Abstract
Low-rate smoking patterns are common, but their pulmonary effects remain poorly known. The study hypothesis was that any level of daily smoking may cause chronic obstructive pulmonary disease (COPD).
We investigated the association between longitudinal smoking patterns and COPD using logistic regressions and survival models adjusted for multiple covariates. Data from Finnish Twin Cohort surveys were used. Participants (n = 21,609) were grouped into categories describing 1981 smoking and change in smoking during 1975–1981. Light smoking was defined as < 5 cigarettes per day, moderate 5–19 cigarettes, and heavy ≥ 20 cigarettes per day. Finland's Social Insurance Institution provided data on inhaled anticholinergics purchases (1995–2008) and diagnoses entitling to special reimbursements (1981–2008). We defined COPD as regular anticholinergic use or special reimbursement eligibility for COPD, emphysema, or chronic bronchitis.
COPD incidence was 2.5% (n = 528). Elevated disease risks were observed in former, moderate, and heavy smokers, in all who increased smoking, and in those who reduced from moderate to light smoking. Increased risk for anticholinergic use was found in former smokers, in constant light, moderate, and heavy smokers, and in increasers. Former, light, moderate, and heavy smoking in 1981 was associated with future development of disease. Our results demonstrate that all daily smoking patterns may impair pulmonary function.
Key words::
Key message
Low-rate daily smoking patterns are associated with chronic obstructive pulmonary disease (COPD) and increased use of anticholinergic medication.
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by progressive, partly irreversible air-flow limitation. A pathological inflammatory reaction destroys lung parenchyma, causing emphysema, and obstructs small airways, causing bronchiolitis (Citation1). Tobacco smoke usually induces this inflammatory state, but also biomass fuels, airway hyper-reactivity, and infections are risk factors for COPD (Citation2). Genetics affects the susceptibility of developing COPD, and disease course varies individually (Citation2,Citation3). Early, mild COPD is often asymptomatic and rarely diagnosed (Citation1). The estimated COPD prevalence in Finland, 5%–9%, is similar to that in other European countries (Citation4,Citation5).
As many as 50% of smokers develop COPD (Citation6), but it remains unclear whether low-rate smoking patterns also increase disease risk. Occasional smoking or consumption of <5 cigarettes per day is associated with cardiovascular diseases and cancer (Citation7,Citation8); however, the pulmonary effects of daily light smoking are unknown. Neither is there consensus on whether reducing moderate smoking improves lung function, although cardiovascular risks and respiratory symptoms may improve (Citation9,Citation10).
For decades, COPD has been treated with short-acting inhaled anticholinergics, ipratropium and oxitropium. In 1993, combinations of ipratropium and beta2 agonists came onto the market in Finland. In 2002, the long-acting anticholinergic tiotropium was introduced in Europe. Generally, no conditions other than COPD are treated with inhaled anticholinergics. They relieve symptoms by reducing vagally mediated bronchoconstriction, a major reversible airway obstruction component in COPD (Citation11). Anticholinergics have a wide therapeutic margin, and their few adverse effects seldom cause treatment discontinuance (Citation12).
Finnish current care guidelines for COPD treatment (Citation13) are in line with international guidelines (Citation14) and are carefully followed (Citation15). Between 1999 and 2003, national guidelines recommended combinations of ipratropium and beta2 agonists for severe COPD, and one or the other for mild or moderate disease. In 2003, tiotropium was added to the guidelines and recommended as an alternative medication. Tiotropium prescriptions (monetary value) soon exceeded ipratropium prescriptions, being in 2008 over 4-fold those of ipratropium (Citation16).
In Finland, the Social Insurance Institution (SII) registers nation-wide all reimbursed medications purchased from pharmacies and the diagnoses entitling to special reimbursements. Basic reimbursement, covering 42% of product price, is granted for all prescribed purchases, whereas special reimbursement, covering 72%, can be allocated only with a specialist's certificate submitted to the SII (see Methods for details).
In Finland, the prevalence of daily smoking decreased from 27% to 19% between 1979 and 2009 (Citation17). Similarly, smokers’ consumption of cigarettes decreased, and quit attempts increased (Citation17). Occasional smoking is becoming more popular (Citation8), and in some populations the proportion of light smokers is also increasing (Citation18).
Wide-spread comprehension of the deleterious effects of smoking is one potential explanation for increased low-rate smoking patterns. Indeed, light and occasional smokers often have a healthier life-style and a higher education than heavier smokers (Citation8,Citation19). Continuous light smokers are possibly motivated more by social and enjoyment factors than by craving (Citation20), whereas heavier smokers might see smoking reduction as an alternative when cessation is perceived as difficult. Whether low-rate smoking can permanently damage lungs remains unclear. Our aim here was to explore the longitudinal associations between different smoking patterns and COPD. We used data on anticholinergics usage and diagnoses entitling to special reimbursements to define COPD.
Materials and methods
Data were collected as part of the Finnish Twin Cohort, comprising all same-sex twins born before 1958 with both members alive in 1967 (Citation21). Questionnaires were collected in 1975 and 1981, with response rates of 89% and 84%, respectively. Those who participated in both years (n = 21,609) comprised this study sample. We performed a record-linkage study by combining the survey data with the SII's nation-wide medical registry data. In this study, we primarily analyzed twins as individuals, with appropriate statistical adjustment for within-pair correlation.
Smoking
Among respondents, we identified never, occasional, former and current daily smokers. Current and former smokers’ average daily cigarette consumption was defined, and the number of pack-years was calculated. According to amount smoked, we collapsed participants into the following groups: never, occasional, former, daily light (<5 cigarettes per day), moderate (5–19 cigarettes per day), and heavy (≥20 cigarettes per day) smokers. The classification was identical in 1975 and 1981 and is described in detail elsewhere (Citation21). We formed specific smoking subgroups describing the change in smoking status and the amount smoked between 1975 and 1981. We also divided all 1981 former smokers into categories according to their smoking history: <5 (n = 1,182), 5–10 (n = 403), and >10 pack-years (n = 2,455).
Confounders
All analyses were adjusted for age and sex. As potential confounders, we tested the following selected characteristics in 1981, all of which were significantly associated with outcome: chronic bronchitis (odds ratio (OR) 3.84; P ≤ 0.001), asthma (OR 2.89; P ≤ 0.001), heavy alcohol consumption (OR 1.74; P ≤ 0.001), high education (OR 0.53; P ≤ 0.001), and physical activity (OR 0.35; P ≤ 0.001). These variables were adjusted for in logistic regressions, discordant pair analyses, and survival analyses. Heavy alcohol use was defined as having six or more drinks on one occasion at least monthly (Citation21). Education was dichotomized as those with lower education and those with at least 12 years of schooling (high school). We categorized the subjects as sedentary, intermediate, or active, based on frequency, duration, and intensity of leisure physical activity (Citation22). Self-reported chronic, productive cough for at least three successive months a year was considered chronic bronchitis (Citation21,Citation23). Those reporting physician-diagnosed asthma were considered to suffer from asthma (Citation21).
Medical register data
Data on diagnoses entitling to special reimbursements (between 1970 and 2008) and reimbursed anticholinergic medication purchase dates (between 1995 and 2008) were received from the SII. We linked register data to study subjects with approval from the SII and according to the guidelines of the Data Protection Ombudsman.
The SII tracks reimbursement eligibilities and medications for ‘Chronic asthma and similar chronic obstructive pulmonary diseases’ together; thus, discrimination between COPD and asthma is possible only if exact diagnoses are registered (Citation24). Diagnoses were collected occasionally until 2000 and systematically since then. Until the 1990s, the terms ‘emphysema’ and ‘chronic bronchitis’ were used alongside ‘COPD’ in Finland (Citation5). Special reimbursement criteria for COPD medication are strict; until December 2007, patients with forced expiratory volume in 1 second (FEV1) <40% of predicted, or arterial pCO2 constantly >6.5 kPa were considered eligible. Since then, FEV1 <50% was accepted if the patient had suffered from exacerbations during bronchodilator treatment (Citation25).
Anticholinergic data include purchase dates of inhaled oxitropium, ipratropium, tiotropium, and combination preparations (ipratropium + salbutamol, ipratropium + fenoterol). A patient's medication cost consists of a fixed deductible and the amount remaining after basic or special reimbursement. It is most economical to buy the maximum amount of the reimbursed drug, a 3-month requirement, at one time because only one fixed deductible is paid irrespective of the number of medication refills (Citation26). The next purchase can be made when the previous amount is almost used (Citation27).
Outcome definition
To find subjects with symptomatic COPD, we defined the main outcome as a diagnosis of COPD, emphysema, or chronic bronchitis entitling to special reimbursement, or as regular usage of basically reimbursed pure anticholinergics/combination preparations (at least two purchases at 99-day intervals). We excluded subjects having moved abroad, deceased, or with a special reimbursement for COPD, chronic bronchitis, or emphysema before 1981 from the analyses of reimbursements. Likewise, subjects with these characteristics before 1995 were excluded from the analyses of medications.
Statistical analyses
We combined some categories describing change in smoking patterns. Categories whose estimates for disease risk could be set as equal in the regression model were grouped together ().
Table I. Formation of categories describing change in smoking patterns 1975–1981. Initial groups (on the left) were grouped together if their regression coefficients could be set equal in the regression model. Final categories are presented in the second column, and number of subjects (n) in each category in the third column.
All analyses were performed with Stata (version 11.0) (Citation28). First, we conducted logistic regression models, where the significances of COPD risks were tested for smoking status in 1975 and 1981, for categories describing change in smoking during 1975–1981, and for former smoker subgroups. The effect of smoking onset age was omitted from analyses because of its high correlation with smoking categories. The correlation between pack-years and smoking status in 1981 was 0.66; thus, only models studying 1975 smoking or the change in smoking included the pack-years effect. We examined 1975 smoking status × 1981 chronic bronchitis interaction to compare the disease risk between all 1975 smokers and those who developed chronic bronchitis. The ORs with 95% confidence intervals (CIs) for COPD versus no COPD were computed. Since observations on individuals within twin pairs are correlated, robust estimators of variance and the cluster option in Stata were used when estimating standard errors (Citation29).
To explore the causal nature of smoking and COPD association, we conducted conditional logistic regressions among twin pairs discordant for COPD using a matched pair case-control design (Citation22). Twin pairs were regarded as discordant if one twin had COPD (case) while the other did not (control), and the analyses were repeated for monozygotic (MZ) and dizygotic (DZ) pairs separately. The advantage of these analyses is the possibility to control for such genetic and shared environmental factors that cause both smoking and COPD. Similar risk estimates in MZ and DZ pairs would suggest that such confounders do not significantly affect the association between smoking and COPD.
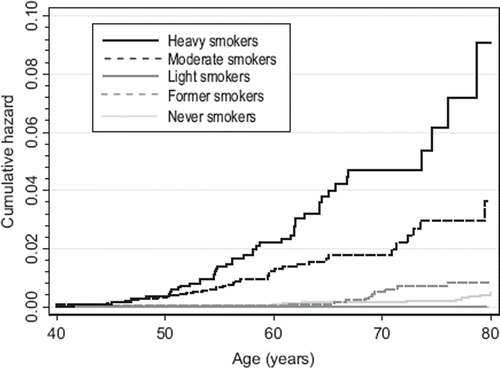
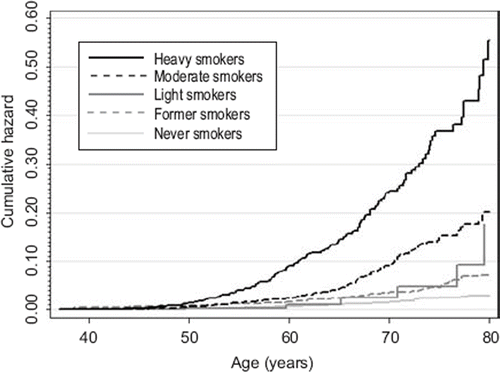
Disease development rates were calculated with survival analyses according to 1981 smoking status and change in smoking during 1975–1981. Because follow-up times for outcomes were different, we conducted analyses separately for both. For anticholinergic medications, the follow-up started on 1 January 1995; for special reimbursement diagnoses, the follow-up started on the response date to the 1981 questionnaire. The follow-up ended on the date of granting of special reimbursement, initiation of regular medication use, migration from Finland, death, or end of the study follow-up (31 December 2008). Hazard ratios (HRs) with 95% CIs associated with disease development were calculated with adjusted Cox proportional hazards regression models. We used non-parametric Nelson–Aalen estimators to illustrate the cumulative hazard estimates for COPD development for 1981 smoking groups.
Results
Characteristics
Participants’ (47.3% men) mean age was 40.2 (SD 13.4) years in 1981. In 1981, 48% of participants were never, 3% occasional, 21% former, 2% light, 17% moderate, and 9% heavy smokers. After exclusion of subjects who had moved abroad (n = 19) or died (n = 241) before 1981, we had 21,349 subjects at risk for COPD at the beginning of the follow-up.
The cumulative incidence of COPD was 2.47% during a 27-year follow-up. Among ever smokers, the incidence was 5.1%. Between 1981 and 2008, we observed 112 COPD cases according to special reimbursement eligibility: 17 persons with chronic bronchitis, 93 with COPD, and 2 with emphysema. The incidence of COPD special reimbursement eligibility was 0.54% during 27 years. An additional 401 persons were granted special reimbursement eligibility due to ‘chronic asthma and similar chronic obstructive pulmonary diseases’ without a specific diagnosis.
From the analysis of regular anticholinergics use, we excluded also subjects who had moved abroad (n = 35) or died (n = 1,829) between 1981 and 1995. Between 1995 and 2008, a total of 416 persons without special reimbursement eligibility for COPD, emphysema, or chronic bronchitis had purchased anticholinergics/combination preparations at least twice at 99-day intervals and were considered COPD cases. The incidence of regular anticholinergic use was 2.15% during 13 years.
COPD by smoking patterns
In adjusted logistic regressions, former, moderate, and heavy smokers in 1975 and 1981 demonstrated elevated COPD risks relative to never smokers (not shown in tables). The model adjusted for 1981 chronic bronchitis was compared with the model adjusted for 1975 smoking status × 1981 chronic bronchitis interaction. The interaction model was better with border-line significance (likelihood ratio test chi-square 7.87; P = 0.049), indicating that smokers in 1975 who had bronchitis in 1981 had a higher outcome risk than smokers in 1975 who did not develop bronchitis.
shows the results of logistic regressions for categories describing change in smoking in 1975–1981. The risk of having ever purchased anticholinergics was also tested among these categories. Former (OR 1.55; P = 0.023), constant light (OR 4.58; P = 0.011), 1981 moderate (OR 3.19; P ≤ 0.001), and constant heavy smokers (OR 4.44; P ≤ 0.001) as well as increasers (OR 3.34; P ≤ 0.001) had purchased combination preparations significantly more often than had constant never smokers. For pure anticholinergics, elevated risks were observed for the above-mentioned groups, except for light smokers.
Table II. Development of COPD, defined by regular anticholinergic medication or special reimbursement eligibility, by daily smoking patterns with number of subjects in each category, proportion of subjects affected, odds ratios (ORs), and 95% confidence intervals (95% CIs).
To examine further the effect of smoking exposure, we conducted adjusted logistic regressions for former smoker subgroups. When compared to those with < 5 pack-years, subjects with 5–10 pack-years (OR 1.07; 95% CI 0.47–2.42) and >10 pack-years (OR 1.48; 95% CI 0.82–2.68) did not have a significantly elevated disease risk. The result of trend test was also non-significant (OR 1.23; 95% CI 0.91–1.67).
Survival analyses
represents risks for COPD development for all categories describing change in smoking patterns in 1975–1981. illustrates risks for COPD for selected categories. Those who reduced from moderate to light smoking have a disease risk (measured as anticholinergic use) similar to constant moderate smokers and smoking increasers. When examining 1981 smoking status, we observed elevated HRs for regular anticholinergic medication in former smokers and all daily smokers (). Only moderate (HR 8.97; P ≤ 0.001) and heavy smokers (HR 22.05; P ≤ 0.001) demonstrated elevated risks for special reimbursement, light smokers being omitted due to lack of observations (not shown in Figures). and represent cumulative hazard estimates for COPD by 1981 smoking using two outcome definitions. Light smokers’ cumulative risk for regular anticholinergic use approached the risk of moderate smokers with increasing age.
Table III. Hazard ratios (HRs) with 95% confidence intervals (95% CIs) for regular anticholinergic medication and special reimbursement eligibilities among different smoking patterns. Number of subjects and affected subjects in each category are presented.
Figure 1. Results of the Cox proportional hazards regression model for regular anticholinergic medication among smoking groups in 1981, with never smokers as a reference category (Ref). Data points represent hazard ratios (HRs) with 95% confidence intervals (CIs) for each smoking group. Continuous line represents the result of the trend test.
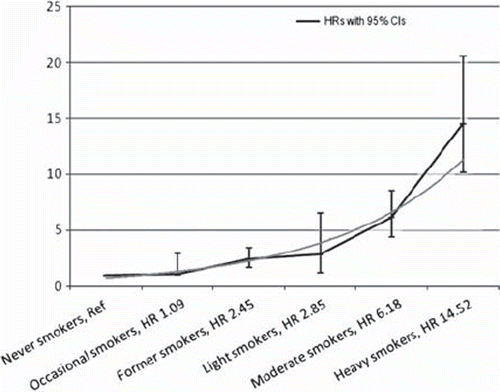
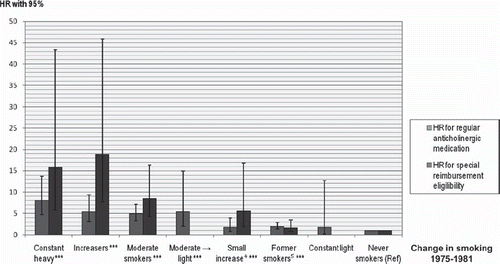
Figure 2. Hazard ratios1 (HRs) with 95% confidence intervals (95% CIs) for regular anticholinergic medication and special reimbursement eligibilities among different smoking patterns, with never smokers as a reference group. Only HR for medication is shown if a group has no observations for special reimbursements. Heavy → light smokers, initiators, and occasional smokers not shown. Notes: 1Hazard ratios (HR) obtained from Cox proportional hazards regression model. 2Hazard ratios results were adjusted for sex, pack-years, chronic bronchitis, asthma, education, physical activity, and alcohol use. 3***P ≤ 0.001 (non-significant results have no asterisks). 4P ≤ 0.001 for special reimbursement; P value for anticholinergic medication non-significant. 5P ≤ 0.001 for anticholinergic medication; P value for special reimbursement non-significant.
Discordant twin pair analyses
Conditional logistic regression models were conducted among 325 twin pairs discordant for outcome. The main results were replicated, COPD risk being significantly elevated among smoking increasers (OR 6.15; P = 0.004), moderate (OR 8.98; P ≤ 0.001), and heavy smokers (OR 10.17; P ≤ 0.001). Analyses conducted separately for MZ (n = 81) and DZ (n = 213) pairs gave clearly elevated risk estimates among the same smoking categories, being, however, statistically significant only among DZ pairs.
Discussion
To our knowledge, this is the first large cohort study estimating COPD risks among various longitudinal smoking patterns. Cox models gave similar risk estimates for smoking increasers, decreasers, and moderate smokers, suggesting that reducing daily consumption to 1–4 cigarettes does not alter COPD risk. Light smoking measured at one point was also associated with an elevated disease risk. With the discordant twin pair analyses, we could control for the common family background, which did not seem to affect the association between smoking patterns and COPD. These results support a causal relationship between smoking and COPD. Non-significant risk estimates among discordant MZ pairs were likely obtained due to small sample size and multiple adjusting variables, decreasing statistical power.
Previous results on the effect of smoking reduction on FEV1 and forced vital capacity (FVC) are contradictory, although reduction may relieve respiratory symptoms (Citation9,Citation10). Even light smoking is associated with respiratory symptoms (Citation30), but only one earlier study found an association between COPD and cross-sectionally measured light smoking (Citation6). When estimating light smokers’ risk, smoking history should also be considered, because this smoking pattern is rarely consistent (Citation19).
Strengths of this study include a large study population, high response rates, long follow-up times, and reliable registry-based outcome measures. Further, we obtained consistent results with different statistical methods. Sufficient statistical power and longitudinally measured smoking allowed us to estimate risks for several accurately defined smoking subgroups. Self-reported smoking seems to be reliable: the proportion of smokers in our sample is similar to that in the general population in the 1980s (Citation17). Finns’ self-reported smoking has been previously shown to be highly consistent with their serum cotinine levels (Citation31). Since acquired smoking patterns are stable in middle age (in our data, the majority of former, moderate, and heavy smokers in 1975 had the same status in 1981), a 6-year follow-up reflects long-term behavior and is highly informative in terms of COPD development. Different follow-up times for reimbursement diagnoses and anticholinergic use do not complicate the interpretation of results, because their outcomes are different. To detect special reimbursements (usually allocated once in a lifetime), a long follow-up is essential. By contrast, once symptomatic COPD develops, it requires constant medication, and even a short follow-up of medication purchases detects diseased subjects.
Because our disease definition likely missed patients using other than anticholinergic treatment, as well as those with mild and subclinical disease, the results cannot be used to estimate total incidence of COPD during the follow-up. However, those with symptomatic COPD were likely well covered. The reimbursement system supports regular usage of prescribed medications (Citation26); thus, the majority of purchases for chronic diseases are registered. Only medications purchased abroad, used during hospitalizations, or purchased without presenting the health insurance card are not tracked by the SII. Reimbursement data possibly missed more patients than medication data for two reasons. First, COPD reimbursement criteria do not encompass mild disease. Second, asthma and COPD commonly coexist in aging populations (Citation32,Citation33). Patients suffering from both more likely have a special reimbursement for asthma, which is usually diagnosed earlier in life. If COPD later develops, no new reimbursement eligibility certificate is needed. In 2000, 20% of Finnish asthmatics aged over 65 used anticholinergics (Citation32), suggesting that they actually also have COPD.
Although multiple confounders were taken into account, one limitation of this study is that passive smoking and occupational exposure were not assessed. Neither was spirometry conducted, which is the reliable way to diagnose COPD (Citation1). It is noteworthy, however, that spirometry follow-up studies always involve drop-outs, whereas national register data drop-outs usually occur only when people move abroad, which is accounted for in the analysis. Moreover, continuous monitoring of diagnosis onset is not possible using spirometry. Although our results could be even stronger if subclinical cases were included, the association between low-rate smoking and moderate or severe COPD is an important finding.
Among those who reduced from moderate to light smoking, chronic bronchitis prevalence decreased from 5.1% (1975) to 2.3% (1981), demonstrating that reduction may relieve current respiratory symptoms. Most affected reducers, however, started regular medications in the twenty-first century, suggesting that reduction does not protect against COPD development. Light smoking preceded by heavier use seems to be strongly associated with COPD, possibly because such smokers relapse to heavier smoking later in life.
Constant light smokers form a very small smoking subgroup. Although we found an increased COPD risk among 1981 light smokers, constant light smokers’ risk was not significant. They had, however, purchased anticholinergics significantly more often than had never smokers. A similar need for medication suggests no difference in respiratory symptoms between light and other daily smokers. Medication usage may reflect chronic bronchitis or early COPD. Our follow-up time was possibly too short to reveal all COPD cases among light smokers, who were relatively young; mean age was 35 years (SD 11) in 1981. Whether a longer follow-up would uncover increased COPD risks also among other low-rate smokers remains to be elucidated.
To conclude, regarding pulmonary function, no safe level of daily smoking exists. Even daily light smoking is associated with certain pulmonary dysfunction, as indicated by increased use of inhaled anticholinergics. Other daily smokers represent clearly elevated COPD risks.
Acknowledgements
The Finnish Twin Cohort study is funded by the Academy of Finland Centre of Excellence in Complex Disease Genetics. The analyses here were supported by a research grant from the Yrjö Jahnsson Foundation. The Social Insurance Institution of Finland is thanked for providing the outcome data on special reimbursements and medication purchases related to COPD.
All authors participated in the research, contributed to several drafts of the manuscript, and approved the final manuscript. JK designed the study and its methods, supervised data collection, and contributed to the analyses and interpretation of data. MH conducted the literature review, summarized related works, wrote the manuscript, and performed the statistical analyses under the supervision of TK. KH provided substantial help with statistical analyses.
JK attests that the study objectives and procedures are honestly disclosed. He reviewed the study execution data and confirms that procedures were followed to an extent that convinces all authors that the results are valid and generalizable to a population similar to that enrolled in this study.
Declaration of interest: The authors have no conflicts of interest to declare.
References
- Global Initiative of Chronic Obstructive Pulmonary Disease (GOLD). Global Strategy for Diagnosis, Management and Prevention of COPD. Updated in 2010. Accessed 11 May 2011.
- Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet. 2007;370:765–73.
- Wan ES, Silverman EK. Genetics of COPD and emphysema. Chest. 2009;136:859–66.
- Pietinalho A, Kinnula VL, Sovijärvi AR, Vilkman S, Säynäjäkangas O, Liippo K, . Chronic bronchitis and chronic obstructive pulmonary disease. The Finnish Action Programme, interim report. Respir Med. 2007;101:1419–25.
- Kotaniemi J. Asthma, chronic obstructive pulmonary disease and respiratory symptoms among adults: prevalence and risk factors—The FinEsS Study in Northern Finland [thesis]. Helsinki: University of Helsinki; 2006. Available at: https://oa.doria.fi/bitstream/handle/10024/2032/asthmach.pdf?sequence=1. Accessed 11 May 2011.
- Lundbäck B, Lindberg A, Lindström M, Rönmark E, Jonsson AC, Jönsson E, . Not 15 but 50% of smokers develop COPD? Report from the Obstructive Lung Disease in Northern Sweden Studies. Respir Med. 2003;97: 115–22.
- Bjartveit K, Tverdal A. Health consequences of smoking 1-4 cigarettes per day. Tob Control. 2005;14:315–20.
- Korhonen T, Broms U, Levälahti E, Koskenvuo M, Kaprio J. Characteristics and health consequences of intermittent smoking: Long-term follow-up among Finnish adult twins. Nicotine Tob Res. 2009;11:148–55.
- Pisinger C, Godtfredsen NS. Is there a health benefit of reduced tobacco consumption? A systematic review. Nicotine Tob Res. 2007;9:631–46.
- Fagerström, K. Smoking reduction in the management of COPD. Monaldi Arch Chest Dis. 2002;57:281–4.
- Tashkin DP. Long-acting anticholinergic use in chronic obstructive pulmonary disease: efficacy and safety. Curr Opin Pulm Med. 2010;16:97–105.
- Gross NJ. Anticholinergic agents in asthma and COPD. Eur J Pharmacol. 2006;533:36–9.
- Kinnula V, Tukiainen P, Katajisto M, Keistinen T, Tikkanen H, Sovijärvi A. Working group appointed by the Finnish Medical Society Duodecim and the Finnish Respiratory Society. Käypä hoito-suositus: Keuhkoahtaumatauti (English summary: Chronic Obstructive Pulmonary Disease, Diagnosis and Treatment). Updated in 2009. Accessed 11 May 2011.
- Pauwels R. Global initiative for chronic obstructive lung diseases (GOLD): time to act. Eur Respir J. 2001;18:901–2.
- Jousilahti P, Komulainen J, Hanski T, Kaila M, Ketola E. Perusterveydenhuollon lääkärit tuntevat hyvin Käypä Hoito-suositukset (English summary: Knowledge of, attitudes to, and use of current care guidelines among Finnish primary health care physicians). Finnish Medical Journal. 2007;62: 3319–23.
- Finnish Statistics on Medicines. Helsinki: National Agency for Medicines and Social Insurance Institution; 1995–2008. ISSN 0786-2180.
- Official Statistics of Finland. Tobacco Statistics 2009. ISSN = 1796-0479. Helsinki: Statistics of Finland (accessed 17 Feb 2011).
- Okuyemi KS, Harris KJ, Scheibmeir M, Choi WS, Powell J, Ahluwalia JS. Light smokers: issues and recommendations. Nicotine Tob Res. 2002;4(Suppl 2):S103–12.
- Hukkinen M, Kaprio J, Broms U, Koskenvuo M, Korhonen T. Characteristics and consistency of light smoking: Long-term follow-up among Finnish adults. Nicotine Tob Res. 2009;11:797–805.
- Shiffman S, Kassel JD, Paty J, Gnys M, Zettler-Segal M. Smoking typology profiles of chippers and regular smokers. J Subst Abuse. 1994;6:21–35.
- Hukkinen M, Korhonen T, Broms U, Koskenvuo M, Kaprio J. Long-term smoking behavior patterns predicting self-reported chronic bronchitis. COPD. 2009;6:242–9.
- Kujala UM, Kaprio J, Koskenvuo M. Modifiable risk factors as predictors of all-cause mortality: the roles of genetics and childhood environment. Am J Epidemiol. 2002;156: 985–93.
- British Medical Research Council; Medical Research Council's Committee on the aetiology of chronic bronchitis. Standardised questionnaires on respiratory symptoms. British Medical Journal. 1960;ii:1665.
- Laitinen LA, Koskela K. Chronic bronchitis and chronic obstructive pulmonary disease: Finnish National Guidelines for Prevention and Treatment 1998–2007. Respir Med. 1999;93:297–332.
- Finland's Social Insurance Institution's decisions on medical conditions for specially reimbursed medications (in Finnish). Updated 6 Sep 2010. Available at: http://www.kela.fi/in/internet/suomi.nsf/NET/270308131720KA?OpenDocument.Accessed 11 May 2011.
- Martikainen J, Rajaniemi S. Drug reimbursement systems in EU Member States, Iceland and Norway. 2002. Available at: http://helda.helsinki.fi/bitstream/handle/10138/13932/Drug_reimbursement.pdf?sequence=1. Accessed 11 May 2011.
- The Social Insurance Institution of Finland: Guide to benefits. 2010. Available at: http://www.kela.fi/in/internet/liite.nsf/NET/180808091909HS/$File/Pahkina_eng.pdf?Open Element. Accessed 11 May 2011.
- StataCorp. Stata Statistical Software: Release 11. College Station, TX: StataCorp LP.; 2009.
- Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56:645–6.
- Amigo H, Oyarzun MG, Bustos P, Rona RJ. Respiratory consequences of light and moderate smoking in young adults in Chile. Int J Tuberc Lung Dis. 2006;10:744–9.
- Vartiainen E, Seppälä T, Lillsunde P, Puska P. Validation of self-reported smoking by serume cotinine measurement in a community-based study. J Epidemiol Community Health. 2002;56:167–70.
- Ikäheimo P, Hartikainen S, Tuuponen T, Kiuttu J, Klaukka T. Comorbidity and medication load in adult asthmatics. Scand J Prim Health Care. 2005;23: 88–94.
- Gibson PG, Simpson JL. The overlap syndrome of asthma and COPD: what are its features and how important is it? Thorax. 2009;64:728–35.