of Medicine and Pharmacology, University of Western Australia, Nedlands, WA, Australia), Cecilia Soderberg (Department of Medicine, Karolinska Institute, Solna, Stockholm, Sweden), Mattias Ekstedt (Division of Gastroenterology and Hepatology, Department of Molecular and Clinical Medicine, University Hospital, Linköping, Sweden), Stergios Kechagias (Division of Internal Medicine, Department of Medicine and Care, University Hospital, Linköping, Sweden), Praveen Guturu (Department of Internal Medicine, UTMB, Galveston, Texas, USA), Raza Malik (Department of Medicine, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, Massachusetts, USA), Hideki Fujii (Department of Hepatology, Osaka City University, Osaka, Japan), Dana Crisan (University of Medicine and Pharmacy ‘Iuliu Hatieganu’, Cluj-Napoca, Romania), Richard Parker (University Hospitals of Bristol, NHS Foundation Trust, Bristol, UK), Noriyuki Obara and Yoshiyuki Ueno (Division of Gastroenterology, Tohoku University Graduate School of Medicine, Aoba, Sendai 980-8574, Japan), and Jason Chang (Department of Gastroenterology & Hepatology, Singapore General Hospital, Singapore).
Declaration of interest: No author has any present or past conflict of interest to report. Giovanni Musso is independent of any commercial funder, performed the statistical analysis, wrote the manuscript, had full access to all the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis. This work was supported in part by the Piedmont Region Funds Comitato Interministeriale per la Programmazione Economica 2008, which were employed for data collection.
References
- Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202–19.
- Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, . Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–8.
- Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dyonisos nutrition and liver study. Hepatology. 2005;42:44–52.
- Targher G, Bertolini L, Padovani R, Rodella S, Tessari R, Zenari L, . Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007;30:1212–8.
- Mathurin P, Hollebecque A, Arnalsteen L, Buob D, Leteurtre E, Caiazzo R, . Prospective study of the long-term effects of bariatric surgery on liver injury in patients without advanced disease. Gastroenterology. 2009;137:532–40.
- Silverman JF, Pories WJ, Caro JF. Liver pathology in diabetes mellitus and morbid obesity. Clinical, pathological, and biochemical considerations. Pathol Annu. 1989;24 Pt 1:275–302.
- Mummadi RR, Kasturi KS, Chennareddygari S, Sood GK. Effect of bariatric surgery on non-alcoholic fatty liver disease (NAFLD): systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2008;6:1396–402.
- Baumeister SE, Völzke H, Marschall P, John U, Schmidt CO, Flessa S, . Impact of fatty liver disease on health care utilization and costs in a general population: a 5-year observation. Gastroenterology. 2008;134:85–94.
- Charlton M. Nonalcoholic fatty liver disease: a review of current understanding and future impact. Clin Gastroenterol Hepatol. 2004;2:1048–58.
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, . Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283:2008–12.
- Ruttmann E, Brant LJ, Concin H, Diem G, Rapp K, Ulmer H. Gamma-glutamyltransferase as a risk factor for cardiovascular disease mortality: an epidemiological investigation in a cohort of 163,944 Austrian adults. Circulation. 2005; 112:2130–7.
- von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007; 147:573–7.
- Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, . Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. Clin Biochem. 2003;36:2–7.
- Leeflang MG, Deeks JJ, Gatsonis C, Bossuyt PMM. Systematic reviews of diagnostic test accuracy. Ann Intern Med. 2008;149:889–97.
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics. 1988;44:837–45.
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6.
- Söderberg C, Stål P, Askling J, Glaumann H, Lindberg G, Marmur J, . Decreased survival of subjects with elevated liver function tests during a 28 year follow up. Hepatology. 2010;51:595–602.
- Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, . The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–21.
- Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–9.
- Ekstedt M, Franzén LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, . Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–73.
- Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J Hepatology. 2008;49:608–12.
- Dunn W, Xu R, Wingard DL, Rogers C, Angulo P, Younossi ZM, . Suspected nonalcoholic fatty liver disease and mortality risk in a population-based cohort study. Am J Gastroenterol. 2008;103:2263–71.
- Jepsen P, Vilstrup H, Mellemkjaer L, Thulstrup AM, Olsen JH, Baron JA, . Prognosis of patients with a diagnosis of fatty liver—a registry-based cohort study. Hepatogastroenterology. 2003;50:2101–4.
- Haring R, Wallaschofski H, Nauck M, Dörr M, Baumeister SE, Völzke H. Ultrasonographic hepatic steatosis increases prediction of mortality risk from elevated serum gamma-glutamyl transpeptidase levels. Hepatology. 2009;50:1403–11.
- Wannamethee G, Ebrahim S, Shaper AG. Gamma-glutamyltransferase: determinants and association with mortality from ischemic heart disease and all causes. Am J Epidemiol. 1995;142:699–708.
- Lee DH, Ha MH, Kim JH, Christiani DC, Gross MD, Steffes M. Gamma-glutamyltransferase and diabetes—a 4 year follow-up study. Diabetologia. 2003;46:359–64.
- Sattar N, Scherbakova O, Ford I, O'Reilly DS, Stanley A, Forrest E, . Elevated alanine aminotransferase predicts new-onset type 2 diabetes independently of classical risk factors, metabolic syndrome, and C-reactive protein in the west of Scotland coronary prevention study. Diabetes. 2004;53:2855–60.
- Nakanishi N, Suzuki K, Tatara K. Serum γ-glutamyltransferase and risk of metabolic syndrome and type 2 diabetes in middle-aged Japanese men. Diabetes Care. 2004;27: 1427–32.
- Schindhelm RK, Dekker JM, Nijpels G, Heine RJ, Diamant M. No independent association of alanine aminotransferase with risk of future type 2 diabetes in the Hoorn study. Diabetes Care. 2005;28:2812.
- Hozawa A, Okamura T, Kadowaki T, Murakami Y, Nakamura K, Hayakawa T, . gamma-Glutamyltransferase predicts cardiovascular death among Japanese women. Atherosclerosis. 2007;194:498–504.
- Wannamethee SG, Shaper AG, Lennon L, Whincup PH. Hepatic enzymes, the metabolic syndrome, and the risk of type 2 diabetes in older men. Diabetes Care. 2005;28:2913–8.
- Kim CH, Park JY, Lee KU, Kim JH, Kim HK. Fatty liver is an independent risk factor for the development of Type 2 diabetes in Korean adults. Diabet Med. 2008;25:476–81.
- Okamoto M, Takeda Y, Yoda Y, Kobayashi K, Fujino MA, Yamagata Z. The association of fatty liver and diabetes risk. J Epidemiol. 2003;13:15–21.
- Shibata M, Kihara Y, Taguchi M, Tashiro M, Otsuki M. Nonalcoholic fatty liver disease is a risk factor for type 2 diabetes in middle-aged Japanese men. Diabetes Care. 2007;30:2940–4.
- Doi Y, Kubo M, Yonemoto K, Ninomiya T, Iwase M, Tanizaki Y, . Liver enzymes as a predictor for incident diabetes in a Japanese population: the Hisayama study. Obesity. 2007;15:1841–50.
- André P, Balkau B, Born C, Royer B, Wilpart E, Charles MA, . Hepatic markers and development of type 2 diabetes in middle aged men and women: a three-year follow-up study. Diabetes Metab. 2005;31:542–50.
- Kim CH, Park JY, Lee KU, Kim JH, Kim HK. Association of serum gamma-glutamyltransferase and alanine aminotransferase activities with risk of type 2 diabetes mellitus independent of fatty liver. Diabetes Metab Res Rev. 2009; 25:64–9.
- Ebrahim S, Sung J, Song YM, Ferrer RL, Lawlor DA, Davey Smith G. Serum cholesterol, haemorrhagic stroke, ischaemic stroke, and myocardial infarction: Korean national health system prospective cohort study. BMJ. 2006;33:22.
- Cho NH, Jang HC, Choi SH, Kim HR, Lee HK, Chan JC, . Abnormal liver function test predicts type 2 diabetes: a community-based prospective study. Diabetes Care. 2007;30:2566–8.
- Ford ES, Schulze MB, Bergmann MM, Thamer C, Joost HG, Boeing H. Liver enzymes and incident diabetes: findings from the European Prospective Investigation Into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes Care. 2008;31:1138–43.
- Jiamjarasrangsi W, Sangwatanaroj W, Lohsoonthorn V, Lertmaharit S. Increased alanine aminotransferase level and future risk of type 2 diabetes and impaired fasting glucose among the employees in a university hospital in Thailand. Diabetes Metab. 2008;34:283–9.
- Monami M, Bardini G, Lamanna C, Pala L, Cresci B, Francesconi P, . Liver enzymes and risk of diabetes and cardiovascular disease: results of the Firenze Bagno a Ripoli (FIBAR) study. Metabolism. 2008;57:387–92.
- Hanley AJ, Williams K, Festa A, Wagenknecht LE, D'Agostino RB Jr, Kempf J, . Elevations in markers of liver injury and risk of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes. 2004;53:2623–32.
- Nannipieri M, Gonzales C, Baldi S, Posadas R, Williams K, Haffner SM, . Liver enzymes, the metabolic syndrome, and incident diabetes: The Mexico City diabetes study. Diabetes Care. 2005;28:1757–62.
- Vozarova B, Stefan N, Lindsay RS, Saremi A, Pratley RE, Bogardus C, . High alanine aminotransferase is associated with decreased hepatic insulin sensitivity and predicts the development of type 2 diabetes. Diabetes. 2002;51:1889–95.
- Sato KK, Hayashi T, Nakamura Y, Harita N, Yoneda T, Endo G, . Liver enzymes compared with alcohol consumption in predicting the risk of type 2 diabetes: the Kansai Healthcare Study. Diabetes Care. 2008;31:1230–6.
- Fraser A, Harris R, Sattar N, Ebrahim S, Davey Smith G, Lawlor DA. Alanine aminotransferase, gamma glutamyltransferase and incident diabetes: The British Women's Heart and Health Study and meta-analysis. Diabetes Care. 2009;32:741–50.
- Targher G, Bertolini L, Rodella S, Tessari R, Zenari L, Lippi G, . Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabetes Care. 2007;30:2119–21.
- Schindhelm RK, Dekker JM, Nijpels G, Bouter LM, Stehouwer CD, Heine RJ, . Alanine aminotransferase predicts coronary heart disease events: a 10-year follow-up of the Hoorn Study. Atherosclerosis. 2007;191:391–6.
- Lee DS, Evans JC, Robins SJ, Wilson PW, Albano I, Fox CS, . Gamma glutamyl transferase and metabolic syndrome, cardiovascular disease, and mortality risk: the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2007;27:127–33.
- Fraser A, Harris R, Sattar N, Ebrahim S, Smith GD, Lawlor DA. Gamma-glutamyltransferase is associated with incident vascular events independently of alcohol intake: analysis of the British Women's Heart and Health Study and Meta-Analysis. Arterioscler Thromb Vasc Biol. 2007;27:2729–35.
- Targher G, Bertolini L, Poli F, Rodella S, Scala L, Tessari R, . Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes. 2005;54:3541–6.
- Lee DH, Silventoinen K, Hu G, Jacobs DR Jr, Jousilahti P, Sundvall J, . Serum gamma-glutamyltransferase predicts non-fatal myocardial infarction and fatal coronary heart disease among 28,838 middle-aged men and women. Eur Heart J. 2006;27:2170–6.
- Rafiq N, Bai C, Fang Y, Srishord M, McCullough A, Gramlich T, . Long-term follow-up of patients with nonalcoholic fatty liver. Clin Gastroenterol Hepatol. 2009; 7:234–8.
- Hamaguchi M, Kojima T, Takeda N, Nagata C, Takeda J, Sarui H, . Nonalcoholic fatty liver disease is a novel predictor of cardiovascular disease. World J Gastroenterol. 2007;13:1579–84.
- Meisinger C, Lowel H, Heier M, Schneider A, Thorand B. Serum γ-glutamyltransferase and risk of type 2 diabetes mellitus in men and women from the general population. J Intern Med. 2005;258:527–35.
- Meisinger C, Doring A, Schneider A, Lowel H. Serum γ-glutamyltransferase is a predictor of incident coronary events in apparently healthy men from the general population. Atherosclerosis. 2006;189:297–302.
- Goessling W, Massaro JM, Vasan RS, D'Agostino RB Sr, Ellison RC, Fox CS. Aminotransferase levels and 20-year risk of metabolic syndrome, diabetes, and cardiovascular disease. Gastroenterology. 2008;135:1935–44.
- Olynyk JK, Knuiman MW, Divitini ML, Davis TM, Beilby J, Hung J. Serum alanine aminotransferase, metabolic syndrome, and cardiovascular disease in an Australian population. Am J Gastroenterol. 2009;104:1715–22.
- Adams LA, Waters OR, Knuiman MW, Elliott RR, Olynyk JK. NAFLD as a risk factor for the development of diabetes and the metabolic syndrome: an eleven-year follow-up study. Am J Gastroenterol. 2009;104:861–7.
- Sookoian S, Pirola CJ. Nonalcoholic fatty liver disease is strongly associated with carotid atherosclerosis: A systematic review. J Hepatology. 2008;49:600–7.
- Adams LA, Harmsen S, St Sauver JL, Charatcharoenwitthaya P, Enders FB, Therneau T, . Nonalcoholic fatty liver disease increases risk of death among patients with diabetes: a community-based cohort study. Am J Gastroenterol. 2010;105:1567–73.
- Dam-Larsen S, Franzmann M, Andersen IB, Christoffersen P, Jensen LB, Sørensen TI, . Long term prognosis of fatty liver: risk of chronic liver disease and death. Gut. 2004; 53:750–5.
- Sanyal AJ, Banas C, Sargeant C, Luketic VA, Sterling RK, Stravitz RT, . Similarities and differences in outcomes of cirrhosis due to nonalcoholic steatohepatitis and hepatitis C. Hepatology. 2006;43:682–9.
- Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. Liver biopsy. Hepatology. 2009;49:1017–44.
- Poynard T, Munteanu M, Imbert-Bismut F, Charlotte F, Thabut D, Le Calvez S, . Prospective analysis of discordant results between biochemical markers and biopsy in patients with chronic hepatitis C. Clin Chem. 2004;50:1344–55.
- Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, . LIDO Study Group. Sampling variability of liver biopsy in non-alcoholic fatty liver disease. Gastroenterology. 2005;128:1898–906.
- Arubn J, Jhala N, Lazenby AJ, Clements R, Abrams GA. Influence of liver biopsy heterogeneity and diagnosis on non-alcoholic steatohepatitis in subjects undergoing gastric bypass. Obesity Surg. 2007;17:155–61.
- Fracanzani AL, Valenti L, Bugianesi E, Andreoletti M, Colli A, Vanni E, . Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: a role for insulin resistance and diabetes. Hepatology. 2008;48:792–8.
- Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with non-alcoholic steatohepatitis. Hepatology. 1999;30:1356–62.
- Ratziu V, Giral P, Charlotte F, Bruckert E, Thibault V, Theodorou I, . Liver fibrosis in overweight patients. Gastroenterology. 2000;118:1117–23.
- Haukeland JW, Konopski Z, Linnestad P, Azimy S, Marit Løberg E, Haaland T, . Abnormal glucose tolerance is a predictor of steatohepatitis and fibrosis in patients with non-alcoholic fatty liver disease. Scand J Gastroenterol. 2005;40:1469–77.
- Ryan MC, Wilson AM, Slavin J, Best JD, Jenkins AJ, Desmond PV. Associations between liver histology and severity of the metabolic syndrome in subjects with nonalcoholic fatty liver disease. Diabetes Care. 2005;28:1222–4.
- Kelleher T, MacFarlane C, de Ledinghen V, Beaugrand M, Foucher J, Castera L, . Risk factors and hepatic elastography (FibroScan) in the prediction of hepatic fibrosis in non-alcoholic steatohepatitis. Gastroenterology. 2006;130:A736.
- Yoneda M, Yoneda M, Mawatari H, Fujita K, Endo H, Iida H, . Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with nonalcoholic fatty liver disease (NAFLD). Dig Liver Dis. 2008;40:371–8.
- Nobili V, Vizzutti F, Arena U, Abraldes JG, Marra F, Pietrobattista A, . Accuracy and reproducibility of transient elastography for the diagnosis of fibrosis in pediatric nonalcoholic steatohepatitis. Hepatology. 2008;48:442–8.
- Wong VW, Vergniol J, Wong GL, Foucher J, Chan HL, Le Bail B, . Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51:454–62.
- Obara N, Ueno Y, Fukushima K, Nakagome Y, Kakazu E, Kimura O, . Transient elastography for measurement of liver stiffness measurement can detect early significant hepatic fibrosis in Japanese patients with viral and nonviral liver diseases. J Gastroenterol. 2008;43:720–8.
- Chang PE, Lui HF, Chau YP, Lim KH, Yap WM, Tan CK, . Prospective evaluation of transient elastography for the diagnosis of hepatic fibrosis in Asians: comparison with liver biopsy and aspartate transaminase platelet ratio index. Aliment Pharmacol Ther. 2008;28:51–61.
- Poynard T, Ratziu V, Charlotte F, Messous D, Munteanu M, Imbert-Bismut F. Diagnostic value of biochemical markers (Nash Test) for the prediction of non-alcoholic steatohepatitis in patients with non-alcoholic fatty liver disease. BMC Gastroenterology. 2006;6:34.
- Wieckowska A, Zein NN, Yerian LM, Lopez AR, McCullough AJ, Feldstein AE. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in non-alcoholic fatty liver disease. Hepatology. 2006;44:27–33.
- Younossi ZM, Jarrar M, Nugent C, Randahawa M, Afendy M, Stepanova M. A novel diagnostic biomarker panel for obesity-related non-alcoholic steatohepatitis (NASH). Obes Surg. 2008;18:1430–7.
- Feldstein AE, Wieckowska A, Lopez AR, Liu YC, Zein NN, McCullough AJ. Cytokeratin-18 fragment levels as noninvasive biomarker for nonalcoholic steatohepatitis: A multicenter validation study. Hepatology. 2009;50:1072–8.
- Yilmaz Y, Dolar E, Ulukaya E, Akgoz S, Keskin M, Kiyici M, . Solubile forms of extracellular cytokeratin 18 may differentiate simple steatosis from non-alcoholic steatohepatitis. World J Gastroenterol. 2007;13:837–44.
- Diab DL, Yerian L, Schauer P, Kashyap SR, Lopez R, Hazen SL, . Cytokeratin 18 fragment levels as a noninvasive biomarker for nonalcoholic steatohepatitis in bariatric surgery patients. Clin Gastroenterol Hepatol. 2008;6:1249–54.
- Malik R, Chang M, Bhaskar K, Nasser I, Curry M, Schuppan D, . The clinical utility of biomarkers and the nonalcoholic steatohepatitis CRN liver biopsy scoring system in patients with nonalcoholic fatty liver disease. J Gastroenterol Hepatol. 2009;24:564–8.
- Zein CO, Edmison JM, Schluchter M, Feldstein AE, Zein NN, McCullough AJ. A NASH predictive index (NPI) for use in patients with non-alcoholic fatty liver disease. Hepatology. 2007;46(S1):747A.
- Campos GM, Bambha K, Vittinghoff E, Rabl C, Posselt AM, Ciovica R, . A clinical scoring system for predicting nonalcoholic steatohepatitis in morbidly obese patients. Hepatology. 2008;47:1916–23.
- Ratziu V, Massard J, Charlotte F, Messous D, Imbert-Bismut F, Bonyhay L, . Diagnostic value of biochemical markers (FibroTest-FibroSURE) for the prediction of liver fibrosis in patients with non-alcoholic fatty liver disease. BMC Gastroenterology. 2006;6:6.
- Adams LA, Gorge J, Rossi E, van der Poorten D, Kench JG, DeBoer B, . Non-invasive prediction of liver fibrosis in nonalcoholic fatty liver disease. Hepatology. 2008;48:520A.
- Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, . The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–54.
- Guha IN, Parkes J, Roderick P, Chattopadhyay D, Cross R, Harris S, . Noninvasive markers of fibrosis in non-alcoholic fatty liver disease: validating the European liver fibrosis panel and exploring simple markers. Hepatology. 2008;47:455–60.
- Tabesh A, Duan Z, Kleiner DE, Wright EC, Loomba R, Liang TJ, . Serum caspase-3 generated cytokeratin 18 fragments (CK-18) as a marker for non-alcoholic steatohepatitis (NASH) and response to therapy. Hepatology. 2008;48(S1):803A.
- Calès P, Lainé F, Boursier J, Deugnier Y, Moal V, Oberti F, . Comparison of blood tests for liver fibrosis specific or not to NAFLD. J Hepatol. 2009;50:165–73.
- Nobili V, Parkes J, Bottazzo G, Marcellini M, Cross R, Newman D, . Performance of ELF serum markers in predicting fibrosis stage in pediatric non-alcoholic fatty liver disease. Gastroenterology. 2009;136:160–7.
- Wong VW, Wong GL, Chim AM, Tse AM, Tsang SW, Hui AY, . Validation of the NAFLD fibrosis score in a Chinese population with low prevalence of advanced fibrosis. Am J Gastroenterol. 2008;103:1682–8.
- Qureshi K, Clements RH, Abrams GA. The utility of the ‘NAFLD fibrosis score’ in morbidly obese subjects with NAFLD. Obes Surg. 2008;18:264–70.
- Harrison SA, Oliver D, Arnold HL, Gogia S, Neuschwander-Tetri BA. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut. 2008;57:1441–7.
- Guturu P, Steffer K, Petersen JR, Snyder N. A risk index for the estimation of fibrosis in non alcoholic fatty liver disease (NAFLD): comparison with the Mayo Score and the AST platelet ratio index (APRI). Hepatology. 2008;48(4S):522A.
- Musso G, Gambino R, Durazzo M, Cassader M. Noninvasive assessment of liver disease severity with liver fat score and CK-18 in NAFLD: Prognostic value of liver fat equation goes beyond hepatic fat estimation. Hepatology. 2010;51:715–7.
- Crisan D, Grigorescu MD, Grigorescu D, Feier D, Saplacan M, Serban A. Cytokeratin 18 fragments levels and dehydroepiandrosterone: valuable markers of nonalcoholic fatty liver disease. 2009 EASL Special Conference on NAFLD/NASH and related metabolic disorders abs 39 Page 122.
- Parker R, Collins P, McCune A. Can clinical scoring systems replace liver biopsy in non-alcoholic fatty liver disease? 2009 EASL Special Conference on NAFLD/NASH and related metabolic disorders abs 101 Page 189.
- Ruffillo GE, Fassio E, Alvarez E, Landeira G, Longo CG, Domnguez N, . Comparison of NAFLD fibrosis score and BARD score in predicting fibrosis in nonalcoholic fatty liver disease. Hepatology. 2009;50(S4):782A.
- Raszeja-Wyszomirska J, Szymanik B, Lawniczak M, Kajor M, Chwist A, Milkiewicz P, . Validation of the BARD scoring system in Polish patients with nonalcoholic fatty liver disease (NAFLD). BMC Gastroenterol. 2010;10:67.
- Sumida Y, Yoneda M, Hyogo H, Yamaguchi K, Ono M, Fujii H, . A simple clinical scoring system using ferritin, fasting insulin, and type IV collagen 7S for predicting steatohepatitis in nonalcoholic fatty liver disease. J Gastroenterol. 2010 Sep 15. [Epub ahead of print]. PMID: 20842510
- Fuji H, Enomoto M, Fukushima W, Tamori A, Sakaguchi H, Kawada N. Application of non-invasive laboratory tests for the assessment of fibrosis staging in Japanese patients with NAFLD. 2009 EASL Special Conference on NAFLD/NASH and related metabolic disorders abs 49 Page 134.
- Munteanu M, Poynard T, Charlotte F, Jacqueminet S, Messous D, Podevin P, . Utility of a combination of non-invasive biomarkers (Fibromax) in assessing the efficacy of rosiglitazone in a one year randomized, double-blind trial in non alcoholic steatohepatitis. Gastroenterology. 2007;132(4 S1):S283.
- Hollebecque A, Monteanu M, Arnalsteen L, Buob D, Leteurtre E, Caiazzo R, . Diagnostic value of liver injury biomarkers, fibrotest(FT), steatotest(ST), NASHtest(NT) in patients without advanced disease undergoing bariatric surgery. Hepatology. 2009;50(4 Suppl):793A.
- American Diabetes Association. Standards of medical care in diabetes—2009. Diabetes Care. 2009;32 Suppl 1:S13–61.
- Sargin M, Uygur-Bayramiçli O, Sargin H, Orbay E, Yayla A. Association of nonalcoholic fatty liver disease with insulin resistance: is OGTT indicated in nonalcoholic fatty liver disease? J Clin Gastroenterol. 2003;37:399–402.
- Su CC, Wang K, Hsia TL, Chen CS, Tung TH. Association of nonalcoholic fatty liver disease with abnormal aminotransferase and postprandial hyperglycemia. J Clin Gastroenterol. 2006;40:551–4.
- Wong VW, Hui AY, Tsang SW, Chan JL, Wong GL, Chan AW, . Prevalence of undiagnosed diabetes and postchallenge hyperglycaemia in Chinese patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2006;24:1215–22.
- Yun JW, Cho YK, Park JH, Kim HJ, Park DI, Sohn CI, . Abnormal glucose tolerance in young male patients with nonalcoholic fatty liver disease. Liver Int. 2009;29:525–9.
- Shiga T, Moriyoshi Y, Nagahara H, Shiratori K. Nonalcoholic fatty liver is a risk factor for postprandial hyperglycemia, but not for impaired fasting glucose. J Gastroenterol. 2009;44:757–64.
- Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, . A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297–307.
- Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, . Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21:93–111.
- Foucher J, Castera L, Bernard PH, Carvalho F, Allaix D, Merrouche W, . Pitfalls of liver stiffness measurement: A 5-year prospective study of 13 369 examinations. Hepatology. 2010;51:828–35.
- de Ledinghen V, Fournier C, Foucher J, Miette V, Vergniol J, Rigalleau V, . New Fibroscan probe for obese patients. A pilot study of feasibility and performances in patients with BMI>30 kg/m2. J Hepatol. 2009;50(Suppl 1):S359.
- Argo CK, Northup PG, Al-Osaimi AM, Caldwell SH. Systematic review of risk factors for fibrosis progression in non-alcoholic steatohepatitis. J Hepatol. 2009;51:371–9.
- Mayo MJ, Parkes J, Adams-Huet B, Combes B, Mills AS, Markin RS, . Prediction of clinical outcomes in primary biliary cirrhosis by serum enhanced liver fibrosis assay. Hepatology. 2008;48:1549–57.
- Ngo Y, Munteanu M, Dessous D, Charlotte F, Imbert-Bismut F, Thabut D, . A prospective analysis of the prognostic value of biomarkers (FibroTest) inpatients with chronic hepatitis C. Clin Chem. 2006;52:1887–96.
- Pimentel SK, Strobel R, Gonçalves CG, Sakamoto DG, Ivano FH, Coelho JCU. Evaluation of the nonalcoholic fat liver disease fibrosis score for patients undergoing bariatric surgery. Arq Gastroenterol. 2010;47:170–3.