Abstract
Background. Few studies have tested differences in relationships between hemoglobin (Hb) and long-term risk of major cardiovascular diseases according to age and gender in healthy subjects as opposed to anemia.
Aims. Such relationships were examined and risk-tested in relation to Hb values in the Apolipoprotein MOrtality RISk (AMORIS) Study.
Methods. Using data from AMORIS and the Swedish hospital discharge and mortality registers, a prospective cohort study of 114,159 subjects with mean follow-up of 11.8 years, the association between Hb and risk of acute myocardial infarction (AMI), ischemic stroke (IS), and congestive heart failure (CHF) by Cox regression analysis according to age and gender was studied.
Results. Elevated Hb levels were associated to acute myocardial infarction (AMI) (HR 1.10 (1.06–1.13) per SD change), mostly confined to men and younger subjects but with greater sex similarity trends for CHF. Slightly increased risks were seen for the lowest Hb levels in the elderly and in females. IS risk was positively and more linearly associated to Hb.
Interpretation. In AMORIS the highest AMI and CHF risks were found in the upper region of the distribution, but different shapes of relationships according to age and gender were found. IS associated positively with Hb. Key words:
| Abbreviations | ||
| AMI | = | acute myocardial infarction |
| AMORIS | = | Apolipoprotein MOrtality RISk study |
| ApoA-1 | = | apolipoprotein A-1 |
| ApoB | = | apolipoprotein B |
| CALAB | = | Central Automation Laboratory AB |
| CHF | = | congestive heart failure |
| CI | = | confidence interval |
| CV | = | coefficient of variation |
| CVD | = | cardiovascular disease |
| Hb | = | hemoglobin |
| HDL-C | = | high-density lipoprotein cholesterol |
| HR | = | hazard ratio |
| Htc | = | hematocrit |
| ICD | = | International Classification of Diseases |
| IS | = | ischemic stroke |
| LDL-C | = | low-density lipoprotein cholesterol |
| MACE | = | major (atherosclerotic) cardiovascular disease |
| Q | = | quartile |
| SAS | = | statistical analysis system |
| SD | = | standard deviation |
| TC | = | total cholesterol |
| TG | = | triglycerides |
Key messages
Elevated hemoglobin levels were associated to acute myocardial infarction.
This was mostly confined to men and younger subjects.
Ischemic stroke increased more linearly by increased hemoglobin levels.
Introduction
Hemoglobin (Hb) levels are used by physicians to diagnose various diseases such as anemia at the lower end and vascular blood clotting at the higher end of the distribution. It is also used in sports medicine to monitor blood oxygen content in athletes performing in endurance sports. The use of Hb or hematocrit (Htc) for monitoring risk of patients with congestive heart failure (CHF) is crucial and well described (Citation1,Citation2). Similarly, patients with acute coronary syndromes also have lower Hb or Htc levels than do healthy subjects (Citation3). Thus, serious cardiovascular complications or death may occur in subjects with extremely low or high Hb levels. In healthy subjects not participating in sports, without anemia or having any sign of or acknowledged cardiovascular disease (CVD), the ability of Hb within normal physiological range to predict long-term development of CVD has been investigated by some groups (Citation4–6). However, its close correlate, Htc, has been explored as a risk factor for CVD by more groups, including the Framingham study, with somewhat mixed results (Citation7–10). The Framingham 34-year follow-up study reported age and gender-specific U or J-shaped risk curves by quintile type of dose-response gradients for a series of end-points and found high risks for several end-points at both low and high ends of the distribution within the physiological range of Htc (Citation7). However, the Puerto Rico study lasting 8 years could not demonstrate a U-shaped risk curve for AMI, only an excess risk at the higher end of the Htc distribution (Citation8). The Japanese study showed that low Htc in women was associated with high adjusted risk of cerebral infarction (Citation9), whereas the Gothenburg study could not find any association between Htc and 10-year development of coronary heart disease (Citation10).
There are several obvious confounders when such relationships are to be explored. Women have clearly lower Hb levels than do men, whereas older subjects have somewhat higher levels (Citation7). Cigarette smoking is also positively associated to Hb levels (Citation8), whereas hypertension and atherogenic lipids do not seem to be strongly associated to Hb in young adults, showing low-degree correlation coefficients (all r < 0.22) (Citation11). However, in women, high-density lipoprotein cholesterol (HDL-C) and iron deficiency anemia seem to be negatively correlated, and ferritin associated even positively with HDL-C (Citation12). Diabetics have lower Hb levels than do non-diabetics (Citation13).
In the Apolipoprotein MOrtality RISk (AMORIS) study, we measured routine markers such as Hb, lipids, etc., and even uric acid and haptoglobin (Hp) (an acute-phase reactant hemoglobin-binding protein in the plasma) in a large series of healthy subjects from the Stockholm area whose morbidity and mortality information was followed for 12 years in the Swedish Hospital Discharge and Cause of Death Registries (Citation14,Citation15). Htc was, however, not included in the present version of the database.
Data demonstrate that free hemoglobin and heme in plasma mediate the oxidative modification of low-density lipoprotein in vivo. After oxidation of plasma hemoglobin, release of heme moieties occurs. In turn, heme is taken up by low-density lipoprotein and endothelium and promotes oxidative modification of low-density lipoprotein and potentiates endothelial cell damage (Citation16,Citation17). Some recent data have also proposed a new possible mode of action explaining why Hb may be a pro-atherogenic factor. Thus, data suggest that HDL particles may become pro-atherogenic at high levels of Hb (Citation12,Citation18). In addition, other studies have indicated that diabetics with haplotype 2–2 of the haptoglobin gene are at excess risk of CVD (Citation19). These ideas merit new studies to find out if high (or low) Hb levels really are indicative of increased CVD risk in large long-term population studies consisting of healthy subjects.
The aims of the present study were to investigate the detailed relationships between a single measurement of Hb covering a wide range and different major atherosclerotic CVD events in healthy subjects, and to formally test to what extent such relationships, adjusted for other risk factors, differed between genders and age-groups, taking into account the occurrence of other cardiovascular events after blood sampling but prior to analyzed end-point. In addition, we wanted to test if there was an interaction between Hb and HDL-C and between Hp and Hb levels on CVD end-points in AMORIS.
Patients and methods
Study population and data collection
The AMORIS study (1985–1996) contains 689,588 men and women, mainly from the greater Stockholm area. They were either healthy individuals referred for clinical laboratory testing as part of health check-ups or were out-patients referred for laboratory testing. The number of requested biochemical variables varied widely per subject depending on the purpose of the blood sample examination. All laboratory analyses were done at the Central Automation Laboratory (CALAB), Stockholm. No individuals were excluded from the database for any possible manifestation of disease or because of treatment. However, patients discharged from hospital prior to blood sampling with hospital-recorded diagnoses of AMI, IS, or CHF were excluded from our analysis population. More detailed descriptions of the AMORIS study are given elsewhere (Citation20–22).
Incident cases of ischemic stroke (IS) (ICD–7: 332; ICD–8: 432–434; ICD–9: 433–434; and ICD–10: I63), acute myocardial infarction (AMI) (ICD–8 and ICD–9: 410 and ICD–10: I21), and CHF (ICD–7: 422, 434.1, 434.2, 434.4, 782.4; ICD–8: 427.0, 428, 429, 782.4; ICD–9: 428; and ICD 10: I50) as well as cause of death were ascertained by record linkage to the Swedish Hospital Discharge register and the Swedish Cause of Death register. The validity of the Swedish Hospital Discharge and Cause of Death data for these diagnoses has previously been evaluated (Citation23,Citation24). Persons with non-fatal stroke, AMI, or CHF as primary or secondary diagnosis prior to blood sampling were excluded. We also analyzed a composite end-point of the three listed with ICD codes above, called major (atherosclerotic) cardiovascular event (MACE). Follow-up time was then defined as the time from blood sampling until first occurrence of IS, AMI, CHF, or MACE (whichever of the components that came first), death, or study closing date (31 December 2002) and was on average 11.8 years.
The analyses for this study were based on 114,159 persons aged 30–85 years who had measurements of Hb, as well as total cholesterol (TC) and triglycerides (TG) recorded at the same date. The AMORIS study did not include Htc as measurement in its current database version. In a subgroup analysis, consisting of 46,691 (41%) subjects, also measurements of apolipoprotein B (apoB) and apolipoprotein A-1 (apoA-1) were available. Since prescriptions could vary widely in the number of variables asked for between subjects, joint measurements were often available only in a more limited number of subjects. In addition, all variables including Hb, lipids, lipoproteins, and apolipoproteins had to be taken at the first visit to the physician. In another subgroup (67%) Hp and uric acid measurements were also available.
Hb was analyzed with routinely used hematology analyzers STKS (Coulter®, Japan). Hp was measured with an immunoturbidimetric method (reagents from Orion Diagnostics, Finland), applied to an automated Hitachi-analyzer (Boehringer, Mannheim, Germany). The total imprecision, calculated with the coefficient of variation (CV), was 5.6% at Hp level 1.1 g/L. Uric acid was measured by enzymatic uricase method. Coefficients of variation for uric acid determinations were less than 2.8% at 164 μmol/L (2.76 mg/dL), 2.3% at 470 μmol/L (7.90 mg/dL), and 1.8% at 624 μmol/L (10.49 mg/dL). TC and TG were measured enzymatically as described previously (Citation25,Citation26). In the subgroup with available measurements of apoB and apoA-1 low-density lipoprotein cholesterol (LDL-C) and HDL-C were calculated (Citation20), and the validation procedures have been reported previously (Citation20,Citation27). To assess diabetes, we also used information on glucose levels when available, which was measured enzymatically with a glucose oxidase/peroxidase method. ApoB and apoA-1 were measured by immunoturbidimetric methods with CV < 7% (Citation25,Citation26). All methods were fully automated with automatic calibration and laboratory facilities accredited (Citation26).
Hypertension status was not recorded in these subjects. However, based on hospital discharge data 1,218 (1.1%) subjects were hospitalized at any time prior to blood sampling with a primary or secondary diagnosis of hypertension (ICD–8: 400–404; ICD–9: 401–405; and ICD–10: I10–I15). Since these are only hospitalized cases with a hypertension diagnosis, this information most likely accounts only for a small proportion of all subjects with hypertension in the study population. Diabetes was considered present in those with fasting glucose >7.0 mmol/L at base-line or with a hospital discharge diagnosis of diabetes (ICD–7: 260; ICD–8: 250.0–259.9; ICD–9: 250; and ICD–10: E10-E14) prior to blood sampling, accounting for 4,867 (4.3%) subjects classified as diabetics. Height and weight were not mandatory information on the lab referral form, were missing for the majority of subjects (>80%), and were not used for analyses in this study, since joint measurements of all variables then would have limited the statistical power too much.
Statistical analysis
Hb was categorized both by quartiles and deciles to get more detailed CVD risk information at the far ends of its distribution. Differences of biomarkers by Hb quartiles were evaluated with an analysis of variance with linear contrasts for continuous variables and chi-square tests of trend for categorical variables. We then analyzed the association between time from blood sampling to first event of IS, AMI, CHF, or MACE and Hb with Cox proportional regression models. Analysis of one of the three end-points used occurrence of the other two prior to analyzed event as time-dependent binary coded covariates. Hazard ratios (HR) for continuous variables were expressed for a one standard deviation (SD) difference, including 95% confidence intervals (95% CI). Analyses with decile categories used lowest decile as reference, and HRs were then calculated for the other deciles. Differences in hazard ratios were evaluated with Z tests for two group comparisons. Interaction tests between Hb and gender (or age-groups) on outcome were done by a likelihood ratio chi-square test with nine degrees of freedom of product terms between Hb deciles and gender (age-group) variables. Similar tests of interaction were done between Hb deciles and Hp in the total data set and between Hb deciles and HDL-C in the subgroup with measured levels of apolipoproteins. In addition, interaction tests (Wald) were also done treating Hb as a continuous variable with a single product term between Hb and each of HDL-C and Hp. In the latter analysis LDL-C was used as adjustment variable for atherogenic lipoproteins. All models were adjusted for age, gender, TG, hospital-diagnosed hypertension, and diabetes status, and TC in the total data set (LDL-C in the subset). In the subgroup analyses with available measurements of apoB and apoA-1, apoB/apoA-1 was used as adjustment variable instead of TC. In another subgroup analysis with available measurements of uric acid and Hp, these were additionally adjusted for in the decile analysis of Hb versus CVD end-points. All analyses were conducted with SAS version 8.1.
Ethics and approvals
This study complied with the Declaration of Helsinki, and the Ethics Review Board of the Karolinska Institutet approved the study.
Results
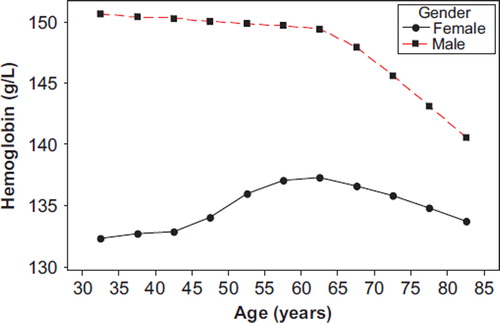
Hb levels according to 5-year age-groups per gender are given in . Males had clearly higher Hb levels than did women in all age-groups, and Hb levels stayed about stable until age 65 and then fell off. Hb levels in females increased until 65 years and then declined gradually, though to a smaller degree than that of men. In risk factors and proportions of atherosclerotic cardiovascular events are given by quartiles (Q) of Hb for all included subjects and in a 41% subsample where also apolipoproteins apoB and apoA-1 were measured. Due to a large sample size all variables were highly significantly associated to increased levels of Hb, except for Hp (P = 0.80), with an inverse direction for HDL-C and apoA-1. Relative differences in risk factor levels between Q4 and Q1 were most pronounced for TG (59%), uric acid (34%), TC/HDL-C (29%), and apoB/apoA-1 (25%). Prevalence of hospital-recorded diabetes was more than doubled from Q1 to Q4, whereas hospital-recorded hypertension differred clearly less. The incidence of new cases of AMI was 98% higher in Q4 than in Q1, 123% higher for IS, and only 9% higher for CHF, showing only weak signs of a J-shaped form. For total MACE the ratio was 51%.
Table I. Gender-adjusted least square means (SE) of continuous and gender-specific categorical risk factors and atherosclerotic cardiovascular events by quartiles of hemoglobin. Total and subgroup with measured apolipoproteins.a
To get insight into the importance of the more extreme levels (high or low) of Hb on cardiovascular risk, Hb was split into deciles. gives hazard ratios of AMI by such deciles, with the lowest as reference, adjusting for age, gender, TC, TG, hospital-diagnosed hypertension, and diabetes and possible occurrence of IS or CHF prior to an AMI by time-dependent Cox regression analysis. In addition, age and gender-specific splits of Hb deciles were used since Hb correlated strongly with gender and also with age. In total, the lowest risk of AMI was observed in decile 3 (130.9–134.2 g/L) and the highest in the two upper deciles. On average the risk of AMI increased 11% per SD (13.1 g/L) difference. This gradient was clearly different between younger and elderly subjects (test of interaction P <0.001), and there was likewise a significant interaction between gender and Hb on AMI (P <0.001). In the elderly subgroup there was no detectable association between increased Hb levels and AMI risk. There were also marked differences between genders when separate deciles per gender were used. In females deciles 2 and 4 had nominally significant lower risk than decile 1 and decile 10, but there was no linear association across the whole Hb distribution range or any increased risk at the high end of the distribution as compared to decile 1. This was different as compared to males where deciles 9 and 10 had elevated such risk. In a small subgroup consisting of 5,046 subjects, among whom 339 experienced a MACE, additional adjustments for body mass index and creatinine were made. In a quartile split of Hb, hazard ratio comparing fourth quartile with first was 1.87 (1.32–2.65) when these factors were additionally adjusted for, whereas it was 1.86 (1.32–2.63) when they were not. This indicates a small impact of added adjustments for these factors. We had no information on smoking in any subject, so this factor could not be investigated in a similar way.
Table II. HR (95% CI) of first occurrence of AMI by age and gender and by deciles of Hb, adjusted for risk factorsa and adjusted for time-dependent occurrence of IS or CHF prior to AMI.
Similar analyses were done for IS, displayed in . In this case there was no reduced risk in any deciles as compared to reference. In total, the IS risk gradient was at least as strong as for AMI. This was mainly due to the fact that elderly subjects also had a significant increased risk by increased Hb levels. The somewhat U-shaped risk curve in females, as seen for AMI, was not present for IS, and gradients were largely similar in the two genders. When CHF was used as end-point, only a weak U-shaped risk curve was observed in the total material (). These shapes were present within both age-groups with about similar and significant risk gradients on average per SD of Hb difference. Again, as for IS, CHF gradients did not differ much between genders. The combined end-point, MACE, showed no major difference between genders but had a significantly steeper gradient in younger as compared to elderly subjects (test of interaction P <0.001), as shown in . Thus, the optimal range seems to vary by age and gender and even end-point. For MACE it seems to be in the range between 20th and 30th percentile.
Table III. HR (95% CI) of first occurrence of IS by age and gender and by deciles of Hb, adjusted for risk factorsa and adjusted for time-dependent occurrence of AMI or CHF prior to IS.
Table IV. HR (95% CI) of first occurrence of CHF by age and gender and by deciles of Hb, adjusted for risk factorsa and adjusted for time-dependent occurrence of AMI or IS prior to CHF.
Table V. HR (95% CI) of first occurrence of MACE by age and gender and by deciles of Hb, adjusted for risk factors.a
Analyses were also repeated on a subgroup (67%) of subjects with available information on uric acid and haptoglobin since they could be potential confounders of Hb in multivariate analyses. Additional adjustments for these two factors did not noteworthily alter associations between Hb and outcomes (data not shown). Another sub-analysis was done when subjects with available measurements of apolipoproteins were included, now adjusting for apoB/apoA-1 instead of TC, since the ratio in previous AMORIS analyses has been found to be the best lipid predictor for AMI and IS (Citation14,Citation15). In these cases, the J-shape at the lower end of the Hb risk curve was somewhat more pronounced for AMI and CHF compared to the total data set analyses, whereas the IS results remained the same. Additionally, the risk increase at the higher end of Hb was slightly less for AMI and CHF than in the main analyses but not for IS (data not shown). We tested if there was an interaction between HDL-C and hemoglobin deciles in the subgroup with available information of HDL-C. For neither of the end-points was there any significant such interaction when LDL-C was used as adjustment factor together with TG, uric acid, hospital-recorded hypertension, and diabetes, for each gender separately or together, adjusting for gender (all P > 0.20).
Interactions between Hb and Hp
Age and gender-specific interaction tests between Hp and Hb were done for each end-point adjusted for the confounding factors as above. For AMI a border-line significant interaction was found in men, chi-square(Citation9) = 16.03, P = 0.066, but not in women. For IS, both men and women showed a significant interaction between Hb and Hp, chi-square(Citation9) = 26.29 (P <0.001) and chi-square(Citation9) = 22.85 (P <0.01), respectively. Interactions were positive in the sense that effect sizes increased monotonically and more than additively on the relative risk scale when both factors increased. When CHF was analyzed similarly, interaction was only found in women. For the combined end-point MACE interactions were again found in both genders, chi-square(Citation9) = 27.94 (P <0.001) in men and chi-square(Citation9) = 22.09 (P <0.01) in women. Analyses by age <65 and ≥65 years separately (adjusted for gender) did not show any significant interaction. Additional age and gender-specific monotonic interaction analyses confirmed some of the findings above but had clearly lower sample sizes within subgroups.
Discussion
This study showed that the pattern of risk according to different levels of Hb varied according to end-point, age, and gender. An elevated risk of CVD at the lower end of the Hb distribution is well known for patients with CHF or in those with acute coronary syndromes (Citation1–3). The elevated risk at the high end of Hb as found in AMORIS has also been observed in healthy subjects when Htc has been used as biomarker (Citation7–9). The major exception of pattern in AMORIS was found for IS as end-point, which had the lowest risk at the lowest Hb levels and had a more positively linear shape of the risk curve. This was at variance with the findings in women in the Framingham study (Citation7), which showed a U-shaped risk curve for total stroke or transient ischemic attacks (IS was not reported), but again only according to Htc in both age-groups (<65 and ≥65 years). However, in the men a similar linearly increased risk as in AMORIS was observed. In a Japanese study Kiyohara et al. also reported increased stroke incidence comparing fifth and third quintiles of Hb in elderly men, and there was a linearly increased stroke risk by increasing Hb levels for all subjects in those <65 years. In older women a U-shaped stroke risk curve was indicated (Citation9). The AMI risk gradient in AMORIS women was more pronounced U-shaped than that of men, with the lowest risks somewhere between the second and fourth deciles. These results are consistent with the gender-specific associations of the Framingham study myocardial infarction end-point. However, CHF as end-point had only weakly U-shaped risk curves, not as marked as those of the Framingham study. This could possibly be due to a more precise measurement schedule of Htc across time than a single value measured at base-line in AMORIS. The Hb levels with the lowest risks in AMORIS seemed to be about 135 g/L for women and 145 g/L for men.
Direct comparisons with the Framingham data must be made with caution since length of follow-up was clearly different (34 versus 12 years), and the possible occurrence of other cardiovascular events after blood sampling but prior to analyzed end-point were adjusted for completely only in AMORIS. Furthermore, Framingham had semi-annual measurements of Htc and not Hb, a more extended set of base-line adjustment factors, and they analyzed data by logistic instead of Cox proportional regression, resulting in slightly exaggerated estimates of relative risks by their odds ratios. In AMORIS, due to its size, a decile type of analysis was made as compared to quintiles in Framingham. However, despite these discrepancies in primary variables, design, and statistical analyses, the main trends of results were essentially similar for the end-points compared.
It is important to acknowledge that the excess risks at both tails of the Hb distribution lie completely within physiological ranges. Subjects in AMORIS were probably not anemic, since they were referred from out-patient clinics or for health check-up reasons. Also, end-point cases, usually above 40 years of age, were hardly competitive athletes in endurance sports, so pathological Hb levels occurring in such sports probably did not confound the relationships. At the high end of Hb, blood viscosity is increased with increased peripheral resistance and diminishing cardiac output. Increased viscosity has an impact on coronary, cerebral, as well as peripheral blood flow and perfusion (Citation28–31). High Hb levels may also stimulate atherogenesis by erythrocyte aggregation, leading to platelet aggregation, adhesion, and aggregation to the arterial wall (Citation31–33). Blood rheology is especially important in the brain microcirculation. Thus, even a small reduction in blood flow may have a huge impact on cerebral function (Citation34). This is consistent with the more linear increase in IS by increasing Hb levels found in AMORIS. There is also a relatively new theory that small amounts of free Hb in plasma may be taken up by the HDL-C particles, thereby changing the anti-atherogenic property of HDL-C to become pro-atherogenic (Citation18). However, in our interaction analyses between HDL-C and Hb deciles on the various end-points, we could not detect any trend towards such interactions. It has also been proposed that high Hb levels may lead to infiltration of small amounts of Hb into the arterial wall where its components heme and other constituents may contribute to increased oxidation of intra-arterial lipids and perhaps also proteins, thereby accelerating the development of plaques (Citation35). Furthermore, the intra-arterial plaque and its debris may be more prone to bleeding and to rupture, which may increase the risk of formation of thrombus material and its clinical consequences (Citation36,Citation37). Since no haplotyping of the haptoglobin gene was done in AMORIS, we could not verify the high CVD risk in diabetics with haplotype 2-2 as suggested by Levy et al. (Citation19). However, since the Hp haplotype 2-2 has a prevalence of about 40% and has clearly higher CVD risk than types 1-1 and 1-2 (Citation19), it should be possible to detect excess CVD risk (of diabetic origin) in a large data set such as in AMORIS. In our interaction tests it was indicated that the combination of high Hp and Hb levels may increase risk on CVD end-points, but it is still unclear whether disturbances in the Hb–Hp complexes could be an explanatory link.
At the low end of Hb an increased risk of AMI or CHF was observed even within physiological ranges. It is known that when Hb is very low, oxygen delivery capacity is not satisfactory, and this anemic deficiency is especially important in patients with acute coronary syndromes or CHF (Citation3). Lipid disturbances may also be involved in anemic subjects (Citation12). In AMORIS we cannot exclude the possibility that non-hospitalized lower-degree CHF or silent AMI may have been present in some subjects, contributing to the elevated J-formed risk at low Hb levels.
The clinical utility of these findings must be put in perspective of proving causality of low and/or high Hb levels. For that purpose randomized controlled trials where manipulations of Hb are investigated versus clinical outcomes are needed. In special populations, such as in anemia or heart failure, some trial results have been published showing promising results (Citation38–41). However, more trials are needed for subjects with high Hb levels.
Study strengths and limitations
The major strength of this analysis is the large number of major cardiovascular end-points in AMORIS. However, major drops in number of subjects may take place in AMORIS when several biomarkers are to be included simultaneously. Special runs on single factor univariate analyses in larger populations did not give substantially different estimates of HRs when compared to those found when the same analyses were made on a much more limited number of subjects due to joint inclusion of many risk factors. Thus, severe selections were probably not involved when analyses with joint inclusion of risk factors were needed. During the study period the all-cause mortality was about 14% lower in the AMORIS population than in the general population of Stockholm county, when taking age, gender, and calendar year into account (Citation21). This ‘healthy cohort effect’ may influence the generalizability of the results, but not the internal validity, i.e. the hazard ratios given for different levels of, for example, Hb. In addition, the impact on the generalizability is likely to be minor since it has been shown that the AMORIS cohort is similar to the general working population of Stockholm county in terms of socio-economic class and ethnicity. A major limitation of this study is the lack of more important risk factors such as body mass index, creatinine, smoking, and more classically derived proportions of hypertension and diabetes. We investigated in a small subgroup the effects of additional adjustments for body mass index and creatinine but found no such effects when MACE was used as end-point. For smoking we had no subgroup data. However, adjustment for these factors, and more as done in the Framingham study, did not change odds ratios markedly as compared to the crude age-adjusted risk gradients per gender (Citation7). Since AMORIS also adjusted for lipids and apolipoproteins, we believe that such extra adjustments would only have changed risk estimates marginally, especially since degree of correlation between smoking, blood pressure, and Hb is relatively low. However, as shown in , many risk factors were associated to Hb, and we cannot rule out the possibility of a residual confounding in these analyses. The quality of AMI classifications in the Swedish Hospital Discharge Register has been evaluated several times and was shown to be of good quality for epidemiological purposes (Citation24). The quality of routine stroke diagnoses in Sweden has been evaluated less extensively and with less consistent results (Citation21). For CHF it is known from a Danish study that the specificity is high but the sensitivity is low, so that an under-reporting of less severe cases may have occurred in our study (Citation42).
Conclusions
This study showed that in healthy persons a single measurement of Hb within a wide range was associated with major atherosclerotic cardiovascular events during 12 years of follow-up. The AMI risk was clearly highest at the upper end of the Hb region, but that was only found in men and in the younger part of the population. The trends were weaker for CHF and more similar between genders. There were slight trends towards increased risk in the lower as compared to the middle Hb region for AMI and CHF in women and in the elderly. Conversely, the IS risk increased more linearly by increased levels of Hb in both genders. There were interactions between Hp and Hb on CVD end-points, requiring further research.
Declaration of interest: The authors state no conflict of interest and have received no payment in preparation of this manuscript.
Acknowledgements
The study was supported by grants from the Gunnar and Ingmar Jungner Foundation for Laboratory Medicine, Stockholm.
References
- Okonko DO, Van Veldhuisen DJ, Poole-Wilson PA, Anker SD. Anaemia of chronic disease in chronic heart failure: the emerging evidence. Editorial. Eur Heart J. 2005;26: 2213–4.
- Sharma R, Francis DP, Pitt B, Poole-Wilson PA, Coats AJS, Anker SD. Haemoglobin predicts survival in patients with chronic heart failure: a substudy of the ELITE II trial. Eur Heart J. 2004;25:1021–8.
- Turner SJ, Ketch TR, Gandhi SK, Sane DC. Routine hematologic clinical tests as prognostic markers in patients with acute coronary syndromes. Am Heart J. 2008;155: 806–16.
- Kannel WB, Gordon T, Wolf PA, McNamara P. Hemoglobin and the risk of cerebral infarction: The Framingham Study. Stroke. 1972;3:409–20.
- Campbell MJ, Elwood PC, Mackean J, Waters WE. Mortality, haemoglobin level and haematocrit in women. J Chron Dis. 1985;38:881–9.
- Abu-Zeid HAH, Chapman JM. The relation between hemoglobin level and the risk for ischemic heart disease: a prospective study. J Chron Dis. 1976;29:395–403.
- Gagnon DR, Zhang T-J, Brand FN, Kannel WB. Hematocrit and the risk of cardiovascular disease—The Framingham Study: A 34-year follow-up. Am Heart J. 1994;127:674–82.
- Sorlie PD, Garcia-Palmieri MR, Costas R Jr, Havlik RJ. Hematocrit and risk of coronary heart disease: the Puerto Rico Heart Health Program. Am Heart J. 1981;101:456–61.
- Kiyohara Y, Ueda K, Hasuo Y, Fujii I, Yanai T, Wada J, . Hematocrit as a risk factor of cerebral infarction: long-term prospective population survey in a Japanese rural community. Stroke. 1986;17:687–92.
- Wilhelmsen L, Wedel H, Tibblin G. Multivariate analysis of risk factors for coronary heart disease. Circulation. 1973;48: 950–8.
- Shimakawa T, Bild DE. Relationship between hemoglobin and cardiovascular risk factors in young adults. J Clin Epidemiol. 1993;46:1257–66.
- Merono T, Sorroche P, Rosso LAG, Casanas L, Boero LE, Arbelbide JA, . Proatherogenic disturbances in lipoprotein profile, associated enzymes and transfer proteins in women with iron deficiency anaemia. Clin Biochem. 2010; 43:416–23.
- Nikolsky E, Aymong ED, Halkin A, Grines CL, Cox DA, Garcia E, . Impact of anemia in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention: Analysis from the Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications (CADILLAC) Trial. J Am Coll Cardiol. 2004;44:547–53.
- Holme I, Aastveit AH, Jungner I, Walldius G. Relationships between lipoprotein components and risk of myocardial infarction: age, gender and short versus longer follow-up periods in the Apolipoprotein MOrtality RISk study (AMORIS). J Intern Med. 2008;264:30–8.
- Holme I, Aastveit AH, Hammar N, Jungner I, Walldius G. Relationships between lipoprotein components and risk of ischaemic and haemorrhagic stroke in the Apolipoprotein MOrtality RISk study (AMORIS). J Intern Med. 2009; 265:275–87.
- Jeney V, Balla J, Yachie A, Varga Z, Vercellotti GM, Eaton JW, . Pro-oxidant and cytotoxic effects of circulating heme. Blood. 2002;100:879–87.
- Balla G, Jacob HS, Eaton JW, Belcher JD, Vercellotti GM. Hemin: A possible physiological mediator of low density lipoprotein oxidation and endothelial injury. Arterioscler Thromb. 1991;11:1700–11.
- Watanabe J, Grijalva V, Hama S, Barbour K, Berger FG, Navab M, . Hemoglobin and its scavenger protein haptoglobin associate with apoA-1 containing particles and influence the inflammatory properties and function of high density lipoprotein. J Biol Chem. 2009;284:18292–301.
- Levy AP, Asleh R, Blum S, Levy NS, Miller-Lotan R, Kalet-Litman S, . Haptoglobin: basic and clinical aspects. Antioxid Redox Signal. 2010;12:293–304.
- Walldius G, Jungner I, Holme I, Aastveit AH, Kolar W, Steiner E. High apolipoprotein B, low apolipoprotein A-1, and improvement in the prediction of fatal myocardial infarction (AMORIS study): a prospective study. Lancet. 2001;358:2026–33.
- Holme I, Aastveit AH, Hammar N, Jungner I, Walldius G. Uric acid and risk of myocardial infarction, stroke and congestive heart failure in 417734 men and women in the Apolipoprotein MOrtality RISk study (AMORIS). J Intern Med. 2009;266:558–70.
- Holme I, Aastveit AH, Hammar N, Jungner I, Walldius G. Haptoglobin and risk of myocardial infarction, stroke, and congestive heart failure in 342,125 men and women in the Apolipoprotein MOrtality RISk study (AMORIS). Ann Med. 2009;41:522–32.
- Medin J, Nordlund A, Ekberg K. Increasing stroke incidence in Sweden between 1989 and 2000 among persons aged 30 to 65 years: evidence from the Swedish Hospital Discharge Register. Stroke. 2004;35:1047–51.
- Hammar N, Alfredsson L, Rosén M, Spetz C-L, Kahan T, Ysberg A-S. A national record linkage to study acute myocardial infarction incidence and case fatality in Sweden. Int J Epidemiol. 2001;30 (Suppl 1):S30–4.
- Jungner I, Walldius G, Holme I, Kolar W, Steiner E. Apolipoprotein B and A-1 in relation to serum cholesterol and triglycerides in 43 000 Swedish males and females. Int J Clin Lab Res. 1992;21:247–55.
- Jungner I, Marcovina SM, Walldius G, Holme I, Kolar W, Steiner E. Apolipoprotein B and A-1 values in 147 576 Swedish males and females, standardized according to the World Health Organization—International Federation of Clinical Chemistry First International Reference Materials. Clin Chem. 1998;44:1641–9.
- Talmud PJ, Hawe E, Miller GJ, Humphries SE. Nonfasting apolipoprotein B and triglyceride levels as a useful predictor of coronary heart disease risk in middle-aged UK men. Arterioscler Thromb Vasc Biol. 2002;22:1918–23.
- Lowe GDO, Forbes CD. Blood rheology and thrombosis. Review. Clin Haematol. 1981;10:343–67.
- Dormandy JA, Hoare E, Colley J, Arrowsmith DE, Dormandy TL. Clinical, haemodynamic, rheological, and biochemical findings in 126 patients with intermittent claudication. Br Med J. 1973;4:576–81.
- Dormandy JA, Gutteridge JMC, Hoare E, Dormandy TL. Effect of clofibrate on blood viscosity in intermittent claudication. Br Med J. 1974;4:259–62.
- Dormandy JA, Hoare E, Khattab AH, Arrowsmith DE, Dormandy TL. Prognostic significance of rheological and biochemical findings in patients with intermittent claudication. Br Med J. 1973;4:581–3.
- Thomas DJ, Marshall J, Russell RW, Wetherley-Mein G, du Boulay GH, Pearson TC, . Effect of haematocrit on cerebral blood-flow in man. Lancet. 1977;2:941–3.
- Wood JH, Kee DB Jr. Hemorheology of the cerebral circulation in stroke. Progress Review. Stroke. 1985;16:765–72.
- Müller R, Lehrach F. Haemorheology and cerebrovascular disease: multifunctional approach with pentoxifylline. Curr Med Res Opin. 1981;7:253–63.
- Nagy E, Eaton JW, Jeney V, Soares MP, Varga Z, Galajda Z, . Red cells, hemoglobin, heme, iron, and atherogenesis. Arterioscler Thromb Vasc Biol. 2010;30:1347–53.
- Paterson JC. Vascularization and hemorrhage of the intima of arteriosclerotic coronary arteries. Arch Pathol. 1936; 22:313–24.
- Barger AC, Beeuwkes R III, Lainey LL, Silverman KJ. Hypothesis: vasa vasorum and neovascularization of human coronary arteries. A possible role in the pathophysiology of atherosclerosis. N Engl J Med. 1984;310:175–7.
- Eschbach JW, Egrie JC, Downing MR, Browne JK, Adamson JW. Correction of the anemia of end-stage renal disease with recombinant human erythropoietin. Results of combined phase I and II clinical trial. N Engl J Med. 1987; 316:73–8.
- Tsakiris D. Morbidity and mortality reduction associated with the use of erythropoietin. Nephron. 2000;85 (Suppl 1): 2–8.
- Drüeke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, . Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006;355:2071–84.
- Voors AA, Belonje AMS, Zijlstra F, Hillege HL, Anker SD, Slart RHJA, . A single dose of erythropoietin in ST-elevation myocardial infarction. Eur Heart J. 2010;31: 2593–600.
- Kümler T, Gislason GH, Kirk V, Bay M, Nielsen OW, Köber L, . Accuracy of a heart failure diagnosis in administrative registers. Eur J Heart Fail. 2008;10:658–60.