Abstract
Background. Current treatment of hyperglycemia in type 2 diabetes (T2DM) is often ineffective and has unwanted effects. Therefore, novel antidiabetic drugs are under development.
Objective. To assess efficacy and safety of the new antidiabetic drugs sodium glucose co-transport-2 (SGLT2) inhibitors in T2DM.
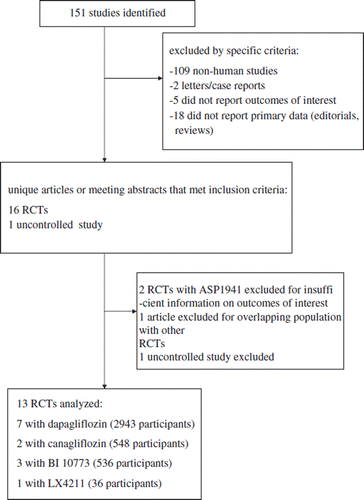
Design and setting. Among 151 articles published on MEDLINE, Cochrane Library, EMBASE, PubMed, International meeting abstracts through December 2010, 13 randomized placebo-controlled trials (RCT) were included.
Measurements. Two reviewers retrieved articles and evaluated study quality by appropriate scores. Main outcomes were pooled using random- or fixed-effects models.
Results. Dapagliflozin significantly reduced HbA1c (weighted mean difference (WMD) −0.52%; 95% CI −0.46, −0.57%; P < 0.00001) fasting plasma glucose (WMD −18.28 mg/dL; 95% CI −20.66, −15.89; P < 0.00001), body mass index (WMD −1.17%; −1.41, −0.92%; P < 0.00001), systolic (WMD −4.08 mmHg; −4.91, −3.24), and diastolic (WMD −1.16 mmHg; −1.67, −0.66) blood pressure, and serum uric acid (WMD −41.50 μmol/L; −47.22, −35.79). Other SGLT2 inhibitors showed similar results. Dapagliflozin treatment increased the risk of urinary (OR 1.34; 1.05–1.71) and genital (OR 3.57; 2.59–4.93) tract infection; it also mildly increased the risk of hypoglycemia (OR 1.27; 1.05–1.53) when co-administered with insulin.
Limitations. Limitations of the literature include the small number, size, and duration of RCTs.
Conclusions. Pending confirmation from larger RCTs, this analysis shows SGLT2 inhibitors are safe and effective for hyperglycemia treatment in T2DM.
| Abbreviations | ||
| ADA | = | American Diabetes Association |
| BP | = | blood pressure |
| EASD | = | European Association for the Study of Diabetes |
| EOT | = | end of treatment |
| FPG | = | fasting plasma glucose |
| GTI | = | genital tract infection |
| HDL | = | high-density lipoprotein |
| LDL | = | low-density lipoprotein |
| OAD | = | oral antidiabetic drugs |
| PRISMA | = | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RCT | = | randomized controlled trials |
| SGLT | = | sodium glucose co-transporter |
| T2DM | = | type 2 diabetes mellitus |
| UKPDS | = | United Kingdom Prospective Diabetes Study |
| UTI | = | urinary tract infection |
| WMD | = | weighted mean difference |
Key messages
Sodium glucose co-transport-2 (SGLT2) inhibitors are a new class of oral antidiabetic drugs that lower plasma glucose by enhancing renal glucose excretion. Available randomized controlled trials evaluate dapagliflozin, canagliflozin, BI 10773, and LX4211 over periods ranging from 2 to 48 weeks.
SGLT2 inhibitors effectively reduce HbA1c and fasting plasma glucose values; they also reduce body mass index, blood pressure, and serum uric acid. With dapagliflozin, doses exceeding 10–20 mg/d do not seem to add further benefit on evaluated outcomes.
Overall, the treatment with SGLT2 inhibitors was safe, without major adverse events. SGLT2 inhibitors yielded a mildly increased risk of mild hypoglycemic events, mostly when superimposed on background insulin therapy, and an increased risk of urinary and genital tract infections, the latter significantly dose-related.
While benefits and safety of these drugs await confirmation in adequately powered RCTs of longer duration, possible strategies to decrease the risk of side-effects include tapering background insulin dose when starting SGLT2 inhibitors, instructing patients to report signs/symptoms of urinary/genital tract infections, and regularly monitoring them for such events.
Introduction
The chronic hyperglycemia of diabetes is associated with long-term damage, dysfunction, and failure of different organs, including eyes, kidneys, nerves, heart, and blood vessels, through mechanisms collectively called ‘glucotoxicity’ (Citation1). Hyperglycemia is considered a major risk factor for microvascular complications in both type 1 and type 2 diabetes, and its reduction is definitely associated with delayed retinopathy and kidney disease progression (Citation2,Citation3). Less evidence is available regarding vascular glucose toxicity (Citation4) and related macrovascular complications that confer a higher risk of cardiovascular disease, premature death, and cardiac failure in type 2 diabetes (T2DM) (Citation5). In prospective epidemiological studies, the incidence of many of these outcomes is directly associated with the degree of hyperglycemia, as assessed by plasma glucose or glycated hemoglobin (HbA1c), the latter reflecting mean blood glucose level during the previous 2–3 months. According to the United Kingdom Prospective Diabetes Study (UKPDS), a 1% increase in HbA1c is associated with an 18% increase in the risk of cardiovascular events and with a 12%–14% increased mortality (Citation2). Similar data were reported in a meta-analysis of 13 observational studies (Citation6).
In line with these data, the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) recommended initial treatment with metformin plus life-style changes at the time of diagnosis of T2DM and subsequent timely augmentation of therapy with additional agents (including early initiation of insulin therapy) to achieve and maintain recommended levels of glycemic control (i.e. HbA1c ≤ 7%) (Citation7). However, the achievement and maintenance of these goals may be at the same time difficult and hazardous with available treatments: in the UKPDS only 25% of patients maintained adequate glycemic control after 9 years, while in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study mortality increased when normoglycemia was pursued with combination pharmacological therapy (Citation8,Citation9). Collectively, these data highlight the need for more effective and safer treatment modalities for T2DM. Recently, a new class of drugs, the inhibitors of sodium glucose co-transporters (SGLTs), has been evaluated in randomized controlled trials (RCTs) (Citation10). SGLTs are a family of active sodium-dependent membrane transport proteins that accomplish the reabsorption of more than 99% of the plasma glucose that filters through the renal glomerulus. Two types of SGLTs have been characterized: the low-capacity, high-affinity SGLT, responsible for most of intestinal glucose absorption and for approximately 10% of renal glucose reabsorption, and the high-capacity, low-affinity transporter SGLT2, located in the proximal segment of the renal proximal tubule, responsible for nearly 90% of the active renal glucose reabsorption (Citation11). Most compounds of this class selectively inhibit SGLT2 and reduce plasma glucose by enhancing renal glycosuria. Importantly, the magnitude of glycosuria induced by SGLT2 inhibition depends on the amount of filtered glucose load, which is a function of plasma glucose concentration; therefore, blood glucose levels cannot be lowered below physiological levels, and the risk of hypoglycemia is theoretically absent with these drugs. Consistent with this hypothesis, subjects affected by familial renal glycosuria, a spontaneous loss-of-function mutation of the SGLT2 gene, have renal glycosuria in the range of range 1–150 g/1.73 m2 per day, are healthy, normoglycemic, with normal kidney function and no increased risk of hypoglycemia or severe hypovolemia (Citation12). Based on these observations and on the evidence for enhanced renal SGLUT2 expression and glucose reabsorption in T2DM patients (Citation13,Citation14), SGLT2 inhibition was proposed as a safe and effective antidiabetic treatment option (Citation14). Current SGLT2 inhibitors represent an evolution of non-selective SGLT inhibitor phlorizin, a natural product found in several fruits (particularly in the bark of apple trees), which normalized plasma glucose and corrected glucotoxicity-induced decline in insulin sensitivity and pancreatic β cell function in animal models of diabetes (Citation15–17). However, several shortcomings of phlorizin prevented its clinical application: poor intestinal absorption and rapid drug degradation by β glycosidase, galactose malabsorption, and diarrhea, due to SGLT1 inhibition in the gastrointestinal tract (Citation11). Following phlorizin, other compounds were subsequently developed and tested in clinical trials: the selective SGLT2 inhibitors dapagliflozin, canagliflozin, and BI 10773, and LX4211, which inhibits both SGLT2 and SGLT1. We meta-analyzed available RCTs evaluating the efficacy and safety of this new class of drugs in T2DM.
Methods
Data sources and searches
Databases searched through December 2010 were: MEDLINE, Ovid MEDLINE In-Process, Cochrane CENTRAL Register of Controlled Trials, Cochrane Database of Systematic Reviews, EMBASE, PubMed, clinicaltrials.gov, and ADA/EASD meeting abstracts, which were subjected to the same assessment as regular articles. Authors were contacted to verify results and methodological quality of retrieved articles.
Search terms. The search terms were: sodium glucose co-transport-2 inhibitors, sodium-glucose transport inhibitors, SGLT2 inhibitors, dapagliflozin, remogliflozin, sergliflozin, canagliflozin, ASP1941, BI 10773, AVE2268, management, therapy, treatment, trial (an example of online strategy run is provided in the online Supplementary Material).
Study selection
Inclusion criteria were: English and non-English articles with participants aged >18 years, of any sex or ethnic origin with T2DM.
Exclusion criteria were: non-human studies, non-randomized trials, letters/case reports, articles not reporting outcomes of interest or primary data (editorials, reviews).
Outcome measures were: primary outcomes assessed were the changes from base-line in mean HbA1c and fasting plasma glucose (FPG) levels. Secondary outcome measures were change in body mass index (BMI) and waist circumference, systolic and diastolic blood pressure, plasma triglyceride and total/high-density (HDL)/low-density (LDL) cholesterol, and serum uric acid. Where available, the changes in parameters of glucose metabolism (insulin sensitivity and pancreatic β cell function) were evaluated. As safety measures, serum creatinine and electrolyte changes and the rate of hypoglycemic and other adverse events were evaluated. All measures of dispersion were converted to standard deviations (SDs). When standard deviations were not reported, estimated base-line and final standard deviations were derived from data from other studies with the same drug and dose.
Data extraction and quality assessment
Data were extracted independently and in duplicate by two authors (GM, GP) using a predefined protocol based on the Cochrane Handbook for Systematic Reviews of Intervention; discrepancies were resolved by mutual discussion. The agreement between the two reviewers for selection and validity assessment of trials was scored by kappa coefficient.
The quality of RCTs was assessed by the Cochrane Risk-of-Bias Tool, attributing 1 point to each item (total score range: 0–8) () (Citation18).
Table I. characteristics of randomized controlled trials included in the meta-analysis.
Data synthesis and analysis
We used WinBUGS 1.4 (WinBUGS 1996–2003, Imperial College of Science & MRC, UK). The analysis was reported according to PRISMA guidelines (Citation18,Citation19). Treatments were evaluated on an intention-to-treat principle. Dichotomous variables were presented as odds ratios (OR) with 95% CI, continuous variables as weighed mean differences (WMD) with 95% CI. The fixed-effect model was used, with significance set at P = 0.05. Statistical heterogeneity was assessed using the I2 statistic: with I2 values ≥50%, we used a random-effects model and explored individual study characteristics and those of subgroups of the main body of evidence. Sensitivity analysis was performed by removing one study at a time and repeating the meta-analysis to assess whether any one study significantly affected pooled estimates. Additionally, we planned a-priori subgroup analysis according to the following criteria: duration of diabetes, initial HbA1c levels (≥ 8.5% versus <8.5%), background therapy (life-style intervention versus metformin versus insulin versus other oral antidiabetic drugs (OADs)), treatment duration (<12 versus ≥12 weeks), different drugs of the same class or different doses of the same drug, and for each item of the Cochrane Risk-of-Bias Tool. When >10 comparisons were available, the effects of different doses of SGLT2 inhibitor, of base-line HbA1c, of treatment duration, and of diabetes duration on each outcome were assessed by meta-regression. The dose variable in the regression equation was treated categorically, with the starting dose coded as the base-line amount and each doubling of a drug dose was a single increment increase. Publication bias was examined using funnel plots.
Results
The agreement between two reviewers for study selection was 0.85 and for quality assessment of trials was 0.86. The flow of study selection is reported in . At the end of selection, 13 RCTs (quality score ranging 7–8), comprising 47 different comparisons, were included in the meta-analysis: seven RCTs assessed dapagliflozin (2,943 participants, duration ranging 2–48 weeks, daily dose ranging 2.5–100 mg) (Citation20–26); two assessed canagliflozin (548 participants, trial duration ranging 2–12 weeks, dose ranging 30–600 mg/d) (Citation27,Citation28); three assessed BI 10773 (536 participants, trial duration ranging 8 days–12 weeks, dose ranging 5–100 mg/d) (Citation29–31); and one RCT assessed LX4211 (36 participants, trial duration 4 weeks, dose ranging 150–300 mg/d) (Citation32) (). The median (range) base-line HbA1c across the study populations was 8% (6%–10%), and background antidiabetic treatment in the studies included: life-style intervention alone in four RCTs, metformin or diet in one RCT, OADs in six RCTs (in two RCTs OADs were discontinued prior to randomization), and insulin with or without OADs in two RCTs.
Renal glucose excretion
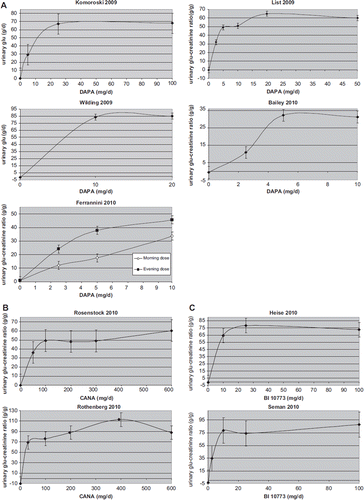
plots the relationship of different doses of dapagliflozin (five RCTs), canagliflozin (two RCTs), and BI 10773 (two RCTs) with urinary glucose excretion measured at the end of RCTs: in all RCTs SGLT2 inhibitors maintained a significant glycosuric effect; the analysis of dose–response relationship suggests little incremental benefit with increasing doses beyond 10–25 mg/d for dapagliflozin, 200–400 mg/d for canagliflozin, and 10–25 mg/d for BI 10773.
HbA1c levels
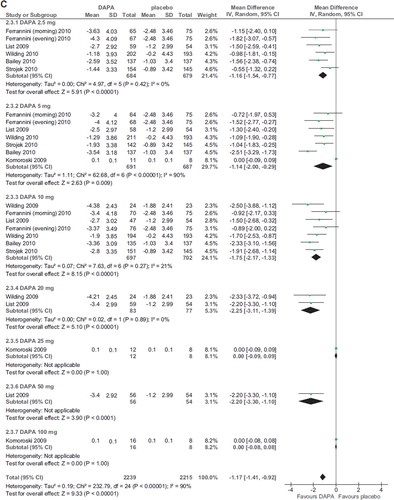
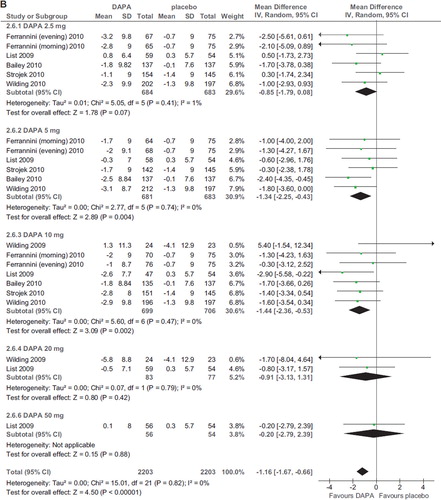
Six RCTs (2,896 participants, duration ranging 12–48 weeks, doses ranging 2.5–50 mg) evaluated dapagliflozin in diabetic patients treated with diet alone (Citation20,Citation25), OADs (Citation24,Citation26), and insulin with or without OADs (Citation22,Citation23) (). Compared with placebo, dapagliflozin at all doses significantly improved HbA1c (WMD −0.52%; 95% CI 0.46–0.57%; P < 0.00001; n comparisons = 22) (). The effect was independent of trial duration, base-line HbA1c, and background antidiabetic therapy. There was little or no heterogeneity in the meta-analysis (I2 = 14%), suggesting a consistent drug effect. HbA1c improvement with dapagliflozin 10 mg/d was significantly higher than with 2.5–5 mg/d (P < 0.01 for all), while higher doses did not improve HbA1c further compared with the 10 mg dose (P > 0.5 for all comparisons).
Figure 3. A: Forest plot of comparison: dapagliflozin (DAPA), outcome: HbA1c changes from base-line (%). B: Forest plot of comparison: dapagliflozin (DAPA), outcome: FPG changes (mg/dL). C: Forest plot of comparison: dapagliflozin (DAPA), outcome: weight changes from base-line (%).
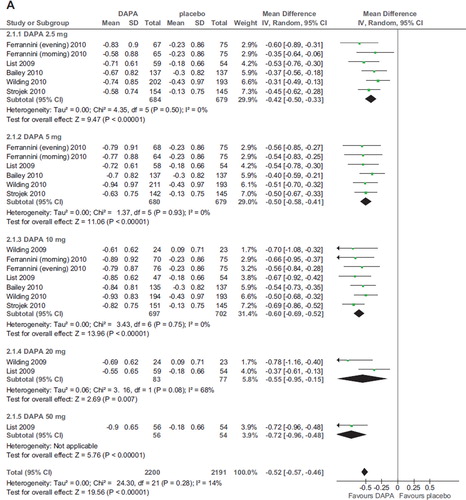
Three RCTs evaluated other SGLT2 inhibitors (one canagliflozin, one BI 10773, and one LX4211; total 895 patients; duration ranging 4–12 weeks): they all found a significant improvement in HbA1c compared to placebo (Citation27,Citation29,Citation32) (; online Supplementary Figure 1A–C).
Fasting plasma glucose (FPG)
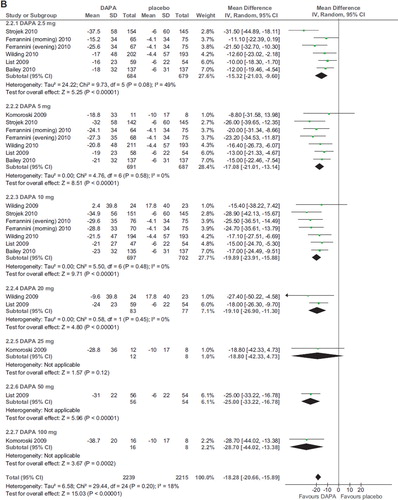
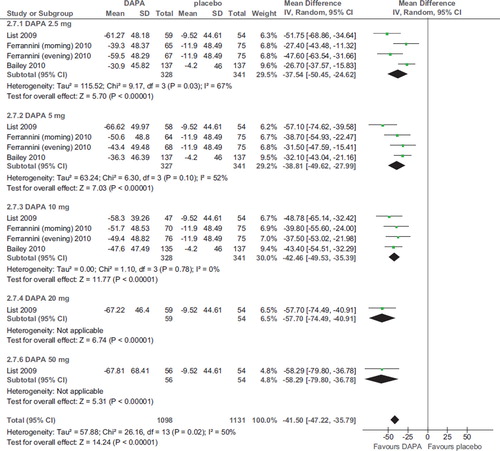
Seven RCTs (2,943 participants, duration ranging 2–48 weeks, doses ranging 2.5–100 mg) evaluated dapagliflozin in diabetic patients treated with diet alone (Citation20,Citation21,Citation25), OADs (Citation24,Citation26), and insulin with or without OADs (Citation22,Citation23) (). Compared with placebo, dapagliflozin significantly and dose-independently reduced FPG (WMD -18.28 mg/dL; 95% CI −20.66, −15.89; P < 0.00001; n comparisons = 25) (). There was little or no heterogeneity in the meta-analysis (I2 = 18%), suggesting a consistent drug effect. The effect was not influenced by trial duration and background antidiabetic therapy. Funnel plots analysis found no strong publication bias.
Six RCTs evaluated other SGLT2 inhibitors (two canagliflozin, three BI 10773, and one LX4211; total 1,120 patients, duration ranging 1–12 weeks) (Citation27–32), finding a significant improvement in FPG compared to placebo. For canagliflozin and BI 10773, the improvement was significant only with doses >30 and 2.5 mg/d, respectively, without dose-dependency (; online Supplementary Figure 2A–C).
Body mass index (BMI) and waist circumference
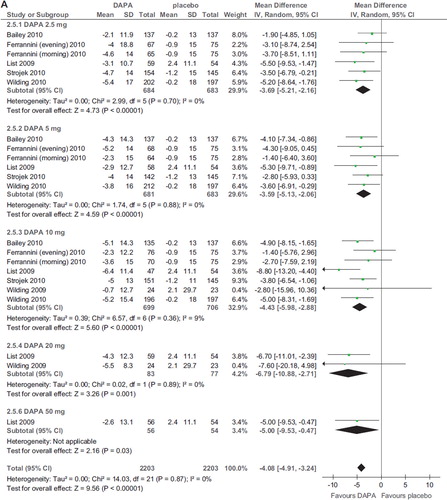
Compared to placebo, dapagliflozin induced a modest but significant BMI reduction (WMD −1.17%; 95% CI −1.41, −0.92; P < 0.00001; n comparisons = 7; I2 = 90%) (). Heterogeneity was not dependent on different doses or background treatment but was abated after exclusion of the RCT with the shortest duration (2 weeks) (Citation21): WMD −1.54%; 95% CI −1.81, −1.28; P < 0.00001; n comparisons = 25; I2 = 30%. There was no significant difference in BMI reduction among different doses (P > 0.4 for all comparisons).
Two RCTs with canagliflozin and one RCT with BI 10773 found similar results (Citation27–29) (online Supplementary Figure 3A–B)
Only two RCTs (Citation20,Citation24) assessed the effect of dapagliflozin on indexes of abdominal obesity, finding a small but significant waist circumference reduction compared to placebo (WMD −1.20 cm; 95% CI −2.00, −0.43; P = 0.004; n comparisons = 7; I2 = 0%).
Blood pressure (BP)
Compared to placebo, dapagliflozin induced a significant reduction in systolic BP at all doses (WMD −4.08 mmHg; 95% CI −4.91, −3.24; P < 0.00001; n comparisons = 22; I2 = 0%) (). There was no significant difference in systolic BP reduction among different doses (P > 0.1 for all comparisons). Diastolic BP was also modestly reduced (WMD −1.16 mmHg; 95% CI −1.67, −0.66; P < 0.00001; n comparisons = 7; I2 = 0%) (). These effects were not associated with an increased incidence of orthostatic hypotension (not shown).
Figure 4. A: Forest plot of comparison: dapagliflozin (DAPA), outcome: sys BP changes (mmHg). B: Forest plot of comparison: dapagliflozin (DAPA), outcome: dia BP changes (mmHg).
In one RCT, 29.5%–37.5% of hypertensive patients with uncontrolled BP at base-line achieved a BP ≤ 130/80 at end of treatment (EOT) with dapagliflozin, compared with 8.8% of patients on placebo (P < 0.007 for all doses) (Citation24).
Effect on serum uric acid level
Compared with placebo, dapagliflozin significantly reduced serum uric acid (WMD −41.50; 95% CI −47.22, −35.79 μmol/L; P < 0.00001; n comparisons = 14; I2 = 50%) (). There was no significant difference in serum uric acid reduction among different doses (P > 0.3 for all comparisons).
β Cell function
Two RCTs (548 participants; duration of 2 and 12 weeks) evaluated the effect of canagliflozin on β cell function, finding a significant improvement with doses ≥100 mg/d (Citation27,Citation28) (online Supplementary Figure 4).
Plasma lipids
Three RCTs (891 participants; duration 12–24 weeks) evaluated the effect of dapagliflozin on fasting plasma lipids, finding a marginally significant improvement in HDL-C, but not in triglyceride, total and LDL-cholesterol (Citation22,Citation24,Citation25) (HDL-C reported in online Supplemental Figure 5).
Hypoglycemia
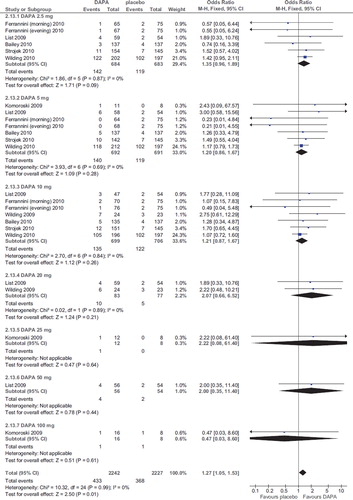
Compared with placebo, dapagliflozin was associated with a higher risk of mild hypoglycemic events (OR 1.27; 95% CI 1.05–1.53; P = 0.01; n comparisons = 25; I2 = 0%) (), not generally leading to drug discontinuation.
The risk was not related to dose, duration, initial HbA1c, initial FPG, changes in HbA1c and FPG, and urinary glucose excretion in a meta-regression model (all P values > 0.5). Sensitivity analysis revealed the risk was not dose-related and was entirely and exclusively explained by co-administration of insulin: after excluding the two RCTs with insulin as background treatment (Citation22,Citation23) the OR for incident hypoglycemia was 1.31 (95% CI 0.93–1.86; P = 0.13; n comparisons = 20; I2 = 0%). Mild hypoglycemic events were particularly frequent (50%–60% of all patients in each arm) when insulin dose was not down-titrated at study entry (Citation23).
The overall incidence of major hypoglycemic events did not exceed 1% and was not significantly increased by dapagliflozin treatment (not shown).
There were too few studies to assess the risk of hypoglycemia with other SGLT2 inhibitors.
Urinary tract infections (UTIs)
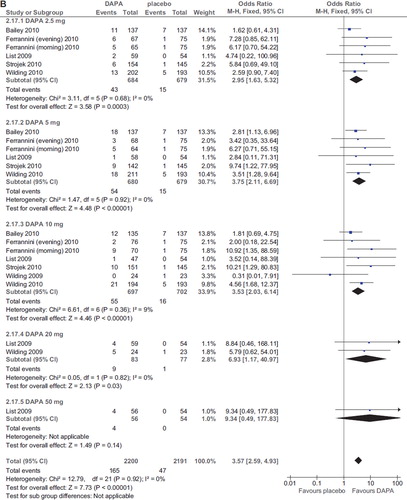
Dapagliflozin treatment was associated with an increased risk of UTI (OR 1.34; 95% CI 1.05–1.71; P = 0.02; n comparisons = 25; I2 = 0%) (). The risk was not related to dose, duration, initial HbA1c, initial FPG, changes in HbA1c and FPG, or urinary glucose excretion in a meta-regression model (all P values > 0.2).
Figure 7. A: Forest plot of comparison: dapagliflozin (DAPA), outcome: incident UTI (%). B: Forest plot of comparison: dapagliflozin (DAPA), outcome: incident GTI (%).
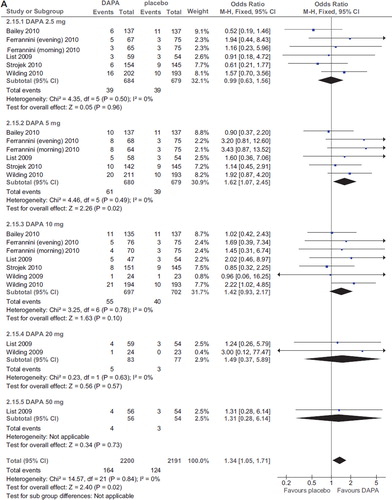
There were two cases of acute pyelonephritis in the 2.5 and 5 mg arm in the RCT of longest duration (48 weeks) (Citation23), responding to antibiotic therapy and not leading to dapagliflozin discontinuation.
Genital tract infections (GTIs)
Dapagliflozin treatment was associated with an increased, dose-related risk of GTI (OR 3.57; 95% CI 2.59–4.93; P < 0.00001; n comparisons = 25; I2 = 0%) (). The risk of GTIs was directly associated with the dose of dapagliflozin (Beta = 0.31; P = 0.02), but not with duration, initial HbA1c, initial FPG, changes in HbA1c and FPG, or urinary glucose excretion in a meta-regression model (all P values > 0.5).
Other side-effects
There was no significant alteration in serum creatinine, Na, K, Ca levels with SGLT2 inhibitors. The incidence of headache, nasopharyngitis, constipation, and diarrhea was not increased by SGLT2 inhibitors (online Supplementary Figure 6A–D)
Discussion
Our analysis disclosed a significant benefit exerted by SGLT2 inhibitors on many glycemic and non-glycemic metabolic outcomes in T2DM. SGLT2 inhibitors represent a substantial paradigm shift in the treatment of diabetes, enhancing a phenomenon that has been so far considered an unwanted effect of diabetes. These drugs consistently improved HbA1c and FPG at all doses, with no clear linear dose-response relationship: in available RCTs, the highest doses did not further increase renal glucose excretion nor yield additional benefit on glycemic parameters. Several studies evaluated the effect of different doses of SGLT2 inhibitors on renal glucose excretion in diabetic and non-diabetic subjects (Citation21,Citation33–36), finding a plateau of 40% −44% renal tubular glucose reabsorption inhibition with dapagliflozin doses > 20 mg/d. As most RCTs assessed urinary glucose excretion at the end of the trial, these findings may be explained by the observation that the absolute amount of urinary filtered glucose load depends on plasma glucose levels and therefore tends to decrease following improved glycemic control over time ().
Animal data suggested that the correction of chronic hyperglycemia and related glucotoxicity may also restore insulin sensitivity in the liver, muscle, and adipose tissue and reduce the decline of pancreatic β cell viability and function (Citation12,Citation37,Citation38). In the few studies evaluating glucose homeostatic indices, SGLT2 inhibitors consistently improved pancreatic β cell function. Further larger RCTs are required to confirm these benefits on glucose homeostasis as well as the optimal dose range of these drugs.
The benefits of this new class of drugs extend beyond glycemic control: SGLT2-treated patients experienced modest but significant BMI reduction with all doses of SGLT2 inhibitors, regardless of background antidiabetic treatment. The mechanisms of weight loss are partially understood. Chronic administration of phlorizin induces lipolysis in lactating cows (Citation22), and dapagliflozin induces reduced adiposity in obese rats (Citation39,Citation40). In the RCT by List (Citation20), weight loss was more pronounced during the initial weeks of treatment, particularly with higher dapagliflozin doses, while in the remaining RCTs all doses induced gradual, progressive weight loss. This observation, coupled with a rapid weight rebound after discontinuation of higher doses, suggests that osmotic diuresis and fluid loss may contribute to early weight loss, whereas continued gradual weight loss represents fat mass reduction induced by glycosuria-mediated steady caloric loss (Citation24,Citation25). The insulin-sparing effect of SGLT2 inhibitors may also have enhanced weight loss in patients on insulin (Citation41). Longer-term clinical and body composition studies will help to establish the relative contribution of diuresis versus adiposity reduction to overall weight loss and will assess whether the visceral or subcutaneous fat compartment is primarily involved.
The glucose-induced osmotic diuresis caused by SGLT2 inhibition may also explain the blood pressure- and uric acid-lowering effect of these drugs. Overall, the fluid loss induced by these drugs was safe and did not increase the risk of orthostatic hypotension and dizziness or altered renal function and serum electrolyte level. The clinical utility of these actions was documented in one RCT, where the addition of dapagliflozin enhanced BP control in previously uncontrolled hypertensive patients (Citation24).
Dapagliflozin treatment was associated with an increased risk of hypoglycemia and urinary and genital tract infections. However, the risk was confined to mild hypoglycemic events and was increased only by co-administration of insulin, particularly when the study protocol did not schedule early insulin dose down-titration. Urinary and genital tract infections were more common with SGLT2 inhibitors, but they were generally mild and responded to standard therapy. Mechanisms underlying the increased risk of urinary and genital tract infections are unclear. The incidence of genital tract infections was linearly dose-related, while the risk of UTIs was not. Enhanced glycosuria may predispose to bacterial and mycotic growth. However, 24-h urinary glucose excretion did not linearly increase in available RCTs, reaching a plateau with moderate–high doses of SGLT2 inhibitors (). A possible explanation for this discrepancy is that the highest doses of SGLT2 inhibitors may cause greater glycosuria earlier in the studies, resulting in a greater decline in glycemia and in lower renal filtered glucose load, such that by the end of the trials urinary glucose excretion had equalized across different dapagliflozin dose groups (). If such was the case, then the excessive incidence of GTI would be expected early after the initiation of SGLT2 inhibitors.
Implications for practice
From the data presented above, it can be derived that an optimal daily dose of dapagliflozin might lie between 10 and 20 mg/day, as higher doses would not seem to further enhance renal glucose excretion, glycemic or metabolic control, while, conversely, they may increase the risk of GTIs. However, these findings require confirmation by larger and longer RCTs before they can impact routine clinical practice.
Regarding side-effects, the risk of mild hypoglycemia seems to be slightly increased by co-administration of insulin, and prompt down-titration of total insulin dose may reduce the risk of hypoglycemic events; the optimal protocol of insulin dose de-escalation needs to be established.
Lastly, diabetic patients on SGLT2 inhibitors should be instructed to promptly report clinical signs and symptoms of UTIs and GTIs to their physicians; regular active monitoring for such events may be warranted in order to prevent more serious infections, including pyelonephritis.
Open issues and future directions
If these data are confirmed by RCTs of adequate power and duration, SGLT2 may represent a useful tool for the treatment of diabetic patients, both as initial therapy in drug-naive patients and as a rescue therapy after failure of OAD and insulin. Its insulin-independent mechanisms of action and insulin-sparing properties may also allow insulin dose reduction and enhance weight loss in obese subjects.
Our analysis highlights the overall short-term safety and efficacy of SGLT2 inhibitors, but these drugs need further assessment. In the longest (48-wk) RCT, two cases of pyelonephritis were observed with dapagliflozin (Citation23): whether recurrent UTIs may predispose to potentially serious infections warrants evaluation in larger RCTs of adequate power and duration. These RCTs should also try to identify risk factors predisposing to the development of UTIs and GTIs.
Future RCTs will need to assess whether these drugs are equally effective and safe in patients with impaired renal function, as available RCTs excluded subjects with even mildly impaired renal function. It will also be important to assess the efficacy of these drugs in type 1 diabetic subjects, and as a uric acid-lowering agent in non-diabetic subjects. Finally, the effects of SGLT2 inhibitors on whole-body inflammation (only one RCT reported marginally significant improvement in CRP with dapagliflozin), hepatic steatosis (elevated liver enzymes were an exclusion criterion in most RCTs), and insulin resistance are also unknown.
http://www.informahealthcare.com/ann/doi/10.3109/07853890.2011.560181
Download PDF (701.5 KB)Acknowledgements
Role of each author: Giovanni Musso: data collection and elaboration, statistical analysis and writing of the manuscript. Roberto Gambino: data collection and discussion, and review of the manuscript. Maurizio Cassader: data collection and discussion, and review of the manuscript. Gianfranco Pagano: data collection and discussion, and writing of the manuscript.
Declaration of interest: No author has any present or past conflict of interest to report. All authors are independent of any commercial funder; Giovanni Musso takes responsibility for the integrity of the data and the accuracy of the data analysis. This work was supported in part by the Piedmont Region Funds Comitato Interministeriale per la Programmazione Economica 2008, which were employed for data collection.
References
- American Diabetes Association. Position statement. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl1);S62–9.
- The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993; 329:977–86.
- Stratton MI, Adler AI, Neil HAW, Matthews DR, Manley SE, Cull CA, . Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000; 321:405–12.
- Brownlee M. The pathobiology of diabetic complications. A unifying mechanism. Diabetes. 2005;54:1615–25.
- Gerstein HC. Glucose: a continuous risk factor for cardiovascular disease. Diabet Med. 1997;14 (Suppl 3):S25–31
- Selvin E, Marinopoulos S, Berkenblit G, Rami T, Brancati FL, Powe NR, . Meta-analysis: Glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med. 2004;141:421–31.
- American Diabetes Association. Position statement. Standards of medical care in diabetes—2010. Diabetes Care. 2010;33 (Suppl1):S23.
- Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA. 1999;281;2005–12.
- ; Action to Control Cardiovascular Risk in Diabetes Study GroupGerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB, . Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–59.
- Chao EC, Henry RR. SGLT2 inhibition: a novel strategy for diabetes treatment. Nat Rev Drug Discov. 2010;9:551–9.
- Bakris GL, Fonseca VA, Sharma K, Wright EM. Renal sodium–glucose transport: role in diabetes mellitus and potential clinical implications. Kidney Int. 2009;75: 1272–7.
- Santer R, Calado J. Familial renal glucosuria and SGLT2: from a mendelian trait to a therapeutic target. Clin Am Soc Nephrol. 2010;5:133–41.
- Rahmoune H, Thompson PW, Ward JM, Smith CD, Hong G, Brown J. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulindependent diabetes. Diabetes. 2005;54:3427–34.
- Polidori D, Sha S, Devineni D, Rothenberg P. The renal glucose threshold is increased in patients with type 2 diabetes: results from a novel method for measuring the renal threshold. ADA 2010 meeting abstract 2186-PO.
- Ehrenkranz JRL, Lewis NG, Kahn CR, Ross J. Phlorizin: a review. Diabetes Metab Res Rev. 2005;21:31–8.
- Rossetti L, Shulman GI, Papachristou D, DeFronzo RA. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest. 1987; 79:1510–5.
- Rossetti L, Giaccari A, DeFronzo RA. Glucose toxicity. Diabetes Care. 1990;13:610–30.
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.0.0. The Cochrane Collaboration; updated February 2008. Available at: http://www.cochrane-handbook.org (accessed 26 May 2009).
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. J Clin Epidemiol. 2009;62: 1006–12.
- List JF, Woo V, Morales E, Tang W, Fiedorek FT. Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care. 2009;32:650–7.
- Komoroski B, Vachharajani N, Feng Y, Li L, Kornhauser D, Pfister M. Dapagliflozin, a novel, selective SGLT2 inhibitor, improved glycemic control over 2 weeks in patients with type 2 diabetes mellitus. Clin Pharmacol Ther. 2009;85:513–9.
- Wilding JP, Norwood P, T'joen C, Bastien A, List JF, Fiedorek FT. A study of dapagliflozin in patients with type 2 diabetes receiving high doses of insulin plus insulin sensitizers: applicability of a novel insulin-independent treatment. Diabetes Care. 2009;32:1656–62.
- Wilding JPH, Woo V, Pahor A, Sugg J, Langkilde A, Parikh S. Sustained effectiveness of dapagliflozin over 48 weeks in patients with type 2 diabetes poorly controlled with insulin. Diabetologia. 2010;53(S1):S348–9.
- Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;375: 2223–33.
- Ferrannini E, Jimenez Ramos S, Salsali A, Tang W, List JF. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase III trial. Diabetes Care. 2010;33:2217–24.
- Strojek K, Hruba V, Elze M, Langkilde A, Parikh S. Efficacy and safety of dapagliflozin in patients with type 2 diabetes mellitus and inadequate glycaemic control on glimepiride monotherapy. Diabetologia. 2010;53(S1):S347–8.
- Rosenstock J, Polidori D, Zhao Y, Sha S, Arbit D, Usiskin K, . Canagliflozin, an inhibitor of sodium glucose co-transporter 2, improves glycaemic control, lowers body weight, and improves beta cell function in subjects with type 2 diabetes on background metformin. Diabetologia. 2010; 53(S1):S349.
- Rothenberg PL, Devineni D, Ghosh A, Polidori AD, Hompesch M, Arnolds S, . Canagliflozin, a novel inhibitor of sodium glucose co-transporter 2, improved glucose control in subjects with type 2 diabetes: Results of a phase 1b study. Diabetologia. 2010;53(S1):S350–1.
- Ferrannini E, Seman LI, Seewaldt-Becker E, Hantel S, Pannetti S, Woerle HJ. The potent and highly selective sodium-glucose co-cransporter (SGLT-2) inhibitor BI 10773 is safe and efficacious as monotherapy in patients with type 2 diabetes mellitus. Diabetologia. 2010;53(S1):S351.
- Heise T, Seewaldt-Becker E, Macha S, Hantel S, Huber K, Pinnetti S, . BI 10773, a Sodium-glucose co-transporter inhibitor (SGLT-2), is safe and efficacious following 4-week treatment in patients with type 2 diabetes. Diabetes ADA 2010 meeting abstract 629-P.
- Seman L, Macha S, Jones P, Marquart A, Port A, Pinnetti S, . Safety and tolerability of BI 10773, a sodium-glucose co-transporter (SGLT-2) inhibitor, following 8-days treatment in patients with type 2 diabetes. Diabetes ADA 2010 meeting abstract 571-P.
- Freiman J, Ruff DA, Frazier KS, Combs K, Turnage A, Shadoan M, . LX4211, a dual SGLT2/SGLT1 inhibitor, shows rapid and significant improvement in glycemic control over 28 days in patients with type 2 diabetes (T2DM). Diabetes ADA 2010 meeting abstract 17-LB.
- Komoroski B, Vachharajani N, Boulton D, Kornhauser D, Geraldes M, Li L, . Dapagliflozin, a novel SGLT2 inhibitor, induces dose-dependent glucosuria in healthy subjects. Clin Pharmacol Ther. 2009;85:520–6.
- Polidori D, Sha S, Sarich T, Devineni D, Rothenberg PL. Canagliflozin lowers the renal threshold for glucose excretion in lean, obese and type 2 diabetic subjects. Diabetologia. 2010;53(Suppl1):S350.
- Sha S, Devineni D, Ghosh A, Polidori D, Chien S, Wexler D, . Canagliflozin, a novel inhibitor of sodium glucose co-transporter 2, induces dose-dependent urinary glucose excretion in healthy subjects. Diabetes ADA 2010 meeting abstract 76-OR.
- Sarich T, Devineni D, Ghosh A, Wexler D, Shalayda K, Blake J, . Canagliflozin, a novel inhibitor of sodium glucose co-transporter 2 (SGLT2), increases 24-h urinary glucose excretion and decreases body weight in obese subjects. Diabetes ADA 2010 meeting abstract 567–P.
- Macdonald FR, Peel JE, Jones HB, Mayers RM, Westgate L, Whaley JM, . The novel sodium glucose transporter 2 inhibitor dapagliflozin sustains pancreatic function and preserves islet morphology in obese, diabetic rats. Diabetes Obes Metab. 2010;12:1004–12.
- Ueta K, Torres TP, Mccoy GL, Farmer TD, Condren AB, Whitfield T, . Normalization of hyperglycemia by inhibiting SGLT2 prevents progressive reduction of hepatic glucokinase (GK) expression and improves hepatic glucose metabolism (HGM) in Zucker diabetic fatty (ZDF) rats. Diabetes ADA 2010 meeting abstract 608–P.
- Bradford BJ, Allen MS. Phlorizin induces lipolysis and alters meal patterns in both early- and late-lactation dairy cows. J Dairy Sci. 2007;90:1810–5.
- Devenney J, Harvey S, Rooney S, Godonis H, Washburn W, Whaley J, . The effect of dapagliflozin, a highly selective SGLT2 inhibitor, on body weight in diet-induced obese rats. Presented at The Obesity Society Annual Scientific Meeting 2007; New Orleans, LA, 2007; Abstract 0384.
- Zhang L, Feng Y, List J, Kasichayanula S, Pfister M. Dapagliflozin treatment in patients with different stages of type 2 diabetes mellitus: effects on glycaemic control and body weight. Diabetes Obes Metab. 2010;12:510–6.