Abstract
Background. The everolimus-eluting stent (EES) is a second-generation drug-eluting stent (DES) which is designed to provide better stent deliverability, deployment, safety, and efficacy. We performed a meta-analysis to evaluate the relative safety and efficacy of the EES compared with the paclitaxel-eluting stent (PES).
Methods. The published literature was scanned by formal searches of electronic databases from January 2001 to August 2010. All randomized trials comparing EES versus PES and reporting the clinical outcomes were examined for analysis.
Results. A total of four randomized trials were included, involving 6,788 patients. EES were superior to PES with respect to the major adverse cardiac events (cardiac death, myocardial infarction (MI), and ischemia-driven target lesion revascularization (TLR)) within 1-year follow-up (OR 0.57; P < 0.001). The 1-year rates of MI, ischemia-driven TLR, and definite or probable stent thrombosis (ST) were also lower with EES than with PES (OR 0.57, P < 0.001 for MI; OR 0.48, P < 0.001 for TLR; OR 0.34, P < 0.001 for ST). There was no significant difference between EES and PES with respect to cardiac mortality (OR 0.93; P = 0.81).
Conclusion. The EES is superior to the PES in terms of 1-year safety and efficacy.
| Abbreviations | ||
| CAD | = | coronary artery disease |
| DES | = | drug-eluting stent |
| EES | = | everolimus-eluting stent |
| MI | = | myocardial infarction |
| OR | = | odds ratio |
| PES | = | paclitaxel-eluting stent |
| RCT | = | randomized controlled trial |
| TLR | = | target lesion revascularization |
Key messages
The everolimus-eluting stent is a second-generation drug-eluting stent which is designed to provide better stent performance, safety, and efficacy.
This meta-analysis shows that the everolimus-eluting stent, as compared with the paclitaxel-eluting stent, resulted in reduced rates of ischemia-driven target lesion revascularization, myocardial infarction, and stent thrombosis and thereby led to a reduction in major adverse cardiac events at 1 year after initial procedure.
First-generation drug-eluting stents (DES), which release sirolimus or paclitaxel, have been demonstrated to be superior to bare-metal stents (BMS) in reducing restenosis and repeat revascularization. However, their success is hampered by some limitations affecting device safety (Citation1). The everolimus-eluting stent (EES) is a second-generation DES manufactured from a flexible cobalt chromium alloy with a multicellular design and 81 μm strut thickness which is coated with a thin (7.8 μm), non-adhesive, durable, biocompatible acrylic polymer and fluorinated co-polymer releasing everolimus (Citation2). Recently, several randomized trials have been performed to compare this new device with the paclitaxel-eluting stent (PES), an extensively used and validated first-generation DES, for the treatment of obstructive coronary artery disease (CAD) (Citation3–6). However, its benefits and safety have not been systematically quantified across different studies. Direct comparison meta-analysis has the potential to increase power and improve precision of treatment effects (Citation7). We therefore performed a meta-analysis of all available randomized clinical trials to evaluate the relative safety and efficacy of the second-generation EES compared with the first-generation PES.
Methods
Data sources and selection criteria
The published literature was scanned by formal searches of electronic databases (PubMed, Medline, EMBASE, and Cochrane Controlled Trials Register) from January 2001 to August 2010. All randomized trials on head-to-head comparison of EES versus PES in CAD patients were examined using the following key words: randomized trial, drug-eluting stent, everolimus-eluting stent, paclitaxel-eluting stent, percutaneous coronary intervention. To be selected for this meta-analysis, studies comparing EES with PES for the treatment of obstructive coronary artery lesions had to be randomized and have their results reported by the trial investigators.
Study end-points and data abstraction
The primary end-point was a composite of major adverse cardiac events (cardiac death, non-fatal myocardial infarction (MI), and ischemia-driven target lesion revascularization (TLR) either by percutaneous or surgical intervention) within 1 year of follow-up. The secondary end-points included all-cause mortality, cardiac death, non-fatal myocardial infarction, ischemia-driven TLR, ischemia-driven target vessel revascularization (TVR), and stent thrombosis (ST) at 1 year. ST was defined according to the definitions provided by the Academic Research Consortium (ARC) (Citation8).
Statistical analysis
All analyses were performed based on the intention-to-treat principle. Odds ratios (OR) with 95% confidence intervals (CI) were computed as summary statistics. The pooled OR was calculated with the Mantel–Haenszel method for fixed effects and the DerSimonian and Laird method for random effects (Citation9,Citation10). To assess heterogeneity across trials, we used Cochran's test (Citation11). A funnel plot as well as the adjusted rank correlation test, according to the method of Begg and Mazumdar (Citation12), was used to assess publication bias with respect to the primary end-point. Results were considered statistically significant at two-sided P < 0.05. Statistical analyses were performed with the Stata version 9 statistical package (Stata Corp., College Station, Texas, USA).
Results
A total of four randomized trials with available 1-year follow-up data were analyzed, involving 6,788 patients (4,247 in EES group and 2,541 in PES group) (Citation9–12). Base-line characteristics of patients in individual studies are shown in . In all trials, a loading dose of 300 to 600 mg of clopidogrel was administered before the procedure. Maintenance therapy for 6 to 12 months with clopidogrel consisted of a daily dose of 75 mg for patients of both DES groups. The compliance with aspirin and clopidogrel at 1 year was similar between two groups in the SPIRIT III, IV, and COMPARE trials (Citation4–6) but not reported in SPIRIT II trial.
Table I. Base-line clinical and angiographic characteristics of the study population.
The primary outcome of interest, a composite of major adverse cardiac events at 1 year follow-up, occurred in 190 (4.5%) of 4,247 patients assigned to the EES group, significantly fewer than 196 (7.7%) of 2,541 patients assigned to the PES group (OR 0.57; 95% CI 0.46–0.70; P < 0.001) by the fixed-effect model (). There was no significant heterogeneity between trials (P = 0.48). No evidence of publication bias with respect to the primary end-point was found using the Begg funnel plot and rank correlation test (P = 0.19). Omission of individual trials from the analysis did not have any relevant influence on the overall results of the analysis.
Figure 1. Odds ratios of major adverse cardiac events (A), Ischemia-driven target lesion revascularization (B), Myocardial infarction (C), and Definite or probable stent thrombosis (D) associated with everolimus-eluting stent versus paclitaxel-eluting stent. The size of the data marker is proportional to the weight of the individual studies, measured as the inverse of the variance in the study. EES = everolimus-eluting stent; PES = paclitaxel-eluting stent; CI = confidence interval.
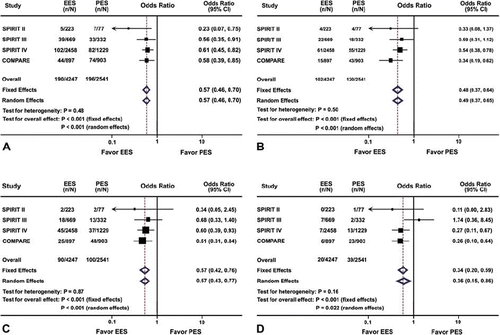
Ischemia-driven TLR was needed in 102 (2.4%) patients in the EES group and 120 (4.7%) patients in the PES group (OR 0.48; 95% CI 0.37–0.64; P < 0.001 (P = 0.50 for heterogeneity)) (). Ischemia-driven TVR was needed in 161 (3.8%) patients in the EES group and 152 (6.0%) patients in the PES group (OR 0.60; 95% CI 0.47–0.75; P < 0.001 (P = 0.18 for heterogeneity)). Risk of 1-year MI in patients with EES was significantly lower than that in patients with PES (2.1% (90/4,247) versus 3.9% (100/2,541); OR 0.57; 95% CI 0.42–0.76; P < 0.001 (P = 0.87 for heterogeneity)) (). Moreover, the differences between the groups were statistically significant in terms of both non-Q-wave MI (1.9% (82/4,247) versus 3.4% (86/2,541); OR 0.60; 95% CI 0.44–0.82; P = 0.001 (P = 0.92 for heterogeneity) and Q-wave MI (0.2% (8/4,247) versus 0.7% (17/2,541); OR 0.33; 95% CI 0.14–0.79; P = 0.02 (P = 0.63 for heterogeneity)). In contrast, patients treated with EES and PES did not differ significantly with respect to either all-cause mortality (1.2% (53/4,247) versus 1.4% (35/2,541); OR 0.95; 95% CI 0.61–1.46; P = 0.81 (P = 0.51 for heterogeneity)) or cardiac mortality (0.6% (26/4,247) versus 0.7% (19/2,541); OR 0.93; 95% CI 0.52–1.68; P = 0.81 (P = 0.61 for heterogeneity)) within 1 year follow-up.
The rate of definite and probable ST for up to 1 year remained significantly lower in the EES group compared with the PES group (0.5% (20/4,247) versus 1.5% (39/2,541); OR 0.34; 95% CI 0.20–0.59; P < 0.001 (P = 0.16 for heterogeneity)) (). Both the early (< 1 month) ST (0.2% (8/4,247) versus 0.9% (22/2,541); OR 0.25; 95% CI 0.11–0.58; P = 0.001) and late (1 month–1 year) ST (0.2% (7/4,247) versus 0.6% (15/2,541); OR 0.37; 95% CI 0.16–0.89; P = 0.03) occurred in fewer patients with EES compared to those with PES.
Discussion
The present meta-analysis shows that a second-generation EES was superior to a first-generation PES, with a significant reduction in the rate of major adverse cardiac events at 1 year. This beneficial effect was associated with a decrease in the risk of MI and ischemia-driven TLR. Moreover, the use of an EES as compared with a PES resulted in significantly lower risks of both early and late ST within a 12-month follow-up period.
Compared with the commonly used first-generation DES, second-generation DES have been designed with the goal of providing better stent performance, safety, and efficacy. They differ from the first-generation stents in terms of the anti-proliferative drug, the polymer layer, the stent frame, and the delivery system (Citation13). With this newer DES, an effective anti-proliferative agent everolimus is released from a thin coating of a biocompatible fluoropolymer on a lower-profile, open-cell, flexible cobalt-chromium stent. Improved efficacy or delivery of the anti-proliferative agent everolimus may result in less neointimal proliferation and restenosis compared with the PES, consequently leading to a further reduced target lesion reintervention. On the other hand, reduction in procedure-related non-Q-wave MI with the EES may result from less side-branch compromise due to the thinner polymer plus stent strut width compared with the PES. Furthermore, improvements in stent structure and polymer biocompatibility may result in better stent apposition, improved endothelialization, and reduced platelet aggregation, thereby reducing the risk of ST and subsequent MI.
Preclinical data have shown more rapid and extensive endothelialization with the second-generation EES compared with the first-generation sirolimus-eluting stent (SES) and PES (Citation14,Citation15). In this meta-analysis, both early and late stent thromboses were noted statistically reduced in the EES group compared to the PES group during 12 months follow-up. However, use of first-generation DES has been associated with increased rates of late ST compared with BMS, and this difference emerges only after 1 year of follow-up (Citation16). In this regard, it is noteworthy that 5-year clinical follow-up from the SPIRIT FIRST trial demonstrates that the safety of EES observed at 1-year follow-up is maintained with no additional thrombotic events between 1 and 5 years follow-up (Citation17), and a recent pooled analysis of the 2-year clinical follow-up from the SPIRIT II and III trials suggested that incidence of ST after 1 year is numerically lower in EES than in PES (Citation18). However, definitive conclusions about ST must await longer follow-up data and further dedicated studies performed in real-world (off-label) complex patient and lesion subgroups (i.e. diabetes mellitus, bifurcation, left main, etc.).
A limitation of our meta-analysis is that all individual trials included were open-label studies given the impossibility of blinding the operator to the stent system used. Another limitation is the fact that SPIRIT II, III, and IV compared the ‘old’ version of PES (Taxus Express) with EES, whereas COMPARE compared the new thin-strut version of PES (Taxus Liberte) with EES. In addition, the clinically relevant differences between EES and another widely used first-generation SES were not evaluated in the present study. Finally, the present meta-analysis is not based on individual patient data, and the time-to-event analyses could not be performed.
In conclusion, this meta-analysis shows that the new-generation EES, as compared with the PES, resulted in reduced rates of ischemia-driven TLR, MI, and ST in patients with CAD and thereby led to a reduction in major adverse cardiac events at 1 year after initial procedure. More and longer follow-up data will certainly provide important additional information.
Declaration of interest: The authors state no conflict of interest and have received no payment in preparation of this manuscript.
References
- Camenzind E, Steg PG, Wijns W. Stent thrombosis late after implantation of first-generation drug-eluting stents: a cause for concern. Circulation. 2007;115:1440–55.
- Serruys PW, Ong AT, Piek JJ, Neumann FJ, van der Giessen WJ, Wiemer M, . A randomized comparison of a durable polymer Everolimus-eluting stent with a bare metal coronary stent: The SPIRIT first trial. EuroIntervention. 2005;1:58–65.
- Ruygrok PN, Desaga M, Van Den Branden F, Rasmussen K, Suryapranata H, Dorange C, . One year clinical follow-up of the XIENCE V everolimus-eluting stent system in the treatment of patients with de novo native coronary artery lesions: the SPIRIT II study. EuroIntervention. 2007;3:315–20.
- Stone GW, Midei M, Newman W, Sanz M, Hermiller JB, Williams J, . Comparison of an everolimus-eluting stent and a paclitaxel-eluting stent in patients with coronary artery disease: a randomized trial. JAMA. 2008;299:1903–13.
- Kedhi E, Joesoef KS, McFadden E, Wassing J, van Mieghem C, Goedhart D, . Second-generation everolimus-eluting and paclitaxel-eluting stents in real-life practice (COMPARE): a randomised trial. Lancet. 2010;375:201–9.
- Stone GW, Rizvi A, Newman W, Mastali K, Wang JC, Caputo R, . Everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease. N Engl J Med. 2010; 362:1663–74.
- Egger M, Ebrahim S, Smith GD. Where now for meta-analysis? Int J Epidemiol. 2002;31:1–5.
- Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, . Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–51.
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327:557–60.
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101.
- Doostzadeh J, Clark LN, Bezenek S, Pierson W, Sood PR, Sudhir K. Recent progress in percutaneous coronary intervention: evolution of the drug-eluting stents, focus on the XIENCE V drug-eluting stent. Coron Artery Dis. 2010; 21:46–56.
- Joner M, Quee SC, Coleman L, Skorija K, Wilson P, Kolodgie FD, . Competitive comparison of reendothelialization in drug eluting stents. Circulation. 2006;114:II–506.
- Joner M, Nakazawa G, Finn AV, Quee SC, Coleman L, Acampado E, . Endothelial cell recovery between comparator polymer-based drug-eluting stents. J Am Coll Cardiol. 2008;52:333–42.
- Stone GW, Moses JW, Ellis SG, Schofer J, Dawkins KD, Morice MC, . Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med. 2007; 356:998–1008.
- Wiemer M, Serruys PW, Miquel-Hebert K, Neumann FJ, Piek JJ, Grube E, . Five-year long-term clinical follow-up of the XIENCE V everolimus eluting coronary stent system in the treatment of patients with de novo coronary artery lesions: the SPIRIT FIRST trial. Catheter Cardiovasc Interv. 2010;75:997–1003.
- Onuma Y, Serruys PW, Kukreja N, Veldhof S, Doostzadeh J, Cao S, . Randomized comparison of everolimus- and paclitaxel-eluting stents: pooled analysis of the 2-year clinical follow-up from the SPIRIT II and III trials. Eur Heart J. 2010;31:1071–8.