Abstract
Atherosclerosis, the underlying cause of atherosclerotic cardiovascular disease (ACVD), develops due not only to a single cardiovascular risk factor but to a variety of complex factors. The concept of the multiple cardiometabolic risk factor clustering syndrome has been proposed as a highly atherogenic state, independent of hypercholesterolemia and smoking. Body fat distribution, especially visceral fat accumulation, is a major correlate of a cluster of diabetogenic, atherogenic, prothrombotic, and proinflammatory metabolic abnormalities referred to as the metabolic syndrome, with dysfunctional adipocytes and dysregulated production of adipocytokines (hypoadiponectinemia). Medical research has focused on visceral adiposity as an important component of the syndrome in Japanese subjects with a mild degree of adiposity compared with Western subjects. For the prevention of ACVD at least in Japan, it might be practical to stratify subjects with multiple risk factors for atherosclerotic cardiovascular disease based on visceral fat accumulation. Visceral fat reduction through health promotion programs using risk factor-oriented approaches may be effective in reducing ACVD events, as well as producing improvement in risks and hypoadiponectinemia. This review article discusses visceral adiposity as a key player in the syndrome. Visceral fat reduction with life-style modification is a potentially useful strategy in the prevention of ACVD in patients with the metabolic syndrome.
| Abbreviations | ||
| AACE | = | American Association of Clinical Endocrinologists |
| ACVD | = | atherosclerotic cardiovascular diseases |
| AHA | = | American Heart Association |
| BIA | = | bioelectrical impedance analysis |
| CT | = | computed tomography |
| EGIR | = | European Group for the Study of Insulin Resistance |
| FFA | = | free fatty acids |
| IAS | = | International Atherosclerosis Society |
| IASO | = | International Association for the Study of Obesity |
| IDF | = | International Diabetes Federation |
| NCEP- | = | National Cholesterol Education Program—Third |
| ATP III | = | Adult Treatment Panel |
| NHLBI | = | National Heart, Lung, and Blood Institute |
| VFA | = | visceral fat area |
| WHF | = | World Heart Federation |
| WHO | = | World Health Organization |
Key messages
For practical prevention of atherosclerotic cardiovascular events, it might be useful to stratify subjects with multiple risk factors based on visceral fat accumulation.
Adoption of the concept of visceral adiposity could facilitate the prevention of future ACVD events in subjects with the metabolic syndrome.
Introduction
Epidemiological, metabolic, and experimental studies conducted over more than half a century have permitted the identification of the major risk factors for cardiovascular disease (Citation1–4). The metabolic syndrome is conceptualized as a complex of interrelated cardiovascular risk factors, beyond the classic risk factors such as hypercholesterolemia and smoking (Citation5–8). These factors include dysglycemia, elevated blood pressure, elevated triglyceride levels, low high-density lipoprotein cholesterol levels, and obesity (Citation9–12). Studies of Japanese-Americans in Hawaii and Seattle also suggest that the Japanese as a race cannot handle glucose metabolism as well as Caucasians do when over-nourished and are liable to develop intolerance and complications even with a mild excess of adiposity (Citation13–17). Especially, obese East and South Asians including Japanese have a mild degree of adiposity, compared with European and American subjects (Citation18–24). Different from the amount of total body fat, body fat distribution, especially accumulation of visceral adipose tissue, has been found to be a major correlate of a cluster of diabetogenic, atherogenic, prothrombotic, and proinflammatory metabolic abnormalities referred to as the metabolic syndrome (Citation25–27). Diagnosis of the metabolic syndrome should allow the physician to recommend visceral fat reduction with life-style modification to reduce multiple risks and prevent atherosclerotic cardiovascular diseases (ACVD). This review discusses visceral adiposity as a target for the management of the metabolic syndrome from a Japanese perspective.
Concept of the visceral fat syndrome
Atherosclerosis, the underlying etiology of various cardiovascular diseases, occurs and develops not because of a single cardiometabolic factor (Citation1–4) but several complex cardiometabolic risk factors (Citation5–8). Among these is hypercholesterolemia, which is well known, particularly elevated serum levels of low-density lipoprotein, to play the most important role in the development of atherosclerosis (Citation1,Citation2). Management of hypercholesterolemia became possible after the development of effective cholesterol-lowering drugs such as statins (Citation28–32). However, it is also true that cardiovascular diseases occur in subjects without hypercholesterolemia (Citation33,Citation34). Although diabetes mellitus, hypertension, and lipid disorders such as hypertriglyceridemia or low levels of high-density lipoprotein cholesterol are recognized risk factors for atherosclerosis, they are considered to be weaker contributing factors than hypercholesterolemia. In the past 20 years, clinical and epidemiological studies have demonstrated that the coexistence of these risk factors is a strong risk factor in itself, and multiple risk factor clustering syndrome has become as important as hypercholesterolemia as an underlying cause of ACVD (Citation35–37). The incidence of obesity has increased worldwide due to the life-style of overeating and physical inactivity.
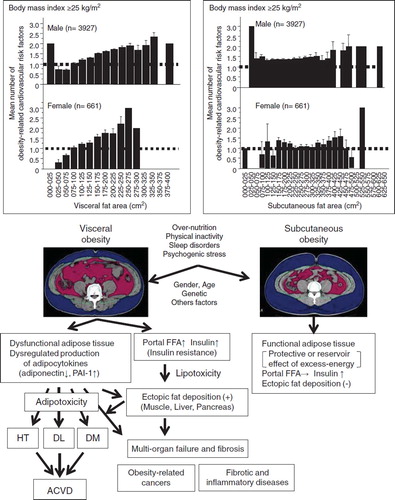
In 1956, Vague (Citation38) was the first to discover the link between adipose tissue distribution and complications in obese subjects. Subsequent clinical research demonstrated that body fat distribution, abdominal obesity, rather than total amount of fat is linked to obesity-related disorders (Citation25,Citation35–38). In 1983, Tokunaga et al. (Citation39) introduced a new method for fat analysis using computed tomography (CT) scanning, which allowed the separate analysis of subcutaneous fat and intra-abdominal visceral fat (representing fat accumulation predominantly in the mesenteric and omental regions). In obese subjects, the mean number of ACVD-related risk factors increases with CT-measured increase in visceral fat area (VFA) but not with increase in subcutaneous fat area () (Citation40). Furthermore, the mean number of risk factors did not correlate with the abdominal subcutaneous fat area in obese people () (Citation40), suggesting a possible protective effect for subcutaneous fat (Citation41–43). Thus, accumulation of intra-abdominal visceral fat, which is a unique adipose tissue both anatomically and metabolically, locates upstream of obesity-related metabolic disorders, including insulin resistance, glucose intolerance, dyslipidemia, and elevated blood pressure, leading to atherosclerosis based on clustering of multiple risk factors () (Citation25,Citation26,Citation36,Citation44–47). This is conceptualized as ‘visceral fat syndrome’ (Citation48,Citation49) or the ‘metabolic syndrome’. On the other hand, reduction of intra-abdominal fat improved metabolic disorders (Citation50,Citation51).
Figure 1. Visceral obesity and subcutaneous obesity. Top: Correlation of visceral fat area and subcutaneous fat area with obesity-related cardiovascular risk factors in obese subjects (body mass index ≥25 kg/m2). Bottom: Schematic diagrams of the complications associated with visceral obesity (left) and subcutaneous obesity (right). Reproduced with permission from Annals of Medicine (Citation40). (HT = hypertension; FFA = free fatty acids; DL = dyslipidemia; DM = diabetes mellitus; ACVD = atherosclerotic cardiovascular disease).
Global and Japanese criteria for the metabolic syndrome
The concept of the metabolic syndrome, which closely corresponds to the ‘multiple risk factors clustering syndrome’ (Citation35–37), has been noted worldwide (Citation5,Citation6,Citation52). Several cohort (Citation53–57) and meta-analysis (Citation58–60) studies have demonstrated that the presence of the metabolic syndrome is associated with increased frequency of ACVD events as well as cardiovascular morbidity and mortality. In this regard, various expert groups have attempted to develop a clear definition for the metabolic syndrome. The first accepted one was proposed by the World Health Organization (WHO), based on type 2 diabetes and insulin resistance (Citation61). Next, other definitions were proposed by various organizations, such as the European Group for the Study of Insulin Resistance (EGIR) (Citation62), the National Cholesterol Education Program—Third Adult Treatment Panel (NCEP-ATP III) (Citation63), the American Association of Clinical Endocrinologists (AACE) (Citation64), the International Diabetes Federation (IDF) (Citation65), and a committee comprising several Japanese medical societies (Citation66,Citation67). In 2009, the IDF Task Force on Epidemiology and Prevention, of the National Heart, Lung, and Blood Institute (NHLBI), American Heart Association (AHA), World Heart Federation (WHF), International Atherosclerosis Society (IAS), and International Association for the Study of Obesity (IASO), prepared a worldwide consensus statement for the criteria of the metabolic syndrome, which did not include abdominal obesity (Citation68). In contrast, central adiposity is considered by the current Japanese definition of the metabolic syndrome a prerequisite for diagnosis, along with any two of the following abnormalities: 1) high blood pressure, 2) dyslipidemia, or/and 3) elevated fasting glucose level. One major difference between the global and Japanese criteria for the definition of the metabolic syndrome is that the Japanese adopted visceral fat accumulation as an important component of the definition (Citation67) because of the ethnic and racial difference in the pattern of adiposity (Citation18–24).
Pathophysiology of visceral obesity— the visceral fat centric hypothesis
Adipose tissue provides free fatty acids (FFA) and glycerol through lipolysis (Citation69). Subcutaneous fat serves as a long-term depot for excess energy as well as maintenance of insulin sensitivity. In fact, insulin sensitivity is not different between individuals with small and large amounts of subcutaneous fat (Citation70–73). In contrast, the hypertrophied adipocytes in intra-abdominal visceral fat exhibit hyperlipolytic activity that is resistant to the antilipolytic effect of insulin (Citation74,Citation75). The resulting hyper-free-fatty-acidemia and hyperglycerolemia in the portal vein (Citation76) increase the production of triacylglycerol-rich lipoproteins (Citation77–79) and glucose in the liver (Citation80–81), leading to insulin resistance in the liver and muscle. Visceral fat tissue is saturated with triglycerides but has limited capacity for storage of energy excess; the triacylglycerol surplus is thus deposited at undesirable sites such as the liver, skeletal muscle, and pancreas, i.e. ectopic fat deposition, as a consequence of hyperinsulinemia/insulin resistance and hyper-free-fatty-acidemia in the portal vein (Citation5,Citation26,Citation82–87) ().
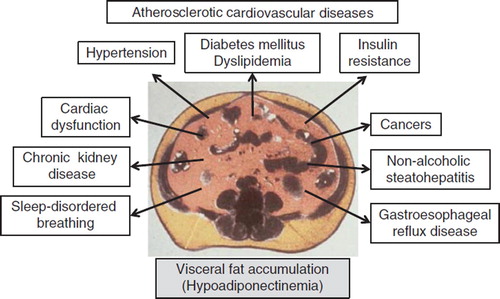
Adipose tissue is not only specialized in the passive storage of excess energy in the form of triglycerides, but it is also a remarkable endocrine organ producing a variety of bioactive substances (Citation88). The latter are conceptualized as ‘adipocytokines’ (Citation89). Our group discovered adiponectin as an adipocytokine in the human adipose cDNA library (Citation90), and subsequent experiments confirmed that it has anti-atherosclerotic properties (Citation91). At the same time, adiponectin was identified independently by three other groups using different approaches and was termed adipocyte complement-related protein of 30 kDa (ACRP30) (Citation92), adipoQ (Citation93), and gelatin-binding protein of 28 kDa (GBP28) (Citation94). The production and secretion of adipocytokines are dynamically regulated mainly by nutritional status. Life-style factors, such as overeating and physical inactivity, induce visceral fat accumulation, which results in adipocyte dysfunction and dysregulated production of adipocytokines (over-production of offensive adipocytokines such as plasminogen activator inhibitor-1, and under-production of defensive adipocytokines such as adiponectin (Citation95)), leading to a state of adipotoxicity. Taken together, abdominal obesity, including dysfunctional visceral fat adipocytes, dysregulated production of adipocytokines, such as hypoadiponectinemia, and ectopic fat deposition () might be the major mechanism of life-style-related diseases accompanied by organ failure and fibrosis (hence the term ‘visceral fat centric hypothesis’, ) (Citation91).
Figure 2. Differences in the pathophysiology of atherosclerotic cardiovascular disease (ACVD) in subjects without (left) and with (right) visceral fat obesity. (HDL-C = high density lipoprotein-cholesterol; PAI-1 = plasminogen activator inhibitor-1; HT = hypertension; DM = diabetes mellitus; DL = dyslipidemia).
Visceral fat reduction as preventative measure against future ACVD events
The control of cardiovascular risk factors is an important strategy to prevent ACVD in the general population. Although measurement of the amount of visceral fat by CT is accurate, this technique is not readily available in clinics, has several problems related to cost-effectiveness and/or radiation exposure, and is not suitable for screening large groups of individuals. Thus, there is a need for a simple and non-invasive method to assess visceral fat accumulation. The bioelectrical impedance analysis (BIA) method, which is based on the difference in electric resistance between fat and components of other organs (Citation96–98), should meet this need. Currently, the visceral fat belt, a new simple device for non-invasive estimation of VFA based on the abdominal BIA method (Citation99), is being co-developed by two companies and our laboratory (we declare no conflict of interest and lack of intellectual property rights). Preliminary studies showed that the VFA measured using this belt correlated significantly with that measured by CT scan (Citation99). Considered collectively, the abdominal BIA method is potentially useful in routine clinical practice for evaluation of visceral fat accumulation associated with the metabolic syndrome.
A health promotion program using this visceral fat belt as a risk factor-oriented approach, not obesity-oriented approach (called ‘Hoken-shido’ in Japanese), was performed by medical personnel, especially health nurses, in the general population (Citation100–106). Visceral fat reduction with life-style modification through this program lessened ACVD events in subjects with abdominal obesity (Citation105), in parallel with reductions in the number of obesity-related cardiovascular risk factors (Citation100) and increases in serum adiponectin levels (Citation102).
Stratification based on visceral fat accumulation to prevent ACVD events
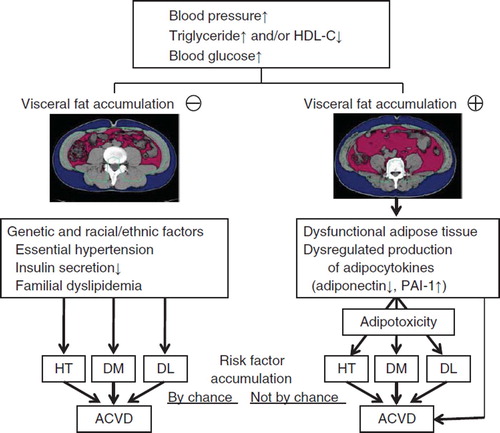
For individuals with hypertension, dyslipidemia, and/or hyperglycemia, it is important to divide them into those with or without fat accumulation for proper management especially prevention of ACVD (). For subjects free of visceral fat accumulation, programs designed to reduce body weight will be futile compared with specific approaches for each risk factor. In this regard, thin subjects with essential hypertension and salt sensitivity (Citation23,Citation107–110), thin subjects with type 2 diabetes and low insulin secretion capacity (Citation23,Citation111–114), and subjects with familial dyslipidemia (Citation115–117) are commonly encountered in East and South Asian countries. On the other hand, loss of body weight and exercise are effective in the visceral fat accumulation group (). For clinically meaningful prevention of future ACVD, it may be important to stratify subjects with multiple risk factors into those with or without visceral fat accumulation, especially for East and South Asians. Various life-style interventions to reduce visceral obesity, such as calorie restriction and exercise, could be implemented to prevent ACVD events. One such program, called ‘Adipo-Do-It’ has already been implemented (Citation95).
Summary
Adoption of the concept of visceral adiposity could facilitate the prevention of future ACVD events in subjects with the metabolic syndrome.
Declaration of interest: This research was supported in part by a Grant-in-Aid for Scientific Research No. (C) 21591177 (to K Kishida) and a Grant-in-Aid for Scientific Research on Innovative Areas No. 22126008 (to T Funahashi and K Kishida). The authors declare no other conflicts of interest.
References
- Kannel WB, Dawber TR, Friedman GD, Glennon WE, Mcnamara PM. Risk factors in coronary heart disease. An evaluation of several serum lipid as predictors of coronary heart disease; The Framingham Study. Ann Intern Med. 1964;61:888–99.
- Stamler J, Wentworth D, Neaton JD. Is relationship between serum cholesterol and risk of premature death from coronary heart disease continuous and graded? Findings in 356,222 primary screenees of the Multiple Risk Factor Intervention Trial (MRFIT). JAMA. 1986;256:2823–8.
- Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, .; INTERHEART Study Investigators. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–52.
- Cullen P, Schulte H, Assmann G. Smoking, lipoproteins and coronary heart disease risk. Data from the Münster Heart Study (PROCAM). Eur Heart J. 1998;19:1632–41.
- Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–7.
- Vitale C, Marazzi G, Volterrani M, Aloisio A, Rosano G, Fini M. Metabolic syndrome. Minerva Med. 2006;97:219–29.
- Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol. 2008;28:629–36.
- Misra A, Khurana L. Obesity and the metabolic syndrome in developing countries. J Clin Endocrinol Metab. 2008; 93:S9–30.
- Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, . Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–9.
- Galassi A, Reynolds K, He J. Metabolic syndrome and risk of cardiovascular disease: a meta-analysis. Am J Med. 2006; 119:812–9.
- Després JP, Arsenault BJ, Côté M, Cartier A, Lemieux I. Abdominal obesity: the cholesterol of the 21st century? Can J Cardiol. 2008;24:7D–12D.
- Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, . The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56:1113–32.
- Fujimoto WY, Bergstrom RW, Boyko EJ, Chen KW, Leonetti DL, Newell-Morris L, . Visceral adiposity and incident coronary heart disease in Japanese-American men. The 10-year follow-up results of the Seattle Japanese-American Community Diabetes Study. Diabetes Care. 1999; 22:1808–12.
- Fujimoto WY, Bergstrom RW, Boyko EJ, Chen K, Kahn SE, Leonetti DL, . Type 2 diabetes and the metabolic syndrome in Japanese Americans. Diabetes Res Clin Pract. 2000;50:S73–6.
- Fujimoto WY, Bergstrom RW, Boyko EJ, Leonetti DL, Newell-Morris LL, Wahl PW. Susceptibility to development of central adiposity among populations. Obes Res. 1995;3: 179S–186S.
- Hayashi T, Boyko EJ, McNeely MJ, Leonetti DL, Kahn SE, Fujimoto WY. Minimum waist and visceral fat values for identifying Japanese Americans at risk for the metabolic syndrome. Diabetes Care. 2007;30:120–7.
- Tong J, Boyko EJ, Utzschneider KM, McNeely MJ, Hayashi T, Carr DB, . Intra-abdominal fat accumulation predicts the development of the metabolic syndrome in non-diabetic Japanese-Americans. Diabetologia. 2007;50: 1156–60.
- Lovejoy JC, de la Bretonne JA, Klemperer M, Tulley R. Abdominal fat distribution and metabolic risk factors: effects of race. Metabolism. 1996;45:1119–24.
- Després JP, Couillard C, Gagnon J, Bergeron J, Leon AS, Rao DC, . Race, visceral adipose tissue, plasma lipids, and lipoprotein lipase activity in men and women: the Health, Risk Factors, Exercise Training, and Genetics (HERITAGE) family study. Arterioscler Thromb Vasc Biol. 2000; 20:1932–8.
- Gill TP. Cardiovascular risk in the Asia-Pacific region from a nutrition and metabolic point of view: abdominal obesity. Asia Pac J Clin Nutr. 2001;10:85–9.
- WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004 Jan 10;363:157–63. Review. Erratum in: Lancet. 2004;363:902.
- Kadowaki T, Sekikawa A, Murata K, Maegawa H, Takamiya T, Okamura T, . Japanese men have larger areas of visceral adipose tissue than Caucasian men in the same levels of waist circumference in a population-based study. Int J Obes (Lond). 2006;30:1163–5.
- Huxley R, James WP, Barzi F, Patel JV, Lear SA, Suriyawongpaisal P, . Ethnic comparisons of the cross-sectional relationships between measures of body size with diabetes and hypertension. Obesity in Asia Collaboration. Obes Rev. 2008;9:53–61.
- Low S, Chin MC, Ma S, Heng D, Deurenberg-Yap M. Rationale for redefining obesity in Asians. Ann Acad Med Singapore. 2009;38:66–9.
- Després JP, Moorjani S, Lupien PJ, Tremblay A, Nadeau A, Bouchard C. Regional distribution of body fat, plasma lipoproteins, and cardiovascular disease. Arteriosclerosis. 1990;10:497–511.
- Després JP, Lemieux I, Bergeron J, Pibarot P, Mathieu P, Larose E, . Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol. 2008;28:1039–49.
- Després JP. Is visceral obesity the cause of the metabolic syndrome? Ann Med. 2006;38:52–63.
- Ylä-Herttuala S, Palinski W, Rosenfeld ME, Parthasarathy S, Carew TE, Butler S, . Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest. 1989;84: 1086–95.
- Brown BG, Zhao XQ, Sacco DE, Albers JJ. Lipid lowering and plaque regression. New insights into prevention of plaque disruption and clinical events in coronary disease. Circulation. 1993;87:1781–91.
- Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, . The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335: 1001–9.
- Assmann G, Schulte H, Cullen P. Pravastatin and coronary heart disease. Circulation. 1998;98:2933–4.
- Archbold RA, Timmis AD. Cholesterol lowering and coronary artery disease: mechanisms of risk reduction. Heart. 1998;80:543–7.
- Austin MA. Plasma triglyceride as a risk factor for coronary heart disease. The epidemiologic evidence and beyond. Am J Epidemiol. 1989;129:249–59.
- Nesto RW. Beyond low-density lipoprotein: addressing the atherogenic lipid triad in type 2 diabetes mellitus and the metabolic syndrome. Am J Cardiovasc Drugs. 2005;5: 379–87.
- Björntorp P. Classification of obese patients and complications related to the distribution of surplus fat. Am J Clin Nutr. 1987;45:112–25.
- Kissebah AH, Vydelingum N, Murray R, Evans DJ, Hartz AJ, Kalkhoff RK, . Relation of body fat distribution to metabolic complications of obesity. J Clin Endocrinol Metab. 1982;54:254–60.
- Kaplan NM. The deadly quartet. Upper-body obesity, glucose intolerance, hypertriglyceridemia, and hypertension. Arch Intern Med. 1989;149:1514–20.
- Vague J. La differenciation sexuelle, feateur determinant des formes de l'obesite. Presse Med. 1947;55:339–40.
- Tokunaga K, Matsuzawa Y, Ishikawa K, Tarui S. A novel technique for the determination of body fat by computed tomography. Int J Obes. 1983;7:437–45.
- Hiuge-Shimizu A, Kishida K, Funahashi T, Ishizaka Y, Oka R, . Absolute value of visceral fat area measured on computed tomography scans and obesity-related cardiovascular risk factors in large-scale Japanese general population (the VACATION-J study). 2010 Oct 22 Ann Med 2010.
- Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, . Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48.
- Porter SA, Massaro JM, Hoffmann U, Vasan RS, O'Donnel CJ, Fox CS. Abdominal subcutaneous adipose tissue: a protective fat depot? Diabetes Care. 2009;32:1068–75.
- Oka R, Miura K, Sakurai M, Nakamura K, Yagi K, Miyamoto S, . Impacts of visceral adipose tissue and subcutaneous adipose tissue on metabolic risk factors in middle-aged Japanese. Obesity (Silver Spring). 2010;18: 153–60.
- Fujioka S, Matsuzawa Y, Tokunaga K, Tarui S. Contribution of intra-abdominal fat accumulation to the impairment of glucose and lipid metabolism in human obesity. Metabolism. 1987;36:54–9.
- Kanai H, Matsuzawa Y, Kotani K, Keno Y, Kobatake T, Nagai Y, . Close correlation of intra-abdominal fat accumulation to hypertension in obese women. Hypertension. 1990;16:484–90.
- Zamboni M, Armellini F, Sheiban I, De Marchi M, Todesco T, Bergamo-Andreis IA, . Relation of body fat distribution in men and degree of coronary narrowings in coronary artery disease. Am J Cardiol. 1992;70:1135–8.
- Nakamura T, Tokunaga K, Shimomura I, Nishida M, Yoshida S, Kotani K, . Contribution of visceral fat accumulation to the development of coronary artery disease in non-obese men. Atherosclerosis. 1994;107:239–46.
- Matsuzawa Y, Shimomura I, Nakamura T, Keno Y, Kotani K, Tokunaga K. Pathophysiology and pathogenesis of visceral fat obesity. Obes Res. 1995;3:187S–94S.
- Matsuzawa Y. Pathophysiology and molecular mechanisms of visceral fat syndrome: the Japanese experience. Diabetes Metab Rev. 1997;13:3–13.
- Fujioka S, Matsuzawa Y, Tokunaga K, Kawamoto T, Kobatake T, Keno Y, . Improvement of glucose and lipid metabolism associated with selective reduction of intra-abdominal visceral fat in premenopausal women with visceral fat obesity. Int J Obes. 1991;15:853–9.
- Kanai H, Tokunaga K, Fujioka S, Yamashita S, Kameda-Takemura KK, Matsuzawa Y. Decrease in intra-abdominal visceral fat may reduce blood pressure in obese hypertensive women. Hypertension. 1996;27:125–9.
- Alberti KG, Zimmet P, Shaw J. Metabolic syndrome—a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23: 469–80.
- Isomaa B, Almgren P, Tuomi T, Forsén B, Lahti K, Nissén M, . Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–9.
- Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, . The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–16.
- Takeuchi H, Saitoh S, Takagi S, Ohnishi H, Ohhata J, Isobe T, . Metabolic syndrome and cardiac disease in Japanese men: applicability of the concept of metabolic syndrome defined by the National Cholesterol Education Program-Adult Treatment Panel III to Japanese men—the Tanno and Sobetsu Study. Hypertens Res. 2005;28: 203–8.
- Ninomiya T, Kubo M, Doi Y, Yonemoto K, Tanizaki Y, Rahman M, . Impact of metabolic syndrome on the development of cardiovascular disease in a general Japanese population: the Hisayama study. Stroke. 2007;38: 2063–9.
- Kokubo Y, Okamura T, Yoshimasa Y, Miyamoto Y, Kawanishi K, Kotani Y, . Impact of metabolic syndrome components on the incidence of cardiovascular disease in a general urban Japanese population: the suita study. Hypertens Res. 2008;31:2027–35.
- Gami AS, Witt BJ, Howard DE, Erwin PJ, Gami LA, Somers VK, . Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403–14.
- Iso H, Sato S, Kitamura A, Imano H, Kiyama M, Yamagishi K, . Metabolic syndrome and the risk of ischemic heart disease and stroke among Japanese men and women. Stroke. 2007;38:1744–51.
- Wu SH, Liu Z, Ho SC. Metabolic syndrome and all-cause mortality: a meta-analysis of prospective cohort studies. Eur J Epidemiol. 2010;25:375–84.
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–53.
- Balkau B, Charles MA. Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR). Diabet Med. 1999;16: 442–3.
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–97.
- Einhorn D, Reaven GM, Cobin RH, Ford E, Ganda OP, Handelsman Y, . American College of Endocrinology position statement on the insulin resistance syndrome. Endocr Pract. 2003;9:237–52.
- Ford ES. Prevalence of the metabolic syndrome defined by the International Diabetes Federation among adults in the U.S. Diabetes Care. 2005;28:2745–9.
- The Examination Committee of the Criteria for Metabolic Syndrome in Japan. Definition and criteria of the metabolic syndrome in Japan. J Jpn Society Intern Med. 2005;94: 188–201 (in Japanese).
- Teramoto T, Sasaki J, Ueshima H, Egusa G, Kinoshita M, Shimamoto K, . Metabolic syndrome. J Atheroscler Thromb. 2008;15:1–5.
- Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, .; International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5.
- Ramsay TG. Fat cells. Endocrinol Metab Clin North Am. 1996;25:847–70.
- Després JP. Abdominal obesity as important component of insulin-resistance syndrome. Nutrition. 1993;9:452–9.
- Kelley DE, Thaete FL, Troost F, Huwe T, Goodpaster BH. Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am J Physiol Endocrinol Metab. 2000; 278:E941–8.
- Ross R, Aru J, Freeman J, Hudson R, Janssen I. Abdominal adiposity and insulin resistance in obese men. Am J Physiol Endocrinol Metab. 2002;282:E657–63.
- Ross R, Freeman J, Hudson R, Janssen I. Abdominal obesity, muscle composition, and insulin resistance in premenopausal women. J Clin Endocrinol Metab. 2002;87:5044–51.
- Smith U, Hammersten J, Björntorp P, Kral JG. Regional differences and effect of weight reduction on human fat cell metabolism. Eur J Clin Invest. 1979;9:327–32.
- Mittelman SD, Van Citters GW, Kirkman EL, Bergman RN. Extreme insulin resistance of the central adipose depot in vivo. Diabetes. 2002;51:755–61.
- Londos C, Brasaemle DL, Schultz CJ, Adler-Wailes DC, Levin DM, Kimmel AR, . On the control of lipolysis in adipocytes. Ann N Y Acad Sci. 1999;892:155–68.
- Kuriyama H, Yamashita S, Shimomura I, Funahashi T, Ishigami M, Aragane K, . Enhanced expression of hepatic acyl-coenzyme A synthetase and microsomal triglyceride transfer protein messenger RNAs in the obese and hypertriglyceridemic rat with visceral fat accumulation. Hepatology. 1998;27:557–62.
- White DA, Bennett AJ, Billett MA, Salter AM. The assembly of triacylglycerol-rich lipoproteins: an essential role for the microsomal triacylglycerol transfer protein. Br J Nutr. 1998; 80:219–29.
- Adeli K, Taghibiglou C, Van Iderstine SC, Lewis GF. Mechanisms of hepatic very low-density lipoprotein overproduction in insulin resistance. Trends Cardiovasc Med. 2001;11:170–6.
- Kuriyama H, Shimomura I, Kishida K, Kondo H, Furuyama N, Nishizawa H, . Coordinated regulation of fat-specific and liver-specific glycerol channels, aquaporin adipose and aquaporin 9. Diabetes. 2002;51:2915–21.
- Maeda N, Funahashi T, Shimomura I. Metabolic impact of adipose and hepatic glycerol channels aquaporin 7 and aquaporin 9. Nat Clin Pract Endocrinol Metab. 2008;4:627–34.
- Unger RH. Lipid overload and overflow: metabolic trauma and the metabolic syndrome. Trends Endocrinol Metab. 2003;14:398–403.
- Klein S. The case of visceral fat: argument for the defense. J Clin Invest. 2004;113:1530–2.
- van Herpen NA, Schrauwen-Hinderling VB. Lipid accumulation in non-adipose tissue and lipotoxicity. Physiol Behav. 2008;94:231–41.
- Lettner A, Roden M. Ectopic fat and insulin resistance. Curr Diab Rep. 2008;8:185–91.
- Szendroedi J, Roden M. Ectopic lipids and organ function. Curr Opin Lipidol. 2009;20:50–6.
- Samuel VT, Petersen KF, Shulman GI. Lipid-induced insulin resistance: unravelling the mechanism. Lancet. 2010;375: 2267–77.
- Maeda K, Okubo K, Shimomura I, Mizuno K, Matsuzawa Y, Matsubara K. Analysis of an expression profile of genes in the human adipose tissue. Gene. 1997;190:227–35.
- Funahashi T, Nakamura T, Shimomura I, Maeda K, Kuriyama H, Takahashi M, . Role of adipocytokines on the pathogenesis of atherosclerosis in visceral obesity. Intern Med. 1999;38:202–6.
- Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1). Biochem Biophys Res Commun. 1996;22:286–9.
- Matsuzawa Y. Establishment of a concept of visceral fat syndrome and discovery of adiponectin. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86:131–41.
- Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–9.
- Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996; 271:10697–703.
- Nakano Y, Tobe T, Choi-Miura NH, Mazda T, Tomita M. Isolation and characterization of GBP28, a novel gelatin-binding protein purified from human plasma. J Biochem. 1996;120:803–12.
- Funahashi T, Matsuzawa Y. Metabolic syndrome: clinical concept and molecular basis. Ann Med. 2007;39:482–94.
- Lukaski HC, Johnson PE, Bolonchuk WW, Lykken GI. Assessment of fat-free mass using bioeletrical impedance measurements of the human body. Am J Clin Nutr. 1985; 41:810–7.
- Lukaski HC, Bolonchuk WW, Hall CB, Siders A. Validation of tetrapolar bioelectrical impedance method to assess human body composition. J Appl Physiol. 1986;60: 1327–32.
- Scharfetter H, Schlager T, Stollberger R, Felsberger R, Hutten H, Hinghofer-Szalkay H. Assessing abdominal fatness with local bioimpedance analysis: basics and experimental findings. Int J Obes. 2001;25:502–11.
- Ryo M, Maeda K, Onda T, Katashima M, Okumiya A, Nishida M, . A new simple method for the measurement of visceral fat accumulation by bioelectrical impedance. Diabetes Care. 2005;28:451–3.
- Okauchi Y, Nishizawa H, Funahashi T, Ogawa T, Noguchi M, Ryo M, . Reduction of visceral fat is associated with decrease in the number of metabolic risk factors in Japanese men. Diabetes Care. 2007;30:2392–4.
- Tamba S, Nishizawa H, Funahashi T, Okauchi Y, Ogawa T, Noguchi M, . Relationship between the serum uric acid level, visceral fat accumulation and serum adiponectin concentration in Japanese men. Intern Med. 2008;47: 1175–80.
- Okauchi Y, Kishida K, Funahashi T, Noguchi M, Ogawa T, Ryo M, . Changes in serum adiponectin concentrations correlate with changes in BMI, waist circumference, and estimated visceral fat area in middle-aged general population. Diabetes Care. 2009;32:e122.
- Tamba S, Nakatsuji H, Kishida K, Noguchi M, Ogawa T, Okauchi Y, . Relationship between visceral fat accumulation and urinary albumin-creatinine ratio in middle-aged Japanese men. Atherosclerosis. 2010;211:601–5.
- Nakatsuji H, Kishida K, Funahashi T, Noguchi M, Ogawa T, Okauchi Y, . One-year reductions in body weight and blood pressure, but not in visceral fat accumulation and adiponectin, improve urinary albumin-to-creatinine ratio in middle-aged Japanese men. Diabetes Care. 2010; 33:e110–1.
- Okauchi Y, Kishida K, Funahashi T, Noguchi M, Morita S, Ogawa T, . 4-year follow-up of cardiovascular events and changes in visceral fat accumulation after health promotion program in the Amagasaki Visceral Fat Study. Atherosclerosis. 2010;212:698–700.
- Okauchi Y, Kishida K, Funahashi T, Noguchi M, Ogawa T, Ryo M, . Absolute value of bioelectrical impedance analysis-measured visceral fat area with obesity-related cardiovascular risk factors in Japanese workers. J Atheroscler Thromb. 2010;17:1237–45.
- Katsuya T, Ishikawa K, Sugimoto K, Rakugi H, Ogihara T. Salt sensitivity of Japanese from the viewpoint of gene polymorphism. Hypertens Res. 2003;26:521–5.
- Flack JM, Ensrud KE, Mascioli S, Launer CA, Svendsen K, Elmer PJ, . Racial and ethnic modifiers of the salt-blood pressure response. Hypertension. 1991;17:I115–21.
- Douglas JG, Thibonnier M, Wright JT Jr. Essential hypertension: racial/ethnic differences in pathophysiology. J Assoc Acad Minor Phys. 1996;7:16–21.
- Brownley KA, Hurwitz BE, Schneiderman N. Ethnic variations in the pharmacological and nonpharmacological treatment of hypertension: biopsychosocial perspective. Hum Biol. 1999;71:607–39.
- Fujimoto WY, Akanuma Y, Kanazawa Y, Mashiko S, Leonetti D, Wahl P. Plasma insulin levels in Japanese and Japanese-American men with type 2 diabetes may be related to the occurrence of cardiovascular disease. Diabetes Res Clin Pract. 1989;6:121–7.
- Ramachandran A. Genetic epidemiology of NIDDM among Asian Indians. Ann Med. 1992;24:499–503.
- Chen KW, Boyko EJ, Bergstrom RW, Leonetti DL, Newell-Morris L, Wahl PW, . Earlier appearance of impaired insulin secretion than of visceral adiposity in the pathogenesis of NIDDM. 5-Year follow-up of initially nondiabetic Japanese-American men. Diabetes Care. 1995; 18:747–53.
- Fujimoto WY. Overview of non-insulin-dependent diabetes mellitus (NIDDM) in different population groups. Diabet Med. 1996;13:S7–10.
- Hoang KC, Le TV, Wong ND. The metabolic syndrome in East Asians. J Cardiometab Syndr. 2007;2:276–82.
- Singh V, Deedwania P. Dyslipidemia in special populations: Asian Indians, African Americans, and Hispanics. Curr Atheroscler Rep. 2006;8:32–40.
- Goff DC Jr, Bertoni AG, Kramer H, Bonds D, Blumenthal RS, Tsai MY, . Dyslipidemia prevalence, treatment, and control in the Multi-Ethnic Study of Atherosclerosis (MESA): gender, ethnicity, and coronary artery calcium. Circulation. 2006;113:647–56.