Abstract
Background and aim. Chronotropic incompetence is risk marker of mortality in various populations, but its value in risk stratification of patients with a recent myocardial infarction (MI) is not known.
Methods. A consecutive series of 494 patients with a recent MI underwent a symptom-limited bicycle ergometer test and echocardiography before discharge from the hospital. Cardiac death was the primary end-point and sudden cardiac death (SCD) the secondary end-point. Heart rate (HR) response to exercise was evaluated using maximal chronotropic response index (CRI = 100 × (peak HR – resting HR) × (220 – age – resting HR)−1).
Results. During 8 years of follow-up, 40 patients (8.1%) experienced cardiac death, of whom 18 died suddenly (3.6%). Abnormal CRI (<39) was the most powerful predictor of the primary end-point with adjusted relative risk (RR) of 5.4 (95% CI 2.9–11.2; P < 0.001) and also a potent risk marker for SCD (adjusted RR 7.3; 95% CI 2.6–20.0; P < 0.001). Adjusted RR of decreased left ventricular ejection fraction (LVEF) (<45%) was 3.4 (95% CI 1.8–6.6; P < 0.001) for cardiac death. In the final predictive model of cardiac death, the removal of CRI decreased c-index from 0.817 to 0.778, whereas c-index was 0.791 after removal of LVEF.
Conclusions. Chronotropic incompetence is a powerful predictor of cardiac mortality among post-MI patients.
| Abbreviations | ||
| ACE | = | angiotensin-converting enzyme |
| AT II | = | angiotensin II |
| CABG | = | coronary artery bypass grafting |
| CI | = | confidence interval |
| CRI | = | maximal chronotropic response index |
| EC | = | exercise capacity |
| HR | = | heart rate |
| LVEF | = | left ventricular ejection fraction |
| MI | = | myocardial infarction |
| NYHA | = | New York Heart Association |
| PTCA | = | percutaneous transluminal coronary angioplasty |
| ROC | = | receiver operating characteristic |
| RR | = | relative risk |
| SCD | = | sudden cardiac death |
Key messages
Chronotropic incompetence is a powerful predictor of cardiac mortality in patients with a recent myocardial infarction.
Introduction
Abnormal cardiac autonomic function is an important risk factor for future cardiac mortality in various patient populations. Measurement of chronotropic response of heart rate (HR) to exercise is a widely used method for quantifying autonomic influences on the sino-atrial node. Previous studies have shown that chronotropic incompetence predicts future adverse cardiac events in both asymptomatic populations as well as in patients with various cardiac diseases (Citation1–9). Chronotropic response of HR to exercise provides information on the ability of both vagal and sympathetic activity to respond to increased metabolic demand via blood pressure regulation during exercise (Citation10–12).
HR response to exercise can be easily obtained from a routine exercise stress test. However, no previous studies have assessed the prognostic significance of chronotropic incompetence exclusively among patients with a recent myocardial infarction (MI) taking beta-blockers. In the current era of invasive treatment of MI, routine symptom-limited exercise testing is not used as frequently after a MI as it was in the past, when it was mainly used to detect exercise-induced myocardial ischemia (Citation13). Thus, the clinical significance of measuring HR behavior during exercise has been largely overlooked in current risk stratification among patients with a recent MI, while the well recognized importance of echocardiographic measures, such as left ventricular ejection fraction (LVEF), has been underscored in clinical practice (Citation14).
The purpose of the present study was to test whether chronotropic incompetence predicts cardiac death and sudden cardiac death (SCD) among patients with a recent MI who are on beta-blocking medication. Our secondary objective was to compare the prognostic significances of chronotropic incompetence and LVEF measured from echocardiography.
Methods
Subjects and study protocol
Consecutive series of patients with an acute MI (≤ 7 days) were enrolled in the Division of Cardiology, University of Oulu, Finland in 1996–2000. The aim was to assess the predictive power of various non-invasive and clinical risk markers of cardiac mortality among post-MI patients whose medical treatment had been optimized according to contemporary guidelines. The present protocol has been described previously in detail (Citation15–18). In the present study we extended the follow-up to increase the number of end-points and thereby the statistical power of the study. The exclusion criteria were inability to perform the exercise stress test, advanced age (> 75 years), unstable angina at the time of recruitment, beta-blockers not prescribed, dementia, alcoholism, drug abuse, or any condition that could impair the subject's capacity to give informed consent. The final study population consisted of a consecutive series of patients (n = 494) (). The Ethical Committee of the Northern Ostrobothnia Hospital District, Oulu, Finland, approved the protocol, and all the subjects gave written informed consent.
Table I. Characteristics of the study population according to maximal chronotropic response index.
All the tests were performed after possible revascularization. Left ventricular systolic function was estimated based on LVEF measured with two-dimensional echocardiography 2 to 7 days after a MI by multiplying the wall motion index by 30, as described earlier (Citation19). A symptom-limited maximal bicycle exercise stress test was performed on the day before discharge (5 to 10 days after the MI). The exercise test was not conducted if the patient had NYHA (New York Heart Association) class IV, planned revascularization, severe chronic obstructive pulmonary disease, or leg amputation. The dose of beta-blocking medication was individually adjusted to obtain a resting heart rate (HR) between 50–60 bpm.
Exercise stress test
The subjects first lay supine in a quiet room for at least 5 min before their resting HR and blood pressure were measured. Blood pressure was monitored using an electronic sphygmomanometer (Dinamap Compact T, GE Medical Systems, Germany) at rest and during exercise testing. The exercise test was started with a work-load of 25 W, followed by 25 W increases every 3 min. The patients were encouraged to reach a symptom-limited maximal work-load. HR at the time of test termination was defined as peak HR (HRpeak). Maximal exercise capacity (EC) in metabolic equivalents (METs) was calculated from the mean work-load during the last 3 min of the test. Predicted exercise capacity (ECpred = 18 – (0.15 × age) for men; ECpred = 14.7 – (0.13 × age) for women) was used to obtain age- and gender-specific reference values for calculation of relative EC (EC% = 100 × EC × ECpred−1) (Citation20). The chronotropic response to exercise was evaluated by maximal chronotropic response index (CRI = 100 × (peak HR – resting HR) × (220 – age – resting HR)−1) (Citation9).
Follow-up and end-points
Follow-up was 8 years after MI and complete for all patients. Cardiac death was the primary end-point and SCD a secondary end-point in the present study. Cardiac deaths were defined as SCD or non-sudden. The criteria for defining the SCD has been previously described in detail (Citation15). It was based on the adjudication of two end-point committees, which reviewed blindly the data related to all deaths obtained from the mortality statistics of the Statistics Finland and the Causes of Death Register, whose quality has been validated previously (Citation21–23). The majority of victims of SCD had had autopsy, and certainly all non-cardiac deaths were excluded as causes of SCD. Cardiac death was defined as sudden if it was: 1) a witnessed death occurring within 60 minutes from the onset of symptoms, unless a cause other that cardiac was obvious, 2) unwitnessed death (<24 hours) in the absence of pre-existing circulatory failure or other cause of death, or 3) death during attempted resuscitation. In this paper, we have extended the follow-up from the previously published papers, but the same criteria have been used in the definition of SCD (Citation15–18). Non-fatal MIs and revascularizations were also registered.
Statistical methods
Values are expressed as mean ± SD or number of subjects (%). Optimal cut-off value for each variable was defined from receiver operating characteristic (ROC) analysis as the maximum sum of sensitivity and specificity below the median (above the median for age and NYHA class) with sensitivity at least 20% using cardiac death as the end-point. Optimal cut-off values were defined because of the absence of previously described values for chronotropic response to exercise in a comparable population of patients on beta-blocking medication. Univariate Cox regression analysis was used to obtain values for relative risk (RR) with 95% confidence intervals (CI) for categorized risk markers. Thereafter, multivariate Cox regression analysis was performed including clinically relevant covariates that were associated to the primary end-point (age, diabetes, history of MI, NYHA class). P value for the addition of each risk marker to a model was calculated. The proportional hazards assumption was verified for each risk marker by plotting Schoenfeld residuals against survival time transformed into natural logarithms. The linearity assumption was confirmed for each continuous variable by plotting Martingale residuals against the linear predictor (X*Beta) and was fulfilled. Potential interaction of CRI with age, diabetes, history of MI, NYHA class, LVEF, and EC% were also assessed in Cox regression models.
To assess the discrimination of the risk markers, c-index and Nagelkerke's binary R2 were calculated for the final model which included age, diabetes, history of MI, NYHA class, LVEF, EC%, and CRI. Thereafter, c-index and Nagelkerke's binary R2 were calculated after removal of one variable at a time. Additional analyses were performed in 250 bootstrap resamplings to obtain 95% CI for c-index of each model. Observed and expected event rates of the risk model were displayed among the patients grouped into deciles of predicted risk. Kaplan-Meier analysis with log rank analysis was used to describe cardiac death-free survival in low- and high-risk groups. A P value < 0.05 was considered statistically significant. The data were analyzed using IBM SPSS Statistics 18.0 (SPSS Inc., Chicago, USA).
Results
Clinical variables, left ventricular ejection fraction, and cardiac mortality
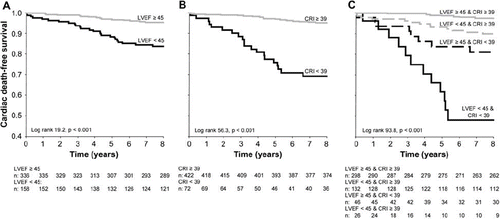
Total mortality was 17.0% (n = 84), cardiac mortality 8.1% (n = 40), and sudden cardiac mortality 3.6% (n = 18) in the present population. Age ≥ 66 years, diabetes, history of MI, and NYHA class ≥ 2 predicted cardiac mortality (RR 2.5, 95% CI 1.3–4.7; RR 2.6, 95% CI 1.3–4.9; RR 3.0, 95% CI 1.6–5.7; and RR 2.3, 95% CI 1.2–4.2, respectively; P < 0.05 for all). Thrombolytic therapy after MI, non-fatal MI during the follow-up, and the use of angiotensin-converting enzyme inhibitors or angiotensin II blockers and diuretics were associated to increased cardiac mortality (RR 1.9, 95% CI 1.0–3.6; RR 3.0, 95% CI 1.5–5.9; RR 2.1, 95% CI 1.1–3.9; and RR 5.2, 95% CI 2.8–9.7, respectively; P < 0.05 for all). Coronary artery bypass grafting or percutaneous transluminal coronary angioplasty during the follow-up and the use of aspirin or warfarin and statins were associated to decreased risk for cardiac death (RR 0.2, 95% CI 0.1–0.6; RR 0.4, 95% CI 0.2–0.8; and RR 0.4, 95% CI 0.2–0.9, respectively; P < 0.05 for all). None of these variables predicted SCD. Gender was not associated with the primary or secondary end-point. Reduced LVEF (<45%) predicted both cardiac death and SCD (RR 3.8, 95% CI 2.0–7.2; and RR 3.6, 95% CI 1.4–9.2, respectively; P < 0.01 for both) () and remained significant after adjustment for clinical variables (RR 3.4, 95% CI 1.8–6.6; and RR 3.7, 95% CI 1.4–9.7, respectively; P < 0.01 for both).
Chronotropic incompetence and cardiac mortality
Decreased CRI (< 39) was a powerful predictor of cardiac death and SCD (RR 7.6, 95% CI 4.1–14.1; and RR 6.2, 95% CI 2.4–15.6, respectively; P < 0.001 for both) (B) and remained significant after adjustments for clinical variables (RR 6.2, 95% CI 2.4–15.6; and RR 7.3, 95% CI 2.6–20.0, respectively; P < 0.001). CRI improved the Cox regression model significantly when predicting cardiac death and SCD (P < 0.001 for both). CRI did not have significant interaction with the other covariates (). Decreased EC% (<57%) also predicted cardiac death and SCD (RR 3.9, 95% CI 2.1–7.3; and RR 3.2, 95% CI 1.3–8.1, respectively; P < 0.05 for both). In multivariate analysis, EC% remained as a significant predictor of cardiac and sudden cardiac death (RR 3.8, 95% CI 1.9–7.4; and RR 3.7, 95% CI 1.4–10.0, respectively; P < 0.01 for both) and improved the risk models for cardiac death and SCD (P < 0.05 for both). CRI improved the discrimination of the risk model most when predicting both cardiac death and SCD (). Observed and expected event rates of the risk model with and without CRI are presented in .
Figure 2. Predicted and observed rates of cardiac mortality before (open circles) and after (solid circles) inclusion of chronotropic incompetence in the risk model for cardiac death in the patients divided into deciles according to predicted risk. Age, diabetes, New York Heart Association classification, history of myocardial infarction, left ventricular ejection fraction, and exercise capacity were included in the initial model. The calibration of the risk model is better when the data points fall closer to the 45° line.
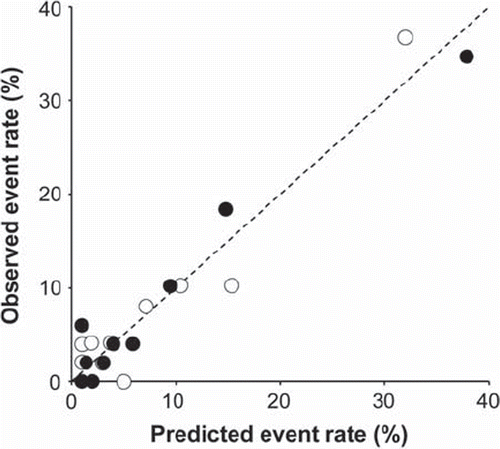
Table II. Association of chronotropic incompetence to cardiac death and sudden cardiac death in subgroups divided according to other predictive variables.
Table III. Discrimination of the final risk model and the models where each risk marker has been removed one at a time using c-index with confidence intervals obtained from 250 bootstrap resamplings and binary R2.
Discussion
This study showed that chronotropic incompetence is a powerful predictor of cardiac mortality and provides important prognostic information beyond LVEF in patients with a recent MI. The data support the view that evaluation of chronotropic response to standard clinical exercise testing should perhaps be included to a greater extent in risk stratification of patients with a recent MI.
Chronotropic incompetence in risk stratification
The initial increase in HR early during exercise is caused by a central withdrawal of vagal inhibition as well as by an increase in sympathetic tone (Citation10–12). Thereafter, there is a further increase in central nervous system sympathetic stimulation as well as in levels of circulating catecholamines as the intensity of exercise increases. The decrease in parasympathetic tone, along with the increase in sympathetic tone, results in increased stimulation of the sinus node and increased HR; thus chronotropic response to exercise reflects both vagal and sympathetic outflow to the sinus node. Chronotropic incompetence is an inability of the HR to increase normally with exercise. In a classic article by Colucci et al. (Citation24), patients with increasing severity of heart failure had a progressively impaired chronotropic response, suggested to be caused by decreased sensitivity of the sinus node to sympathetic stimulation due to an elevated baseline sympathetic tone.
Several studies have shown that chronotropic incompetence predicts future adverse cardiac events in asymptomatic populations and patients with coronary artery disease (Citation1–9). Yet, the present study provides novel aspects to the current knowledge. Firstly, there are no studies assessing the prognostic significance of HR response to exercise early after recovery from MI. Secondly, chronotropic response to exercise has been evaluated mainly among populations who are not on beta-blocking medication, and only a portion of the patients have had a history of coronary artery disease or previous myocardial infarction (Citation1,Citation5–9). It is obvious that the optimal cut-off values for defining chronotropic incompetence differ according to clinical status and medical treatment, as also suggested by Khan et al. (Citation5). Thirdly, we included cardiac death and also SCD as end-points of the study, while a majority of previous studies have used all-cause mortality as an end-point (Citation1,Citation5–7). In agreement with present observations, one prior study also showed that chronotropic incompetence is a powerful predictor of SCD in the general population (Citation3). Pathophysiology of increased cardiac mortality among post-MI patients with chronotropic incompetence is beyond the scope of this study, and it remains speculative if there is causal relationship between chronotropic incompetence and cardiac mortality. It is also likely that chronotropic incompetence is a surrogate marker of some unknown cardiovascular pathology or pathophysiology, which then predisposes to fatal cardiac events (Citation25,Citation26).
Study limitations
The patients were treated according to contemporary guidelines in the late 1990s, when percutaneous coronary intervention was not routinely used in all MI patients. Similarly, angiotensin-converting enzyme inhibitors, angiotensin II blockers, and statins were not prescribed for all post-MI patients at that time. The prognostic significance of chronotropic incompetence was sustained even after adjustments for treatment strategies (data not shown). Still, the results may not be directly applicable to current post-MI patients. All patients in the current study were on beta-blocking medication which decreases the chronotropic response of HR. It may be difficult to determine individual effects of different beta-blocker type and dosage on exercise HR. Therefore, the dosing of beta-blocking medication was adjusted according to resting HR. Cessation of medication is not feasible in clinical practice in the early post-MI phase, and washout for beta-blockers can last 4–14 days. Due to the optimal cut-off values and small number of end-points the present results may suffer from over-optimism and over-fitting, especially when predicting SCD. To reduce over-fitting, we included only the clinically most relevant variables as covariates in multivariate analyses. We also used bootstrap resampling to increase the confidence on the results. Finally, the present results apply only to patients who are able to perform an exercise stress test after MI. Inability to perform an exercise stress test is also associated to increased mortality (Citation27,Citation28), which was observed also in the present population (data not shown).
Clinical implications
Exercise stress testing has been routinely used and recommended in the past for patients in the convalescent phase of an acute MI, mainly to assess residual ischemia and indication for coronary angiography. In the current invasive era, exercise testing is infrequently used, because a majority of patients undergo invasive evaluation/treatment of coronary artery disease. The present study suggests a novel indication for using the exercise test in this patient group. Those with chronotropic incompetence showed an increased risk of SCD, perhaps being candidates for implantable cardioverter-defibrillator therapy. However, the present results carry the limitations and biases inherent to non-randomized studies and therefore need confirmation from other study designs.
Acknowledgements
Jari Tapanainen, MD, PhD, Jari Laukkanen, MD, PhD, Pirkko Huikuri, RN, Päivi Karjalainen, RN, and Ms Anne Lehtinen are sincerely acknowledged. The authors appreciate the technical support received from Heart Signal Co. (Oulu, Finland).
Declaration of interest: This study was supported by grants from the Research Council for Health, Academy of Finland (Helsinki, Finland), the Finnish Foundation of Cardiovascular Research (Helsinki, Finland), the Finnish Technology Development Centre (Tekes, Helsinki, Finland), and the Sigrid Juselius Foundation (Helsinki, Finland). The authors declare no other conflicts of interest.
References
- Nishime EO, Cole CR, Blackstone EH, Pashkow FJ, Lauer MS. Heart rate recovery and treadmill exercise score as predictors of mortality in patients referred for exercise ECG. JAMA. 2000;284:1392–8.
- Savonen KP, Lakka TA, Laukkanen JA, Rauramaa TH, Salonen JT, Rauramaa R. Usefulness of chronotropic incompetence in response to exercise as a predictor of myocardial infarction in middle-aged men without cardiovascular disease. Am J Cardiol. 2008;101:992–8.
- Jouven X, Empana JP, Schwartz PJ, Desnos M, Courbon D, Ducimetiere P. Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med. 2005;352:1951–8.
- Leeper NJ, Dewey FE, Ashley EA, Sandri M, Tan SY, Hadley D, . Prognostic value of heart rate increase at onset of exercise testing. Circulation. 2007;115:468–74.
- Khan MN, Pothier CE, Lauer MS. Chronotropic incompetence as a predictor of death among patients with normal electrograms taking Beta blockers (metoprolol or atenolol). Am J Cardiol. 2005;96:1328–33.
- Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341:1351–7.
- Vivekananthan DP, Blackstone EH, Pothier CE, Lauer MS. Heart rate recovery after exercise is a predictor of mortality, independent of the angiographic severity of coronary disease. J Am Coll Cardiol. 2003;42:831–8.
- Myers J, Tan SY, Abella J, Aleti V, Froelicher VF. Comparison of the chronotropic response to exercise and heart rate recovery in predicting cardiovascular mortality. Eur J Cardiovasc Prev Rehabil. 2007;14:215–21.
- Azarbal B, Hayes SW, Lewin HC, Hachamovitch R, Cohen I, Berman DS. The incremental prognostic value of percentage of heart rate reserve achieved over myocardial perfusion single-photon emission computed tomography in the prediction of cardiac death and all-cause mortality: superiority over 85% of maximal age-predicted heart rate. J Am Coll Cardiol. 2004;44:423–30.
- Tulppo MP, Makikallio TH, Takala TE, Seppanen T, Huikuri HV. Quantitative beat-to-beat analysis of heart rate dynamics during exercise. Am J Physiol. 1996;271(1 Pt 2):H244–52.
- Sheriff DD, O'Leary DS, Scher AM, Rowell LB. Baroreflex attenuates pressor response to graded muscle ischemia in exercising dogs. Am J Physiol. 1990;258(2 Pt 2):H305–10.
- Fukuma N, Oikawa K, Aisu N, Kato K, Kimura-Kato YK, Tuchida T, . Impaired baroreflex as a cause of chronotropic incompetence during exercise via autonomic mechanism in patients with heart disease. Int J Cardiol. 2004;97:503–8.
- Gibbons RJ, Balady GJ, Bricker JT, Chaitman BR, Fletcher GF, Froelicher VF, . ACC/AHA 2002 guideline update for exercise testing: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). J Am Coll Cardiol. 2002;40:1531–40.
- Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, . Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–83.
- Huikuri HV, Tapanainen JM, Lindgren K, Raatikainen P, Makikallio TH, Juhani Airaksinen KE, . Prediction of sudden cardiac death after myocardial infarction in the beta-blocking era. J Am Coll Cardiol. 2003;42:652–8.
- Tapanainen JM, Thomsen PE, Kober L, Torp-Pedersen C, Makikallio TH, Still AM, . Fractal analysis of heart rate variability and mortality after an acute myocardial infarction. Am J Cardiol. 2002;90:347–52.
- Kiviniemi AM, Tulppo MP, Wichterle D, Hautala AJ, Tiinanen S, Seppanen T, . Novel spectral indexes of heart rate variability as predictors of sudden and non-sudden cardiac death after an acute myocardial infarction. Ann Med. 2007;39:54–62.
- Makikallio TH, Barthel P, Schneider R, Bauer A, Tapanainen JM, Tulppo MP, . Prediction of sudden cardiac death after acute myocardial infarction: role of Holter monitoring in the modern treatment era. Eur Heart J. 2005;26:762–9.
- Berning J, Steensgaard-Hansen F. Early estimation of risk by echocardiographic determination of wall motion index in an unselected population with acute myocardial infarction. Am J Cardiol. 1990;65:567–76.
- Kim ES, Ishwaran H, Blackstone E, Lauer MS. External prognostic validations and comparisons of age- and gender-adjusted exercise capacity predictions. J Am Coll Cardiol. 2007;50:1867–75.
- Pajunen P, Koukkunen H, Ketonen M, Jerkkola T, Immonen-Raiha P, Karja-Koskenkari P, . The validity of the Finnish Hospital Discharge Register and Causes of Death Register data on coronary heart disease. Eur J Cardiovasc Prev Rehabil. 2005;12:132–7.
- Mahonen M, Salomaa V, Torppa J, Miettinen H, Pyorala K, Immonen-Raiha P, . The validity of the routine mortality statistics on coronary heart disease in Finland: comparison with the FINMONICA MI register data for the years 1983–1992. Finnish multinational MONItoring of trends and determinants in CArdiovascular disease. J Clin Epidemiol. 1999;52:157–66.
- Rapola JM, Virtamo J, Korhonen P, Haapakoski J, Hartman AM, Edwards BK, . Validity of diagnoses of major coronary events in national registers of hospital diagnoses and deaths in Finland. Eur J Epidemiol. 1997;13:133–8.
- Colucci WS, Ribeiro JP, Rocco MB, Quigg RJ, Creager MA, Marsh JD, . Impaired chronotropic response to exercise in patients with congestive heart failure. Role of postsynaptic beta-adrenergic desensitization. Circulation. 1989;80: 314–23.
- Chaitman BR. Abnormal heart rate responses to exercise predict increased long-term mortality regardless of coronary disease extent: the question is why? J Am Coll Cardiol. 2003;42:839–41.
- Routledge HC, Townend JN. Why does the heart rate response to exercise predict adverse cardiac events? Heart. 2006;92:577–8.
- Chaitman BR, McMahon RP, Terrin M, Younis LT, Shaw LJ, Weiner DA, . Impact of treatment strategy on predischarge exercise test in the Thrombolysis in Myocardial Infarction (TIMI) II Trial. Am J Cardiol. 1993;71: 131–8.
- Villella A, Maggioni AP, Villella M, Giordano A, Turazza FM, Santoro E, . Prognostic significance of maximal exercise testing after myocardial infarction treated with thrombolytic agents: the GISSI-2 data-base. Gruppo Italiano per lo Studio della Sopravvivenza Nell'Infarto. Lancet. 1995;346: 523–9.