Abstract
Background. Non-steroidal anti-inflammatory drugs are associated with poor upper gastrointestinal (UGI) tolerability and increased ulcer risk, but patient adherence to gastroprotective co-therapy is frequently inadequate. A fixed-dose combination of enteric-coated naproxen 500 mg and immediate-release esomeprazole magnesium 20 mg was evaluated: efficacy is reported by Hochberg et al. (Curr Med Res Opin 2011;27:1243–53); tolerability findings are reported here.
Patients and methods. In two 12-week double-blind, placebo-controlled, multicenter, phase III studies (PN400-307 and PN400-309), patients aged ≥ 50 years with symptomatic knee osteoarthritis randomly (2:2:1) received naproxen/esomeprazole magnesium BID, celecoxib 200 mg QD, or placebo. Tolerability end-points included: modified Severity of Dyspepsia Assessment (mSODA); heartburn severity; and UGI adverse events (AEs).
Results. Overall, 619 (PN400-307) and 615 (PN400-309) patients were randomized; mSODA scores improved (baseline to week 12) in each group, with no significant treatment differences between naproxen/esomeprazole magnesium and celecoxib (95% CIs: PN400-307: –0.4, 1.9; PN400-309: –1.8, 0.6). Naproxen/esomeprazole magnesium-treated patients reported significantly more heartburn-free days versus celecoxib (95% CIs: PN400-307: 2.1, 12.7; PN400-309: 2.5, 13.4). UGI AE incidence (PN400-307: 17.3%; PN400-309: 20.3%) was similar between treatment groups. UGI AEs resulted in few discontinuations (< 4%, either study).
Conclusions. Naproxen/esomeprazole magnesium has comparable UGI tolerability to celecoxib in patients with osteoarthritis.
| Abbreviations | ||
| ACR | = | American College of Rheumatology |
| AE | = | adverse event |
| ANCOVA | = | analysis of covariance |
| BID | = | twice daily |
| BMI | = | body mass index |
| CEL | = | celecoxib |
| CI | = | confidence interval |
| COX | = | cyclo-oxygenase |
| EC | = | enteric-coated |
| FDC | = | fixed-dose combination |
| GI | = | gastrointestinal |
| IR | = | immediate-release |
| LDA | = | low-dose aspirin |
| LS | = | least squares |
| MedDRA | = | Medical Dictionary for Regulatory Activities |
| mITT | = | modified intent-to-treat |
| mSODA | = | modified Severity of Dyspepsia Assessment |
| NAP/ESO | = | naproxen/esomeprazole magnesium |
| NSAID | = | non-steroidal anti-inflammatory drug |
| OA | = | osteoarthritis |
| OTE-DP | = | Overall Treatment Evaluation of Dyspepsia |
| PBO | = | placebo |
| PGA | = | Patient Global Assessment |
| PP | = | per protocol |
| PPI | = | proton pump inhibitor |
| QD | = | once daily |
| SAE | = | serious adverse event |
| SODA | = | Severity of Dyspepsia Assessment |
| UGI | = | upper gastrointestinal |
| WOMAC | = | Western Ontario and McMaster Universities Osteoarthritis Index |
Key messages
NSAIDs are widely used to manage the signs and symptoms of osteoarthritis but are sometimes associated with poor upper gastrointestinal (UGI) tolerability. In addition to NSAID-associated ulcers and complications, more prevalent side-effects such as dyspepsia and heartburn are important because they have a significant day-to-day impact on patient quality of life, increase burden of care, and can result in discontinuation of NSAID therapy.
Gastroprotective proton pump inhibitor co-therapy can improve NSAID tolerability, but patient adherence is often suboptimal.
A fixed-dose combination of naproxen 500 mg and esomeprazole magnesium 20 mg reduces symptoms associated with osteoarthritis and has comparable UGI tolerability to celecoxib 200 mg.
Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) are a mainstay of treatment for pain associated with osteoarthritis (OA) (Citation1–6), but their use can be associated with poor upper gastrointestinal (UGI) tolerability, including symptoms of dyspepsia, heartburn, nausea, and abdominal pain in up to 60% of patients, and serious ulcer complications (bleeding, perforation, and obstruction) in 2%–4% of NSAID users (Citation7,Citation8). Dyspepsia, defined by the American Gastroenterological Association as ‘chronic or recurrent pain or discomfort centered in the upper abdomen’ (Citation9), can be considered to be one of the most clinically relevant NSAID-associated UGI adverse events (AEs) based on its prevalence, impact on patient quality of life, and substantial economic burden of care (Citation10,Citation11). Up to one-quarter of NSAID patients have been reported to discontinue therapy as a result of dyspeptic symptoms (Citation10).
Currently recommended strategies to reduce the incidence of NSAID-associated UGI toxicity and complications in patients with risk factors, for example, advanced age, history of gastroduodenal ulcers or UGI symptoms, or concomitant use of aspirin, anticoagulants, or corticosteroids (Citation3,Citation12), include the use of cyclo-oxygenase (COX)-2-selective NSAIDs and/or co-therapy with gastroprotective agents (Citation3,Citation13–16). In particular, proton pump inhibitor (PPI) co-therapy with non-selective NSAIDs may offer greater risk reduction for dyspepsia than COX-2-selective inhibitor therapy (Citation11,Citation17). Esomeprazole is a PPI which has demonstrated efficacy for the resolution of heartburn, acid regurgitation, and upper abdominal pain, burning, or discomfort in continuous users of NSAIDs, including COX-2-selective inhibitors (Citation18,Citation19), and addition of a PPI to either non-selective or COX-2-selective NSAID therapy has been shown to improve patient quality of life in a cost-effective manner (Citation20).
However, the clinical effectiveness of concomitant gastroprotection is often undermined by low physician utilization and poor patient adherence (Citation21–26). One possible strategy to address this issue is the development of fixed-dose combinations of NSAIDs and gastroprotective agents. A fixed-dose combination of enteric-coated (EC) naproxen 500 mg and immediate-release (IR) esomeprazole magnesium 20 mg is approved by the US Food and Drug Administration for the relief of signs and symptoms of OA, rheumatoid arthritis, and ankylosing spondylitis, and to decrease the risk of gastric ulcers in patients who are at risk of developing NSAID-associated gastric ulcers (Citation27). Positive agreement for approval in Europe has also been granted for use in patients who are at risk for developing NSAID-associated gastric and/or duodenal ulcers, and where treatment with lower doses of naproxen or of other NSAIDs is not considered sufficient (Citation28).
In this paper, the tolerability findings from two identical phase III studies designed to compare the efficacy, tolerability, and safety of naproxen/esomeprazole magnesium with celecoxib 200 mg, a COX-2-selective inhibitor, and placebo in patients with OA of the knee are reported. Efficacy findings from these studies are reported in detail elsewhere (Citation29).
Patients and methods
Study design
Two identical, 12-week randomized, double-blind, parallel-group, placebo-controlled, multicenter, phase III studies (PN400-307, NCT00664560 and PN400-309, NCT00665431) were conducted in 157 centers in the United States between April and December 2008. Both studies were reviewed and approved by an independent ethics committee (Copernicus Group Institutional Review Board), and all patients gave written, informed consent in accordance with the 1996 Declaration of Helsinki.
The primary objective was to evaluate the efficacy of naproxen/esomeprazole magnesium compared with celecoxib 200 mg and placebo for the treatment of the signs and symptoms of OA of the knee. UGI tolerability and safety were secondary end-points. The studies consisted of an initial screening visit, where patients provided consent and were assessed for medical history and eligibility, a wash-out period of 7–14 days, a baseline visit (following a flare in OA pain), and follow-up visits at weeks 1, 6, and 12 for efficacy and tolerability assessments.
Patients were randomly assigned (2:2:1) at baseline to treatment with naproxen/esomeprazole magnesium, celecoxib, or placebo via the interactive web response system, according to unique patient numbers and a randomization list produced by a third party under the direction of POZEN Inc. Patients, investigators, study site staff, those performing study assessments, POZEN staff, and analysts remained blinded throughout the study except in the case of emergency.
Patients
Eligible patients were aged at least 50 years with a 6-month history of symptomatic, clinically diagnosed OA of the knee (American College of Rheumatology (ACR) functional class rating I, II, or III), who had been receiving a stable dose of NSAIDs, COX-2-selective inhibitors, or other oral analgesic therapy for at least 6 weeks.
Exclusion criteria included a history of hypersensitivity, allergic reaction, or intolerance to any PPI or any NSAID (including aspirin), a presence of any uncontrolled acute or chronic medical illness, any gastrointestinal (GI) disorder or surgery, a history of peptic ulcer disease within 6 months prior to screening, or recent history (in the past 3 months) of alcohol or drug abuse. Excluded concomitant medications included any other NSAID (other than low-dose aspirin (LDA)) or gastroprotective agent, parenteral steroids, lithium, glucosamine and/or chondroitin sulfate, and anticoagulants.
Incidental use of rescue antacid (≤ 6 tablets per day) and supplemental use of rescue acetaminophen (≤ 3 g/day) were allowed during the study. Concomitant use of oral prednisone (≤ 7.5 mg/day), LDA (≤ 325 mg/day), and antiplatelet agents (non-concomitant with LDA) were also allowed.
Study treatment
Patients received one of the following treatments for 12 weeks: naproxen/esomeprazole magnesium tablets twice daily (BID), celecoxib 200 mg capsules once daily (QD), or placebo. To maintain blinding, patients in the naproxen/esomeprazole magnesium treatment group were also administered placebo capsules with identical packaging and appearance to celecoxib, while those in the celecoxib treatment group also received placebo tablets with identical packaging and appearance to naproxen/esomeprazole magnesium.
Study treatment was taken orally 30–60 minutes before a meal in the morning (one tablet and one capsule) and evening (one tablet) until the end of study or discontinuation in the event of withdrawal of consent, pregnancy, or any significant safety risk at the discretion of the investigator. Emergency or inadvertent unblinding also resulted in discontinuation.
Patients were also provided with open-label antacid and acetaminophen at baseline and week 4 for rescue/supplemental use only in the event that their symptoms were intolerable.
Study assessments
As previously reported, efficacy was assessed as the change from baseline to week 12 in three co-primary end-points: the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain subscale, WOMAC function subscale, and Patient Global Assessment (PGA) of OA (Citation29).
UGI tolerability
UGI tolerability (secondary end-point) was assessed by the modified Severity of Dyspepsia Assessment (mSODA), heartburn severity, the incidence of predefined NSAID-associated UGI AEs, and discontinuations as a result of any AE or predefined NSAID-associated UGI AE. Additionally, use of rescue antacid medication to relieve symptoms of upper abdominal discomfort was assessed by pill counts at each visit.
The mSODA tool consisted of six questions assessing dyspepsia-related upper abdominal pain intensity (Citation30). This instrument was developed as a derivative of SODA, a validated patient-reported outcome that evaluates the multidimensional nature of dyspepsia using three scales: pain, non-pain symptoms, and satisfaction with dyspepsia-related health (Citation31,Citation32). The pain intensity scale alone, designed to evaluate the primary symptom of dyspepsia, namely upper abdominal pain, has been validated in patients using NSAIDs to manage the signs and symptoms of arthritis (Citation33,Citation34). This SODA pain intensity scale was modified for use in these studies by introducing an electronic mode of administration and replacing a 7-day recall period with a 24-hour time-frame, with the aim of improving compliance and accuracy (Citation30). Take-home electronic diaries were used to assess mSODA on a daily basis from baseline to week 12.
A heartburn severity questionnaire was used to rate the symptoms of heartburn (defined as a burning feeling rising from the stomach or lower part of the chest towards the neck) as none, mild, moderate, or severe. Heartburn severity was assessed daily from baseline to week 12 using take-home electronic diaries and a 24-hour recall period.
The following predefined UGI AE preferred terms typically observed in NSAID users, as compiled by a panel of two gastroenterologists and one rheumatologist based on literature and medical expertise, were recorded: abdominal discomfort, abdominal pain, abdominal tenderness, duodenal ulcer, dyspepsia, nausea, gastritis, gastroesophageal reflux disease, GI hemorrhage, hyperchlorhydria, stomach discomfort, upper abdominal pain, and vomiting.
Safety
AEs were assessed via patient-volunteered information and non-directive questioning at each visit. All AEs occurring from the start of treatment to the final visit (treatment-emergent) were recorded and assessed for severity and whether related to study treatment. Serious adverse events (SAEs) were recorded from the time of informed consent and were defined as being fatal or life-threatening, resulting in persistent/significant disability or incapacity, constituting a congenital anomaly/birth defect, resulting in hospitalization or prolonged existing hospitalization, or being considered medically significant.
Safety was also evaluated based on the following: measurement of vital signs (sitting heart rate and a single reading of systolic and diastolic blood pressure) at each visit; physical examination at screening and week 12; and clinical laboratory assessments (creatinine, alanine aminotransferase, aspartate aminotransferase, bilirubin, blood urea nitrogen, and complete blood count) performed at screening, baseline, and each subsequent visit.
Statistical analysis
A target population of approximately 570 patients for each study, with approximately 228 in each active treatment group and 114 in the placebo group, was designed to support the primary non-inferiority end-point, as previously reported (Citation29).
Analyses of mSODA findings, heartburn severity, and rescue antacid use were based on the modified intent-to-treat (mITT) population (all randomized patients who received at least one dose of study medication and provided at least one post-baseline efficacy evaluation). Mean daily mSODA pain scores over 6-week and 12-week treatment periods were calculated, and changes from baseline were analyzed using an analysis of covariance (ANCOVA) model that used baseline scores as covariates and treatment as a factor in the analysis model. Least-squares (LS) mean changes from baseline were estimated for each treatment group, and the difference between naproxen/esomeprazole magnesium and celecoxib was tested against the null hypothesis of no difference, using a two-tailed F test. Analysis of mSODA was also conducted in subgroups of LDA users and non-users at randomization. LDA users were defined as those taking ≤ 325 mg aspirin; no minimum pre-existing or on-study duration of LDA use was pre-specified.
The proportion of heartburn-free days was calculated for each patient by dividing the number of days without heartburn (score of ‘none’ on the heartburn severity questionnaire) by the total number of days with heartburn data. The results were presented descriptively for each treatment group. LS mean changes from baseline were calculated, and a two-sided 95% confidence interval (CI) was constructed for each pair-wise treatment difference. Rescue antacid use was summarized by: proportion of patients using rescue antacid, proportion of study days rescue antacid used, time to first use (days), total number of doses taken, and average doses taken per month, with corresponding two-sided 95% CIs for treatment differences.
Analyses of all AEs, UGI AEs, and additional safety end-points were based on the safety population (all randomized patients who received at least one dose of study medication). AEs were coded for system organ class and preferred term according to the Medical Dictionary for Regulatory Activities (MedDRA) version 10.1. AEs or pre-specified UGI AEs, and resulting discontinuations, were summarized by treatment group. Clinical laboratory values, physical examination findings, and vital signs were summarized using descriptive statistics.
All available data up to the time of discontinuation were included in the statistical analyses for those patients who withdrew prematurely. For week 6 and 12 mSODA analyses, missing data were imputed using a last observation carried forward approach. AEs with a partial or missing start date were treated as treatment-emergent unless the partial date excluded that possibility. AEs with missing severity or relationship to study medication data were treated as severe, and as treatment-related, respectively.
Results
Patients
A total of 619 patients were randomized in PN400-307 (Study 307); 614 were treated, and 521 completed the study. In PN400-309 (Study 309), 615 patients were randomized; 610 were treated, and 489 completed the study (). The premature discontinuation rate was comparable between the naproxen/esomeprazole magnesium, celecoxib, and placebo treatment groups in Study 307: 16.1%, 15.8%, and 15.3%, respectively; and in Study 309: 16.8%, 23.9%, and 21.0%, respectively (). AEs were the most frequent cause of discontinuation in Study 307 (approximately 6%–8%), and two patients in this study were lost to follow-up (celecoxib group). In Study 309, a higher proportion of patients treated with placebo (12.1%) and celecoxib (10.1%) withdrew consent compared with those treated with naproxen/esomeprazole magnesium (7.0%), and a slightly higher proportion of patients in the celecoxib group discontinued due to AEs (8.9%) compared with the naproxen/esomeprazole magnesium (6.6%) or placebo (4.8%) groups. Few patients in any treatment group of Study 309 were lost to follow-up (0.8%–1.2%).
Figure 1. Study disposition. (AE = adverse event; CEL = celecoxib; mITT = modified intent-to-treat; NAP/ESO = naproxen/esomeprazole magnesium; PBO = placebo; PP = per protocol.) Hochberg et al, Curr Med Res Opin, 2011; 27: 1243-53, copyright © 2011, Informa Healthcare. Reproduced with permission of Informa Healthcare.
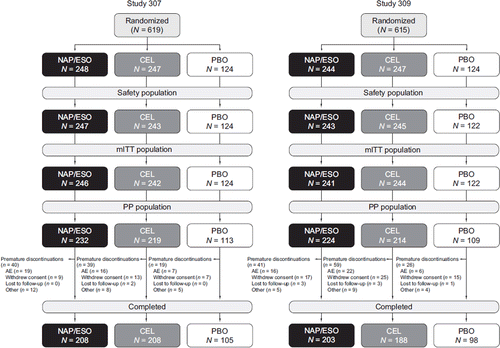
Baseline demographics were similar between treatment groups in both studies (); over 60% of patients were women, and mean age was 62 years. At randomization, LDA was used by 23% of patients in both studies. Approximately 90% of patients had greater than 70% compliance to study medication (took more than 70% of scheduled doses) from baseline to last dose in either study.
Table I. Patient demographics and baseline characteristics.
Modified Severity of Dyspepsia Assessment (mSODA)
At baseline, mean mSODA scores were similarly low between treatment groups in both studies (Study 307: 10.9, 11.8, and 11.9; Study 309: 12.4, 11.6, and 10.5, in the naproxen/esomeprazole magnesium, celecoxib, and placebo groups, respectively), indicating mild dyspepsia-related pain in the study populations prior to randomization. At week 12, similar levels of improvement in mean mSODA scores were observed across all three treatment groups in both studies (Study 307: 7.5, 7.0, and 7.9; Study 309: 8.0, 8.3, and 7.0, in the naproxen/esomeprazole magnesium, celecoxib, and placebo groups, respectively). Corresponding mean change from baseline to week 12 mSODA scores were –3.5, –4.8, and –4.0 (Study 307), and –4.5, –3.3, and –3.5 (Study 309) in the naproxen/esomeprazole magnesium, celecoxib, and placebo groups, respectively. The LS mean change in mSODA scores from baseline to week 12 was also similar in each treatment group (). There were no significant differences between naproxen/esomeprazole magnesium and celecoxib in Study 307 (95% CI on treatment difference: –0.4, 1.9; P = 0.1828) or Study 309 (95% CI on treatment difference: –1.8, 0.6; P = 0.2979). A similar pattern was observed at week 6. Among the subgroup of patients using LDA, there were no significant differences between naproxen/esomeprazole magnesium and celecoxib at week 12 in either study ().
Figure 2. Change from baseline to week 12 in mSODA pain scores (LS mean) in patients treated with naproxen/esomeprazole magnesium, celecoxib, and placebo (mITT population with last observation carried forward). 95% CI on treatment difference for naproxen/esomeprazole magnesium minus celecoxib in Study 307: −0.4, 1.9; P = 0.1828; and for Study 309: −1.8, 0.6; P = 0.2979. (CEL = celecoxib; mITT = modified intent-to-treat; LS = least squares; mSODA = modified Severity of Dyspepsia Assessment; NAP/ESO = naproxen/esomeprazole magnesium; PBO = placebo.)
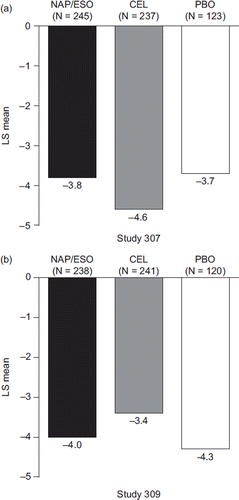
Table II. Mean change from baseline to week 6 and week 12 in mSODA pain scores in LDA users and non-users (mITT population).
Heartburn
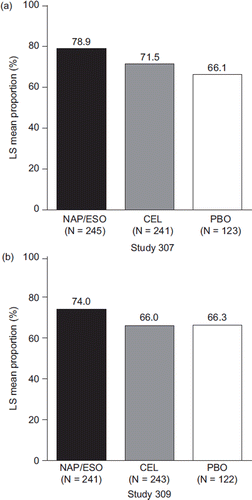
The greatest proportion of heartburn-free days (score of ‘none’ on the heartburn severity questionnaire) between baseline and week 12 was observed in the naproxen/esomeprazole magnesium treatment groups of both studies (Study 307: 83.9% for naproxen/esomeprazole magnesium versus 75.8% for celecoxib and 68.5% for placebo; Study 309: 78.4% for naproxen/esomeprazole magnesium versus 72.1% for celecoxib and 71.1% for placebo). Patients in the naproxen/esomeprazole magnesium treatment group experienced a significantly greater proportion of heartburn-free days over 12 weeks compared with those treated with celecoxib in Study 307 (95% CI on treatment difference: 2.1, 12.7) and Study 309 (95% CI on treatment difference: 2.5, 13.4) (). Similar findings were observed at week 6.
Figure 3. Proportion of heartburn-free days (LS mean %) from baseline to week 12 in patients treated with naproxen/esomeprazole magnesium, celecoxib, and placebo (mITT population). 95% CI on treatment difference for naproxen/esomeprazole magnesium minus celecoxib in Study 307: −2.1, 12.7; and for Study 309: 2.5, 13.4. (CEL = celecoxib; mITT = modified intent-to-treat; LS = least squares; NAP/ESO = naproxen/esomeprazole magnesium; PBO = placebo.)
Rescue antacid use
In Study 307, rescue antacid was used by 43.1%, 55.4%, and 49.2% of patients in the naproxen/esomeprazole magnesium, celecoxib, and placebo groups, respectively. The proportion of patients using rescue antacid was significantly lower in the naproxen/esomeprazole magnesium treatment group versus the celecoxib treatment group (95% CI on treatment difference: –21.1, –3.5). In Study 309, rescue antacid was used by 49.8%, 52.9%, and 53.3% of patients in the naproxen/esomeprazole magnesium, celecoxib, and placebo groups, respectively. There were no significant differences between naproxen/esomeprazole magnesium and celecoxib in this study (95% CI on treatment difference: –12.0, 5.8). Rescue antacid was used on 19.5%, 28.1%, and 37.8% of study days by patients treated with naproxen/esomeprazole magnesium, celecoxib, and placebo, respectively, in Study 307, and on 23.8%, 33.3%, and 38.6% of study days, respectively, in Study 309. In Study 307, the mean number of rescue antacid tablets used per month was significantly lower in the naproxen/esomeprazole magnesium group compared with celecoxib (9.3 versus 13.9; 95% CI –8.2, –1.1). In Study 309, there was no significant difference between active treatments in the mean number of rescue antacid tablets used per month (13.4 versus 20.9; 95% CI –16.9, 1.8).
UGI AEs and discontinuations
The overall proportion of patients reporting predefined NSAID-associated UGI AEs was 17.3% in Study 307 and 20.3% in Study 309. The incidence was similar between treatment groups in both studies (). The most common (reported by at least 3% of patients in any treatment group of either study) were dyspepsia, nausea, upper abdominal pain, and vomiting. Across both studies, the proportion of patients who discontinued as a result of UGI AEs was less than 4% ().
Table III. Summary of predefined UGI AEs and discontinuations as a result of UGI AEs or any AE (safety population).
The proportion of patients discontinuing due to any AE was similar across treatment groups in Study 307 (approximately 6%–7%). In Study 309, fewer patients receiving placebo discontinued due to any AE compared with naproxen/esomeprazole magnesium and celecoxib ().
Safety
The overall incidence of AEs was approximately 50% and was similar between treatment groups in both studies (). GI disorders accounted for the majority of treatment-emergent AEs. Few SAEs were observed; 2% of patients (n = 10) in Study 307 (five patients in each of the naproxen/esomeprazole magnesium and celecoxib groups), and 1% of patients (n = 7) in Study 309 (three patients in each of the active treatment groups, and one in the placebo group). Only one SAE (anaphylactic reaction) was related to treatment, reported in the celecoxib treatment group of Study 307. There were no deaths in either study. As reported previously, changes from baseline in clinical laboratory values and vital signs were small, and the majority of these changes were not clinically significant (Citation29).
Table IV. Treatment-emergent AEs: Overall and reported by ≥ 3% of patients in any treatment group of either study (safety population).
Discussion
These two randomized, controlled, phase III studies were designed to assess the efficacy and tolerability of a fixed-dose combination of EC naproxen and IR esomeprazole magnesium compared with celecoxib and placebo in patients with OA of the knee. Based on the analyses reported in this paper, treatment with naproxen/esomeprazole magnesium had comparable UGI tolerability to celecoxib in terms of severity of dyspepsia, incidence of UGI AEs, and discontinuations as a result of UGI or any AE. Furthermore, naproxen/esomeprazole treatment was associated with the highest proportion of heartburn-free days during the study; and also with less antacid use than celecoxib.
The improvements in dyspepsia-related pain, as measured by mSODA, experienced by patients treated with naproxen/esomeprazole magnesium treatment for 12 weeks in these studies were modest (Citation30,Citation31) but similar to those observed in patients treated with celecoxib and placebo. The findings are also consistent with SODA pain scale scores from two earlier randomized, controlled, phase III studies that compared naproxen/esomeprazole magnesium with EC naproxen 1000 mg/day alone over 6 months in patients requiring long-term NSAID therapy who were at risk of NSAID-associated ulcers (Citation35). In these earlier 6-month studies, a correlation was observed between SODA scores and the findings of a second assessment, the Overall Treatment Evaluation of Dyspepsia (OTE-DP), which assessed the degree and importance of changes in upper abdominal pain and/or discomfort to patients since start of treatment (Citation36). While the symptoms captured by each assessment may be different, this finding supports the validity of the SODA and mSODA tools and the clinical relevance of their findings.
Tolerability data from the 3-month studies reported in this paper are also consistent with the findings from the earlier 6-month studies in terms of comparable proportions of heartburn-free study days among patients treated with naproxen/esomeprazole magnesium (> 70%). The incidence of discontinuations due to any AE in the naproxen/esomeprazole magnesium arm of these studies was also similar to that observed by Goldstein et al. (approximately 7%–10%). However, a higher incidence of predefined NSAID-associated UGI AEs (> 50%) and resulting discontinuations (> 3%) was previously observed, although this may be due to the inclusion of additional predefined UGI AEs, such as duodenitis, esophagitis, gastric mucosal lesions, and GI erosions, which may have been identified endoscopically in the 6-month studies (Citation35).
It is also worth noting that the tolerability data reported here for the 3-month studies compare favorably with those reported in an earlier phase III open-label study that evaluated the long-term (≥ 12 months) safety of naproxen/esomeprazole magnesium in patients at risk of NSAID-associated UGI ulcers (Citation37).The overall incidence of any AEs in the naproxen/esomeprazole magnesium arms of these 3-month studies was lower (52%–54% versus > 70% in the 12-month study), as was the incidence of discontinuations due to any AE (6%–8% in the 3-month studies versus > 18.8% in the 12-month study). However, a higher incidence of AEs and discontinuations due to AEs may be expected in the 12-month study due to its open-label design, lack of comparator, and the higher GI risk patient population included. Interpretation of any comparisons made between tolerability findings from these studies is therefore limited.
Improved UGI tolerability with esomeprazole treatment in continuous NSAID users has previously been demonstrated in other studies, where reduced levels of heartburn, acid regurgitation, and upper abdominal pain, discomfort, or burning have been observed (Citation18,Citation19). The findings from the present studies may suggest that the efficacy of esomeprazole to improve NSAID-associated UGI tolerability is not adversely affected by its combination with EC naproxen. In conjunction with the tolerability findings from earlier studies evaluating naproxen/esomeprazole magnesium, the analyses reported here provide substantial evidence to support the UGI tolerability of this fixed-dose combination. However, the mild symptoms observed at baseline in the studies reported here could be considered a limitation that may have contributed to the modest changes in mSODA scores that were achieved. Further limitations may also result from the somewhat homogeneous patient population enrolled, which could limit extrapolation of the findings for a more diverse population, and the use of patient-reported outcome assessments in these studies, which may not always differentiate between pain of dyspepsia and heartburn, depending on patient understanding of these sometimes interchangeably described symptom complexes. In addition, as all patients included in these studies were informed of possible AEs associated with the use of NSAIDs, including COX-2-selective inhibitors, prior to receiving study treatment, this may have contributed towards an increase in the number of certain AEs reported. However, given the randomized, double-blind nature of these studies, this is unlikely to have led to any differences in the number of AEs reported between treatment groups.
Despite these potential limitations, treatment with naproxen/esomeprazole magnesium over 12 weeks was associated with improvements in upper abdominal pain from baseline that were comparable with celecoxib, and with more heartburn-free days than were observed in patients treated with celecoxib or placebo.
Dyspeptic symptoms are a prevalent and important side-effect of NSAID use for both patients and physicians in terms of negative effect on quality of life, resource utilization required for diagnosis and management, discontinuation of NSAID therapy resulting from poor tolerability, and, consequently, their substantial impact on the clinical and economic burden of care (Citation10,Citation11). A meta-analysis of studies comparing COX-2-selective inhibitor and non-selective NSAID therapy has provided indirect evidence that the use of NSAIDs plus PPIs offers greater risk reduction for dyspepsia than COX-2-selective inhibitors (Citation11); however, when co-prescribed, patient adherence to gastroprotective therapy is often limited (Citation22–26). Combining a PPI with an NSAID in a single tablet may offer one potential strategy to improve gastroprotective adherence rates among patients at risk of NSAID-associated complications, or who are experiencing UGI symptoms, and, consequently, improve clinical outcomes, tolerability, and patient persistence with NSAID therapy.
In conclusion, naproxen/esomeprazole magnesium has comparable UGI tolerability and a similar safety profile to celecoxib, as demonstrated consistently in these two 12-week studies in patients with OA of the knee, and may offer an alternative treatment option for patients with OA.
Declaration of interest: Dr Byron L. Cryer is a consultant to AstraZeneca, Horizon Therapeutics, NiCox Inc., PLx Pharma, Pfizer Inc., and POZEN Inc.
Dr Marc C. Hochberg is a consultant to and/or receives speaker fees from AstraZeneca, Bayer Health Care LLC, Bioiberica SA, CombinatoRx, Eli Lilly, Endo Pharmaceuticals, GlaxoSmithKline, Merck, Merck Serono International, NicOx SA, Novartis, Pfizer, POZEN Inc., and sanofi-aventis, and research grants from the National Institutes of Health.
Dr John G. Fort is an employee of POZEN Inc. with stock ownership. Dr Mark B. Sostek, Dr Ola Svensson, and Ms Clara Hwang are employees of AstraZeneca.
Acknowledgment
These studies were sponsored and conducted by POZEN Inc. Statistical analysis was conducted under the direction of Cemal Unal, formerly of POZEN Inc., and additional statistical support was provided by Ying Zhang (POZEN Inc.). We thank Lynsey Stevenson, of Complete Medical Communications, who provided medical writing support funded by AstraZeneca.
References
- Altman R, Hochberg MC, Moskowitz RW, Schnitzer TJ. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum. 2000;43:1905–15.
- Desai SP, Solomon DH, Abramson SP, Buckley L, Crofford L, Cush JJ, . Recommendations for use of selective and nonselective nonsteroidal antiinflammatory drugs: an American College of Rheumatology white paper. Arthritis Rheum. 2008;59:1058–73.
- Lanza FL, Chan FK, Quigley EMM. Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol. 2009;104:728–38.
- National Institute for Health and Clinical Excellence. The care and management of osteoarthritis in adults. NICE clinical guideline 59. Available at: http://www.nice.org.uk/nicemedia/pdf/CG59NICEguideline.pdf (accessed 17 December 2010).
- Zhang W, Doherty M, Arden N, Bannwarth B, Bijlsma J, Gunther K-P, . EULAR evidence based recommendations for the management of hip osteoarthritis: report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2005;64:669–81.
- Zhang W, Moskowitz RW, Nuki G, Abramson S, Altman RD, Arden N, . OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16:137–62.
- Straus WL, Ofman JJ, MacLean C, Morton S, Berger ML, Roth EA, . Do NSAIDs cause dyspepsia? A meta-analysis evaluating alternative dyspepsia definitions. Am J Gastroenterol. 2002;97:1951–8.
- Silverstein FE, Graham DY, Senior JR, Davies HW, Struthers BJ, Bittman RM, . Misoprostol reduces serious gastrointestinal complications in patients with rheumatoid arthritis receiving nonsteroidal anti-inflammatory drugs. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1995;123:241–9.
- Talley NJ; American Gastroenterological Association. American Gastroenterological Association medical position statement: evaluation of dyspepsia. Gastroenterology. 2005;129: 1753–5.
- Scheiman JM. The impact of nonsteroidal anti-inflammatory drug-induced gastropathy. Am J Manag Care. 2001;7: S10–14.
- Spiegel BMR, Farid M, Dulai GS, Gralnek IM, Kanwal F. Comparing rates of dyspepsia with coxibs vs NSAID + PPI: a meta-analysis. Am J Med. 2006;119:448.e27–36.
- Laine L, Curtis SP, Cryer B, Kaur A, Cannon CP. Risk factors for NSAID-associated upper GI clinical events in a long-term prospective study of 34 701 arthritis patients. Aliment Pharmacol Ther. 2010;32:1240–8.
- Antman EM, Bennett JS, Daugherty A, Furberg C, Roberts H, Taubert KA. Use of nonsteroidal antiinflammatory drugs: an update for clinicians: a scientific statement from the American Heart Association. Circulation. 2007;115:1634–42.
- Bhatt DL, Scheiman J, Abraham NS, Antman EM, Chan FK, Furberg CD, . ACCF/ACG/AHA 2008 Expert Consensus Document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use. A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation. 2008;118: 1894–909.
- Chan FKL, Abraham NS, Scheiman JM, Laine L. Management of patients on nonsteroidal anti-inflammatory drugs: a clinical practice recommendation from the first international working party on gastrointestinal and cardiovascular effects of nonsteroidal anti-inflammatory drugs and anti-platelet agents. Am J Gastroenterol. 2008;103:2908–18.
- Scheiman JM, Fendrick AM. Summing the risk of NSAID therapy. Lancet. 2007;369:1580–1.
- Lai K-C, Chu K-M, Hui W-M, Wong BCY, Hu WHC, Wong W-M, . Celecoxib compared with lansoprazole and naproxen to prevent gastrointestinal ulcer complications. Am J Med. 2005;118:1271–8.
- Hawkey CJ, Jones RH, Yeomans ND, Scheiman JM, Talley NJ, Goldstein JL, . Efficacy of esomeprazole for resolution of symptoms of heartburn and acid regurgitation in continuous users of non-steroidal anti-inflammatory drugs. Aliment Pharmacol Ther. 2007;25:813–21.
- Hawkey C, Talley NJ, Yeomans ND, Jones R, Sung JJ, Langstrom G, . Improvements with esomeprazole in patients with upper gastrointestinal symptoms taking non-steroidal antiinflammatory drugs, including selective COX-2 inhibitors. Am J Gastroenterol. 2005;100:1028–36.
- Latimer N, Lord J, Grant RL, O'Mahony R, Dickson J, Conaghan PG, . Cost effectiveness of COX 2 selective inhibitors and traditional NSAIDs alone or in combination with a proton pump inhibitor for people with osteoarthritis. BMJ. 2009;339:b2538.
- Sturkenboom MCJM, Burke TA, Dieleman JP, Tangelder MJD, Lee F, Goldstein JL. Underutilization of preventive strategies in patients receiving NSAIDs. Rheumatology (Oxford). 2003;42 (Suppl 3):iii23–31.
- Goldstein JL, Howard KB, Walton SM, McLaughlin TP, Kruzikas DT. Impact of adherence to concomitant gastroprotective therapy on nonsteroidal-related gastroduodenal ulcer complications. Clin Gastroenterol Hepatol. 2006;4:1337–45.
- Sturkenboom MCJM, Burke TA, Tangelder MJD, Dieleman JP, Walton S, Goldstein JL. Adherence to proton pump inhibitors or H2-receptor antagonists during the use of non-steroidal anti-inflammatory drugs. Aliment Pharmacol Ther. 2003;18:1137–47.
- Abraham NS, El-Serag HB, Johnson ML, Hartman C, Richardson P, Ray WA, . National adherence to evidence-based guidelines for the prescription of nonsteroidal anti-inflammatory drugs. Gastroenterology. 2005;129:1171–8.
- Abraham NS, Hartman C, Castillo D, Richardson P, Smalley W. Effectiveness of national provider prescription of PPI gastroprotection among elderly NSAID users. Am J Gastroenterol. 2008;103:323–32.
- van Soest EM, Sturkenboom MCJM, Dieleman JP, Verhamme KMC, Siersema PD, Kuipers EJ. Adherence to gastroprotection and the risk of NSAID-related upper gastrointestinal ulcers and haemorrhage. Aliment Pharmacol Ther. 2007;26:265–75.
- Food and Drug Administration (FDA). VIMOVO™ prescribing information. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022511lbl.pdf (accessed 17 December 2010).
- Electronic Medicines Compendium (eMC). Summary of Product Characteristics. VIMOVO 500 mg/20 mg modified-release tablets. Available at: http://www.medicines.org.uk/EMC/medicine/23883/SPC/VIMOVO+500+mg+20+mg+modified-release+tablets (accessed 17 December 2010).
- Hochberg MC, Fort JG, Svensson O, Hwang C, Sostek M. Fixed-dose combination of enteric-coated naproxen and immediate-release esomeprazole has comparable efficacy to celecoxib for knee osteoarthritis: two randomized trials. Curr Med Res Opin. 2011;27:1243–53.
- Welle J, Fort J, Crawley J, Cryer B, Dickerhoof R, Turner MP, . Modified Severity of Dyspepsia Assessment pain scale: a new tool for measuring upper abdominal pain in osteoarthritis patients taking NSAIDs. Patient Relat Outcome Meas. 2011;2:135–43.
- Rabeneck L, Cook KF, Wristers K, Souchek J, Menke T, Wray NP. SODA (severity of dyspepsia assessment): a new effective outcome measure for dyspepsia-related health. J Clin Epidemiol. 2001;54:755–65.
- Rabeneck L. Measuring dyspepsia-related health in randomized trials: the Severity of Dyspepsia Assessment (SODA) and its use in treatment with NSAIDs and COX-2-specific inhibitors. Rheumatology (Oxford). 2003;42 (Suppl 3):iii32–9.
- Rabeneck L, Goldstein JL, Vu A, Mayne TJ, Rublee DA. Valdecoxib is associated with improved dyspepsia-related health compared with nonspecific NSAIDs in patients with osteoarthritis or rheumatoid arthritis. Am J Gastroenterol. 2005;100:1043–50.
- Rabeneck L, Wristers K, Goldstein JL, Eisen G, Dedhiya SD, Burke TA. Reliability, validity, and responsiveness of Severity of Dyspepsia Assessment (SODA) in a randomized clinical trial of a COX-2-specific inhibitor and traditional NSAID therapy. Am J Gastroenterol. 2002;97:32–9.
- Goldstein JL, Hochberg MC, Fort JG, Zhang Y, Hwang C, Sostek M. Clinical trial: incidence of NSAID-associated endoscopic gastric ulcers in patients treated with PN 400 (naproxen plus esomeprazole magnesium) vs. enteric-coated naproxen alone. Aliment Pharmacol Ther. 2010;32: 401–13.
- Goldstein JL, Hwang C, Datto C. Correlation of two patient-reported outcomes to assess dyspepsia: patients listening to their gut feeling. Poster presented at the American College of Gastroenterology Annual Scientific Meeting, San Antonio, TX, USA, 15–20 October 2010.
- Sostek M, Fort J, Estborn L, Vikman K. Long-term safety of naproxen and esomeprazole magnesium fixed-dose combination: phase III study in patients at risk for NSAID-associated gastric ulcers. Curr Med Res Opin. 2011;27: 847–54.