Abstract
Background. Little is known about the association of rheumatic heart disease (RHD) with incident heart failure (HF) among older adults.
Design. Cardiovascular Health Study, a prospective cohort study.
Methods. Of the 4,751 community-dwelling adults ≥ 65 years, free of prevalent HF at baseline, 140 had RHD, defined as self-reported physician-diagnosed RHD along with echocardiographic evidence of left-sided valvular disease. Propensity scores for RHD, estimated for each of the 4,751 participants, were used to assemble a cohort of 720, in which 124 and 596 participants with and without RHD, respectively, were balanced on 62 baseline characteristics.
Results. Incident HF developed in 33% and 22% of matched participants with and without RHD, respectively, during 13 years of follow-up (hazard ratio when RHD was compared to no-RHD 1.60; 95% confidence interval 1.13–2.28; P = 0.008). Pre-match unadjusted, multivariable-adjusted, and propensity-adjusted hazard ratios (95% confidence intervals) for RHD-associated incident heart failure were 2.04 (1.54–2.71; P < 0.001), 1.32 (1.02–1.70; P = 0.034), and 1.55 (1.14–2.11; P = 0.005), respectively. RHD was not associated with all-cause mortality (HR 1.09; 95% CI 0.82–1.45; P = 0.568).
Conclusion. RHD is an independent risk factor for incident HF among community-dwelling older adults free of HF, but has no association with mortality.
Key words::
Key messages
Rheumatic heart disease (RHD) is a major risk factor for cardiovascular morbidity and mortality among younger adults in the developing world.
Little is known about the association between RHD and HF; most evidence is based on small cross-sectional studies.
The findings of the current study demonstrate that in community-dwelling older adults without HF at baseline, the presence of mild to moderate RHD was associated with increased risk of new-onset HF.
This report is unique as it is based on a large meticulously conducted prospective cohort study of community-dwelling older adults free of prevalent HF at baseline, from a developed nation.
Introduction
Rheumatic heart disease (RHD) is highly prevalent in developing nations, where it is the leading cause of cardiovascular morbidity including heart failure (HF) (Citation1–6). Most evidence of the cardiovascular effect of RHD is based on small cross-sectional studies of younger adults from developing nations (Citation5,Citation7). However, little is known about the effect of chronic RHD on incident HF in older adults. We used a public-use copy of the Cardiovascular Health Study (CHS) data sets obtained from the National Heart, Lung, and Blood Institute (NHLBI) to test the hypothesis that a history of RHD would be associated with increased risk of incident HF in a propensity-matched population of community-dwelling older adults free of HF at baseline.
Methods
Study design and participants
The CHS is an on-going epidemiologic study of cardiovascular disease in older adults in the United States, the details of the rationale, design, and implementation of which have been previously reported (Citation8). Briefly, 5,888 community-dwelling adults ≥ 65 years were recruited from four counties, one of each from North Carolina, California, Maryland, and Pennsylvania. We restricted our analysis to the 5,201 participants in the original cohort that was recruited during 1989–1990. Data on baseline echocardiography were not available from the second cohort of 687 African-Americans recruited during 1992–1993. Of the 5,201 participants in the original cohort, data on 5,125 were available in the de-identified public-use copy of the data set (76 participants did not consent to be included in the public-use data). Of 5,125 participants, echocardiographic data on baseline valvular disease were available for 5,100, of whom data on self-reported physician-diagnosed RHD were available from 4,961 participants. Of these, 4,751 participants were free of prevalent HF at baseline and were included in our main analysis.
RHD and other baseline measurements
Of the 4,751 participants, 140 (2.9%) had RHD, defined as self-reported physician-diagnosed RHD, plus echocardiographic evidence of left-sided valvular disease, namely, mitral regurgitation (MR), aortic regurgitation (AR), mitral stenosis (MS), and aortic stenosis (AS). Of the 140 participants with RHD, 68% had MR, 56% had AR, 5% had MS, and 11% had AS. On the other hand, among the 4,611 participants without RHD, 28% had MR, 18% had AR, 0% had MS, and 1% had AS. Data on socio-demographic, clinical, subclinical, and laboratory variables were collected at baseline and have been described previously in detail (Citation8,Citation9).
Outcomes
The primary outcome for this study was definite new-onset HF during a median follow-up of 12 years. The process of adjudication of HF in CHS has been well documented in the literature (Citation10,Citation11). Briefly, participants were asked about a physician diagnosis of HF during semi-annual visits. The CHS Events Committee later adjudicated the diagnosis of HF through the examination of participants’ medical records for a constellation of symptoms, physical signs, and other supporting findings suggestive of HF, use of medications commonly used for HF, and follow-up surveillance. Secondary outcomes were all-cause mortality, new-onset acute myocardial infarction, angina pectoris, stroke, transient ischemic attack, and peripheral arterial disease.
Assembly of a balanced study cohort
Because of significant differences in key baseline characteristics between participants with and without RHD ( and ), we used propensity score matching to assemble a population in which those with and without RHD would be well balanced on all measured baseline covariates (Citation12,Citation13). Propensity score for RHD for a participant is that person's conditional probability of having RHD given her/his measured baseline characteristics. Propensity score matching has emerged as a popular tool that makes it possible to design observational studies like randomized clinical trials in several key ways (Citation13). First, it allows investigators to assemble a study cohort, in which exposed and unexposed participants are well balanced on all measured baseline characteristics. Second, it allows investigators to measure objectively baseline covariate balance and present them in a visually pleasant manner. Finally, and perhaps most importantly, as in a randomized clinical trial, investigators remain blinded to outcomes during this design phase of the study (Citation13). Although propensity score matching is often used to balance two treatment groups (Citation14,Citation15), it can also be used to balance patients across non-treatment exposures (Citation16–19).
Figure 1. Absolute standardized differences before and after propensity score matching comparing covariate values between rheumatic heart disease (RHD) and non-RHD participants. (ACE = angiotensin-converting enzyme; CABG = coronary artery by-pass surgery; COPD = chronic obstructive pulmonary disease; LV = left ventricular; NSAID = non-steroidal anti-inflammatory drug).
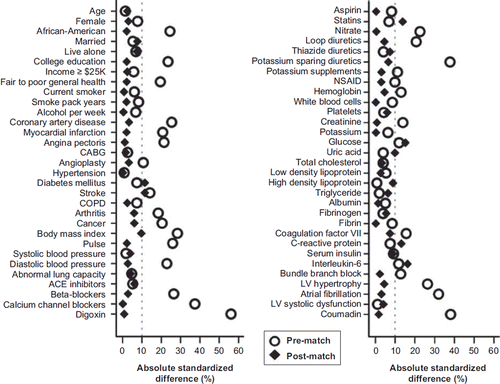
Table I. Baseline characteristics, by rheumatic heat disease (RHD), before and after propensity score matching.
We estimated propensity scores for each of the 4,751 participants using a non-parsimonious multivariable logistic regression model (Citation14–20). In the model, RHD was used as the dependent variable, and 62 baseline characteristics displayed in were entered as covariates. Using a matching protocol that matched each RHD participant with up to five different no-RHD participants who had similar propensity scores, we were able to match 124 (89% of the 140) and 596 participants with and without RHD, respectively. Our matching algorithm first tried to match each RHD participant to a no-RHD participant with an identical propensity score to five decimal places. Then we removed matched patients (i.e. one RHD patients with up to five no-RHD patients with similar propensity scores) and repeated the process matching to four, three, two, and one decimal place (Citation14–20). Absolute standardized differences for all 62 covariates were estimated to assess pre-match imbalances and post-match balances achieved between participants with and without RHD, and are presented in Love plots (Citation14–22). An absolute standardized difference of 0% on a covariate indicates no residual bias for that covariate, and values <10% indicate inconsequential imbalance.
Statistical analysis
For descriptive analyses, Pearson's chi-square tests, Wilcoxon rank-sum tests, and paired sample t tests were used as appropriate for pre- and post-match between-group comparisons. To estimate the association between RHD and outcomes, we used Kaplan-Meier and Cox proportional hazard analyses. Proportional hazards assumptions were checked using log-minus-log scale survival plots. To determine if the association between RHD and incident HF was homogeneous across various subgroups of matched patients, we conducted subgroup analyses and formally tested for interactions using Cox regression models. We also examined the association of RHD and incident HF in the full pre-match cohort of 4,751 participants using three different approaches: 1) unadjusted, 2) multivariable-adjusted (entering all covariates displayed in ), and 3) propensity score-adjusted. All statistical tests were two-tailed, and a P value < 0.05 was considered significant. SPSS for Windows Release 15 (1 April 2009) was used for all data analysis (SPSS Inc. (an IBM Company), Chicago, IL).
Sensitivity analyses
Even though our matched participants with and without RHD were well balanced on 62 measured baseline characteristics, bias due to imbalances in unmeasured covariates is possible. As such, we conducted a formal sensitivity analysis to quantify the degree of a hidden bias that would need to be present to invalidate our main conclusions (Citation23).
Results
Baseline characteristics
Overall, matched participants had a mean (±SD) age of 73 (±5) years, 58% were women, and 1% were African-Americans. Compared to participants without RHD, those with RHD were more likely to have coronary artery disease (CAD), stroke, atrial fibrillation (AF), and left ventricular hypertrophy (LVH) and receive digoxin, diuretics, and warfarin. However, these and other pre-match imbalances were balanced after matching ( and ). Among matched RHD patients, 69%, 56%, 10%, and 2% had MR, AR, AS, and MS, respectively. Among those with MR, 48%, 19%, and 2% had mild, moderate, and severe MR, respectively, and among those with AR, 15%, 40%, and 1% had mild, moderate, and severe AR, respectively. None had severe MS, and only 1 had severe AS.
RHD and incident HF
Overall, 174 (20%) matched participants developed incident HF during 6,894 person-years of follow-up. Incident HF occurred in 33% and 22% participants with and without RHD, respectively (hazard ratio (HR) when RHD was compared with no-RHD 1.60; 95% confidence interval (CI) 1.13–2.28; P = 0.008) ( and ). In the absence of hidden bias, a sign-score test for matched data with censoring provides strong evidence (P < 0.001) that older adults with RHD clearly had more incident HF than those without RHD. A hidden binary covariate that is a near-perfect predictor of incident HF would need to increase the odds of RHD by 28% to explain away this association. The association between RHD and incident HF was homogeneous across a wide spectrum of participants (). Unadjusted, multivariable-adjusted, and propensity-adjusted association between RHD and incident HF among the 4,751 pre-match participants are displayed in .
Figure 2. Kaplan-Meier plots for incident heart failure by rheumatic heart disease (RHD). (CI = confidence interval; HR = hazard ratio).
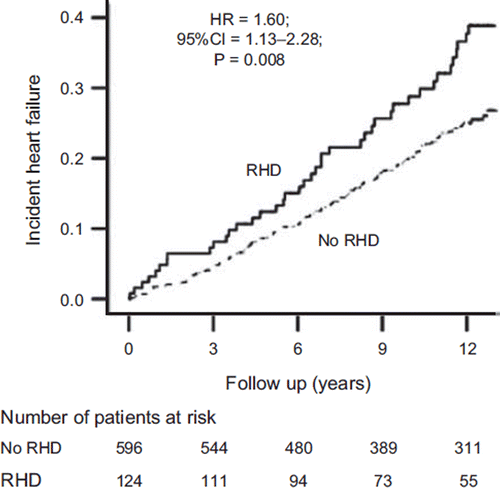
Figure 3. Association of rheumatic heart disease (RHD) with incident heart failure (HF) in subgroups of propensity score-matched partici pants in the Cardiovascular Health Study. (CI = confidence interval; HR = hazard ratio).
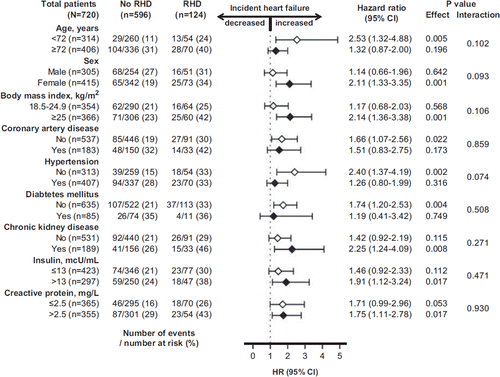
Table II. Association of rheumatic heart disease (RHD) with incident heart failure.
RHD and other outcomes
All-cause mortality occurred in 47% and 43% of matched participants with and without RHD, respectively (HR 1.09; 95% CI 0.82–1.45; P = 0.568) (). Associations of RHD with other outcomes are displayed in .
Table III. Association of rheumatic heart disease (RHD) with other outcomes in the matched cohort.
Discussion
The findings from the current analysis demonstrate that among community-dwelling older adults in the US, the prevalence of RHD was relatively high and comprised mostly a mild to moderate form of valvular disease. A history of RHD was associated with an increased risk of incident HF in this population but had no association with all-cause mortality or other cardiovascular outcomes. Although RHD is no longer a public health problem in the developed world, these findings are important because RHD is highly prevalent in developing nations where it is the leading cause of cardiovascular morbidity and mortality. These findings are also important because, as life expectancy increases in developing nations, the prevalence of older adults with RHD is likely to increase. Our data suggest that older adults, who remained HF-free despite RHD at younger ages, are at a significantly increased risk for incident HF.
The significant bivariate association between RHD and incident HF before matching may be explained by imbalances in various baseline characteristics between the groups. For example, those with RHD had a higher prevalence of CAD, AF, and LVH, all of which are risk factors for incident HF. Attenuation of the association between RHD and incident HF after multivariable and propensity score adjustments suggests that those risk factors may have confounded in part the unadjusted association. However, the association remained statistically significant, suggesting an intrinsic association, which was further confirmed in the propensity-matched cohort. Although regression-based multivariable models can account for confounders, they may not necessarily ensure that the prevalences of those confounders are balanced at baseline between the groups (Citation24). However, replication of the association between RHD and incident HF in our propensity-matched cohort suggests that this association may not be explained by the pre-match imbalances in any of the 62 measured baseline characteristics that included CAD, AF, and LVH. It is, however, possible that more RHD patients subsequently developed CAD, AF, or LVH during follow-up, and these conditions were more severe in patients with RHD, which then increased their risk of incident HF.
Because RHD is primarily a disease of heart valves, any differences in outcomes between participants with and without RHD are likely to be explained by the presence, type, or severity of valvular disease. Although valvular disease was present in all patients in the RHD group, nearly half of the non-RHD patients also had valvular disease. Mild MR and moderate AR were the predominant forms of valvular disease in both groups. These forms of valvular disease are known to remain stable for many years in the majority of non-RHD patients (Citation25,Citation26). However, little is known about the natural history of mild MR and moderate AR in patients with RHD. It is also possible that milder forms may progress at a disproportionately faster rate to more severe forms in those with RHD (Citation25). For example, damage to the mitral subvalvular apparatus, as seen in RHD, may lead to the rapid progression of mild MR to a more severe form (Citation27). It is also possible that RHD patients have subclinical myocardial dysfunction that may have increased their susceptibility to milder forms of valvular disease. Furthermore, a damaged mitral subvalvular apparatus may also lead to left ventricular diastolic and systolic impairment (Citation28). Scarring and retraction of mitral valve leaflet and chordae may restrict leaflet motion, particularly during diastole (Citation29,Citation30). Finally, the slow progression of a moderate AR may be accelerated by age-related dilatation of the aorta (Citation31–34) and vascular stiffness, resulting in a more rapid progression to clinical HF.
Although RHD is considered a risk factor for cardiovascular morbidity, there are limited data about its association with HF. Current evidence of this association is mostly based on a few cross-sectional survey-based studies from developing nations (Citation6). A recent review article on RHD reported several RHD-related cardiovascular outcomes, but did not provide any data on RHD-related incident HF (Citation4). This may be due to the difficulty with the adjudication of HF in the community in general, which may be more difficult in the setting of developing nations. In contrast, in the CHS, the diagnosis of HF was centrally adjudicated using stringent criteria.
To the best of our knowledge, this is the first study to report an association between RHD and incident HF among community-dwelling older adults from a developed nation. Even though RHD is no longer a public health problem in developed nations, it is still common in the developing world, where it is a major risk factor for cardiovascular morbidity and mortality. With economic prosperity and better management of other cardiovascular risk factors such as hypertension and CAD, the life expectancy of people in the developing nations is increasing, and, as such, the prevalence of older adults with RHD is likely to increase. Findings from the current study highlight the importance of RHD as a major risk factor for incident HF among older adults who remained free of RHD-related complications for decades.
Several limitations of our study must be acknowledged. As with most baseline cardiovascular morbidities in CHS, a diagnosis of RHD was made based on a self-reported physician diagnosis. Although our use of echocardiographic documentation of baseline valvular disease has helped us assemble a cohort of true RHD patients, we had no echocardiographic findings confirming RHD-specific valvular disease. Further, since the current study was based on a public-use copy of the de-identified CHS data, validation through review of medical records was not possible. It is also possible that some participants with rheumatoid arthritis may have been misclassified as RHD. Although we had no data on baseline rheumatoid arthritis, the prevalence of arthritis in general was balanced in our matched cohort. Additionally, any random misclassification of persons without RHD as having RHD and vice versa is likely to result in dilution regression and under-estimation of the observed association, thus not posing any serious threat to the validity of our findings (Citation35). Although post-match absolute standardized difference for diabetes, stroke, statin use, serum glucose, C-reactive protein, and insulin were >10%, their prevalence or mean were higher in those without RHD, suggesting any confounding due to these residual imbalances would under-estimate the observed association between RHD and incident HF. In fact, when adjusted for those covariates, the association between RHD and incident HF among matched participants became stronger. Finally, these findings based on older adults from a developed nation may not be generalizable to younger RHD patients from developing nations.
In conclusion, among community-dwelling older adults free of HF at baseline, despite the presence of mostly mild to moderate valvular disease, RHD had a significant association with incident HF. These findings highlight the importance of RHD as a major risk factor for incident HF among older adults who remained free of RHD-related complications for decades. These findings need to be replicated in developing nations, and future well designed prospective studies are needed to develop and test interventions to reduce the risk of HF in patients with RHD.
Acknowledgements
The Cardiovascular Health Study (CHS) was conducted and supported by the NHLBI in collaboration with the CHS Investigators. This manuscript was prepared using a public-use data set obtained from the NHLBI and does not necessarily reflect the opinions or views of the CHS or the NHLBI.
A. Ahmed conceived and designed the study. M. Mujib, R. Desai, M. I. Ahmed, and A. Ahmed analyzed and interpreted the data and drafted the manuscript. All authors interpreted the data, critically revised the draft, and gave final approval of the version to be published.
An abstract based on the current analysis was presented at an oral session at the American Heart Association 2009 Scientific Sessions in Orlando, Florida on 17 November 2009.
Declaration of interest: A. Ahmed is supported by the NIH through grants (R01-HL085561 and R01-HL097047) from the NHLBI and a generous gift from Ms Jean B. Morris of Birmingham, Alabama. The other authors declare no conflicts of interest.
References
- Eisenberg MJ. Rheumatic heart disease in the developing world: prevalence, prevention, and control. Eur Heart J. 1993;14:122–8.
- Marijon E, Ou P, Celermajer DS, Ferreira B, Mocumbi AO, Jani D, . Prevalence of rheumatic heart disease detected by echocardiographic screening. N Engl J Med. 2007;357: 470–6.
- Padmavati S. Rheumatic heart disease: prevalence and preventive measures in the Indian subcontinent. Keywords: rheumatic heart disease; rheumatic fever. Heart. 2001;86:127.
- Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5:685–94.
- Damasceno A, Cotter G, Dzudie A, Sliwa K, Mayosi BM. Heart failure in sub-Saharan Africa: time for action. J Am Coll Cardiol. 2007;50:1688–93.
- WHO. The World Health Report: 2004. Geneva: World Health Organization; 2004.
- Carapetis JR. Rheumatic heart disease in developing countries. N Engl J Med. 2007;357:439–41.
- Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, . The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–76.
- Psaty BM, Furberg CD, Kuller LH, Borhani NO, Rautaharju PM, O'Leary DH, . Isolated systolic hypertension and subclinical cardiovascular disease in the elderly. Initial findings from the Cardiovascular Health Study. JAMA. 1992;268: 1287–91.
- Gottdiener JS, Arnold AM, Aurigemma GP, Polak JF, Tracy RP, Kitzman DW, . Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35:1628–37.
- Psaty BM, Kuller LH, Bild D, Burke GL, Kittner SJ, Mittelmark M, . Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1995;5:270–7.
- Rosenbaum PR, Rubin DB. The central role of propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55.
- Rubin DB. Using propensity score to help design observational studies: Application to the tobacco litigation. Health Services and Outcomes Research Methodology. 2001;2:169–88.
- Ahmed A, Husain A, Love TE, Gambassi G, Dell'Italia LJ, Francis GS, . Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J. 2006;27:1431–9.
- Ahmed A, Rich MW, Love TE, Lloyd-Jones DM, Aban IB, Colucci WS, . Digoxin and reduction in mortality and hospitalization in heart failure: a comprehensive post hoc analysis of the DIG trial. Eur Heart J. 2006;27:178–86.
- Ahmed A, Perry GJ, Fleg JL, Love TE, Goff DC Jr, Kitzman DW. Outcomes in ambulatory chronic systolic and diastolic heart failure: a propensity score analysis. Am Heart J. 2006;152: 956–66.
- Ahmed A, Rich MW, Sanders PW, Perry GJ, Bakris GL, Zile MR, . Chronic kidney disease associated mortality in diastolic versus systolic heart failure: a propensity matched study. Am J Cardiol. 2007;99:393–8.
- Ahmed A, Zannad F, Love TE, Tallaj J, Gheorghiade M, Ekundayo OJ, . A propensity-matched study of the association of low serum potassium levels and mortality in chronic heart failure. Eur Heart J. 2007;28:1334–43.
- Ahmed A, Aban IB, Vaccarino V, Lloyd-Jones DM, Goff DC Jr, Zhao J, . A propensity-matched study of the effect of diabetes on the natural history of heart failure: variations by sex and age. Heart. 2007;93:1584–90.
- Ekundayo OJ, Allman RM, Sanders PW, Aban I, Love TE, Arnett D, . Isolated systolic hypertension and incident heart failure in older adults: a propensity-matched study. Hypertension. 2009;53:458–65.
- D'Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–81.
- Normand ST, Landrum MB, Guadagnoli E, Ayanian JZ, Ryan TJ, Cleary PD, . Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54:387–98.
- Rosenbaum PR. Sensitivity to hidden bias. Rosenbaum PR. Observational studies. 1. New York: Springer-Verlag; 2002. 105–70.
- Fitzmaurice G. Confounding: regression adjustment. Nutrition. 2006;22:581–3.
- Enriquez-Sarano M, Schaff HV, Tajik AJ, Frye RL. Chronic mitral regurgitation. Alpert JS, Dalen JE, Rahimtoola SH. Valvular Heart Disease. 3rd. Philadelphia: Lippincott Williams & Wilkins; 2000:113–142.
- Bonow RO, Carabello BA, Chatterjee K, de Leon AC Jr, Faxon DP, Freed MD, . 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;52: e1–142.
- Libby P. Braunwald's Heart disease: a textbook of cardiovascular medicine. 8th. Philadelphia: Saunders Elsevier; 2008:1657–68. Available at MD Consult at www.mdconsult.com/books Accessed October 23 2010.
- DeAnda A Jr, Komeda M, Nikolic SD, Daughters GT 2nd, Ingels NB, Miller DC. Left ventricular function, twist, and recoil after mitral valve replacement. Circulation. 1995;92:II458–66.
- Acar C, de Ibarra JS, Lansac E. Anterior leaflet augmentation with autologous pericardium for mitral repair in rheumatic valve insufficiency. J Heart Valve Dis. 2004;13:741–6.
- Enriquez-Sarano M, Akins CW, Vahanian A. Mitral regurgitation. Lancet. 2009;373:1382–94.
- Spagnuolo M, Kloth H, Taranta A, Doyle E, Pasternack B. Natural history of rheumatic aortic regurgitation. Criteria predictive of death, congestive heart failure, and angina in young patients. Circulation. 1971;44:368–80.
- Feinstein AR, Wood HF, Spagnuolo M, Taranta A, Jonas S, Kleinberg E, . Rheumatic fever in children and adolescents. A long-term epidemiologic study of subsequent prophylaxis, streptococcal infections, and clinical sequelae. VII. Cardiac changes and sequelae. Ann Intern Med. 1964;60 Suppl 5:87–123.
- Roberts W. Morphological features of the elderly heart. Tresch D, Aronov WS. Cardiovascular disease in the elderly patient. 2nd. New York: Marcel Decker; 1999.
- Roberts WC, Ko JM, Moore TR, Jones WH 3rd. Causes of pure aortic regurgitation in patients having isolated aortic valve replacement at a single US tertiary hospital (1993 to 2005). Circulation. 2006;114:422–9.
- Clarke R, Shipley M, Lewington S, Youngman L, Collins R, Marmot M, . Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. Am J Epidemiol. 1999;150:341–53.
