Abstract
Classical risk scores may underestimate the risk of cardiovascular events in specific risk groups suitable for early prevention, such as asymptomatic hypertensive subjects. Arterial stiffness and wave reflection are now well accepted as the most important determinants of increasing systolic and pulse pressures in aging societies, thus affording a major contribution to stroke and myocardial infarction. A major reason for measuring arterial stiffness in hypertensive patients comes from the demonstration that arterial stiffness has a predictive value for cardiovascular events, beyond classical cardiovascular risk factors. Aortic stiffening also gives direct evidence of target organ damage, and improves the determination of the overall cardiovascular risk of asymptomatic hypertensive subjects. In clinical practice, the measurement of aortic stiffness may avoid patients being mistakenly classified as at low or moderate risk, when they actually have an abnormally high aortic stiffness placing them within a higher-risk group. The present mini-review successively addresses the concept of ‘tissue’ biomarker, applies it to arterial stiffness, describes the methodology of measurement, gives some pathophysiological links in order to explain the occurrence of stroke and myocardial infarction in patients with high arterial stiffness, and raises the issue of whether arterial stiffness is a surrogate marker.
Key messages
Tissue biomarkers are more predictive than circulating biomarkers in asymptomatic hypertensives.
A simple tissue marker is aortic stiffness, easily measured through carotid-femoral pulse wave velocity in clinical practice.
Aortic stiffness, which has demonstrated an added predictive value for cardiovascular events, is listed among subclinical organ damage by the 2007 ESH-ESC guidelines for the management of hypertension.
Introduction
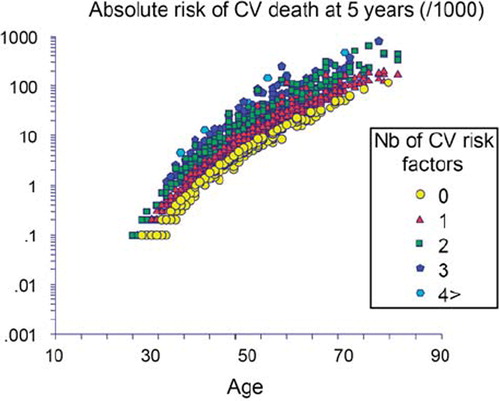
Cardiovascular (CV) disease manifestations still pose a substantial threat to public health. In asymptomatic hypertensive subjects, the European Society of Hypertension (ESH) guidelines for the management of hypertension (Citation1) recommend to screen for classical CV risk factors and to control with life-style advice or drug therapy, in order best to prevent CV disease. However, the role of aging is prominent, compared to the classical CV risk factors, as exemplified in : we calculated the CV risk according to the SCORE equation in 1980 hypertensive patients attending the hypertension clinic of Broussais Hospital (Citation2). At any given age, the increased risk due to an increment from 0 to 4 CV risk factors equals 10 years of aging only.
Figure 1. The CV risk was calculated according to the SCORE equation in 1980 hypertensive patients (Citation2). The role of aging is prominent. At any given age, the increased risk due to an increment from 0 to 4 CV risk factors equals 10 years of aging only.
As the risk of CV disease still represents a challenge in spite of prevention and all treatment efforts, there is a need for new pathophysiological models to better understand cardiovascular risk and its treatment, based on new concepts (Citation3–5). The present mini-review will successively address the concept of ‘tissue’ biomarker, apply it to arterial stiffness, describe the methodology of measurement, give some pathophysiological links in order to explain the occurrence of stroke and myocardial infarction in patients with high arterial stiffness, and raise the issue of arterial stiffness as a surrogate marker.
‘Circulating’ biomarkers versus ‘tissue’ biomarkers
Classical risk scores (i.e. Framingham risk score (Citation6), European SCORE (Citation7)) are quite effective for predicting CV events in patients with several CV risk factors. However, they may underestimate the risk of CV events in other risk groups suitable for early prevention. The utilization of sophisticated biomarkers was suggested for increasing the individual prediction of CV risk. A variety of biomarkers were proposed, the most popular being homocysteine or high-sensitivity C-reactive protein (hs-CRP). However, this approach has been generally deceiving. For instance, Wang et al. demonstrated that the added value of using multiple biomarkers was negligible since their use, either individually or in any combination, did not improve the prediction of outcome in the Framingham study (Citation8). Subsequently, popular but disputed biomarkers such as hs-CRP were withdrawn from current European guidelines for the management of hypertension (Citation1). It remains doubtful whether any other refinement of the biomarker approach will lead to a better individual prediction of cardiovascular risk, except in specific populations (Citation9).
By contrast to the ‘circulating’ biomarkers, target organ damage (Citation1) can be used as ‘tissue’ biomarker together with (or preferentially independently of) classical risk factors and may help to identify patients at high risk of developing CV disease. This strategy has a strong background since target organ damage integrates the cumulative effects of CV risk factors with aging and can be detected before clinical events occur, at a stage when intervention may reverse damage. Numerous target organ damage categories have been identified, such as the presence of left ventricular hypertrophy, microalbuminuria, reduction in glomerular filtration rate, and white matter cerebral lesions.
The damage of the arterial tree—increased arterial stiffness, central pulse pressure, carotid intima-media thickness, and endothelial dysfunction—raises increasing interest (Citation10). Recent studies showed a close relationship between microvascular damage in the heart, brain, retina, and kidney and arterial stiffness. Particularly, in cross-sectional studies and after adjustment for classical CV risk factors, aortic stiffness remained associated with either silent cerebral small-vessel disease (every standard deviation (SD) of pulse wave velocity (PWV) was associated with a 1.78-fold higher likelihood of silent lacunar infarcts in middle-aged hypertensive patients) (Citation11) or decline in cognitive function (every SD of PWV was associated with a 1.73-fold higher likelihood of Alzheimer's disease and 3.52-fold higher likelihood of vascular dementia) (Citation12). In a longitudinal study in older individuals (79 ± 6years), aortic stiffness was a significant predictor of loss in cognitive function independently of age, sex, education, and traditional CV risk factors, each 1 m/s increase in PWV being associated with a 0.74 per-year decrease in MMSE (Mini Mental State Examination) score (Citation13).
Arterial stiffness in general and aortic stiffness in particular can be considered as a measure of the cumulative influence of CV risk factors with aging on the arterial tree. Indeed, arterial stiffness reflects the true arterial wall damage, whereas blood pressure, glycemia, and lipids, which fluctuate during follow-up, may not. A temporal dissociation exists between the observed values of classical and sophisticated CV risk factors (i.e. ‘circulating’ biomarkers), which can be considered as ‘snapshots’, and arterial stiffness, which integrates the long-lasting effects of all identified and non-identified CV risk factors and thus may be considered as a ‘tissue’ biomarker. Measurement of arterial stiffness may avoid patients being mistakenly classified as at low or moderate risk, when they actually have an abnormally high arterial stiffness, placing them within a higher-risk group.
Methods of measurement
An expert consensus document has reviewed the methodological agreements for measuring arterial stiffness (Citation14). In contrast to systemic arterial stiffness, which can only be estimated from models of the circulation, regional and local arterial stiffness can be measured directly, and non-invasively, at various sites along the arterial tree. The measurement of PWV is generally accepted as the most simple, non-invasive, robust, and reproducible method with which to determine arterial stiffness (Citation14).
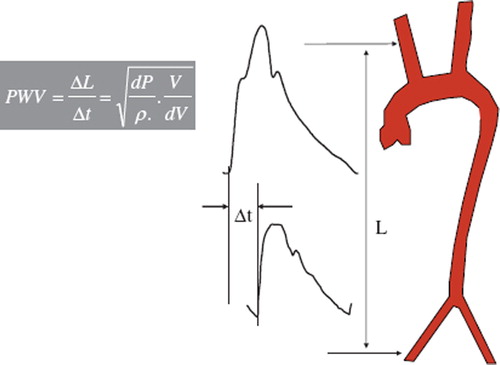
Carotid-femoral PWV is a direct measurement of aortic stiffness, and it corresponds to the widely accepted propagative model of the arterial system. Measured along the aortic and aorto-iliac pathway, it is the most clinically relevant, since the aorta and its first branches are what the left ventricle ‘sees’ and are thus responsible for most of the pathophysiological effects of arterial stiffness. Carotid-femoral PWV is usually measured using the foot-to-foot velocity method, as the ratio of the distance between the measurements sites (common carotid and common femoral arteries) and the transit time between the feet of the carotid and femoral pressure waveforms () (Citation14).
Figure 2. Carotid-femoral PWV is usually measured using the foot-to-foot velocity method, as the ratio of the distance (L) between the measurements sites (common carotid and common femoral arteries) and the transit time (Δt) between the feet of the carotid and femoral pressure waveforms, according to the Bramwell–Hill equation.
Pathophysiology of CV events
A generally accepted mechanistic view is that an increase in arterial stiffness causes a premature return of reflected waves in late systole, increasing central pulse pressure (PP), thus systolic blood pressure (SBP). SBP increases the load on the left ventricle, increasing myocardial oxygen demand (Citation10). In addition, arterial stiffness is associated with increased sympathetic nerve activity (Citation15) and left ventricular hypertrophy. The increase in central PP and the decrease in diastolic blood pressure may directly cause subendocardial ischemia (Citation10).
An increased arterial stiffness can increase the risk of stroke through several mechanisms, including an increase in central PP. Indeed, increased central PP influences arterial remodeling both at the site of the extracranial and intracranial arteries. A higher central PP is associated with an increase in carotid wall thickness, and a higher prevalence of carotid stenosis, carotid plaques, and cerebral white matter lesions (Citation16). Finally, coronary heart disease and heart failure, which are favored by high PP and arterial stiffness, are also risk factors for stroke.
Predictive value of arterial stiffness and central BP
In the late 1990s and early 2000s, some epidemiological studies (Citation2,Citation17,Citation18) showed that aortic stiffness had independent predictive value for all-cause and CV mortality. Currently, as many as 19 studies—some of them were included in a recent meta-analysis (Citation19)—consistently showed the independent predictive value of aortic stiffness for fatal and non-fatal CV events in various populations. Aortic stiffness can thus be considered as an intermediate end-point for CV events. Although the relationship between aortic stiffness and events is continuous, a threshold > 12 m/s has been suggested as a conservative estimate of significant alterations of aortic function in middle-aged hypertensives and was included in the 2007 ESH guidelines for the management of hypertension (Citation1). High aortic PWV may thus represent target organ damage, which needs to be detected during estimation of CV risk in hypertensives.
Clinical application: arterial stiffness as a surrogate end-point
In order for a biomarker to be considered as a ‘surrogate’ marker of CV events, several steps should be completed, according to international guidelines (Citation20). The first four steps have already been completed, in the case of arterial stiffness.
Step 1. Proof of concept: Do novel marker levels differ between subjects with and without outcome? Yes, since several diseases have been associated with an increase in arterial stiffness.
Step 2. Prospective validation: Does the novel marker predict development of future outcomes in a prospective cohort or nested case-cohort study? Yes, since the predictive value of arterial stiffness has been largely demonstrated, as detailed above.
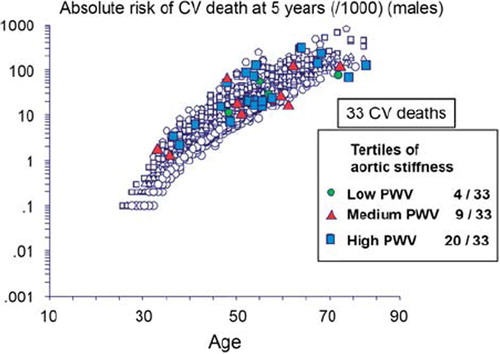
Step 3. Incremental value: Does the novel marker add predictive information to established, standard risk markers? Yes, since the independent predictive value of aortic stiffness has been demonstrated after adjustment to classical cardiovascular risk factors, including brachial PP. This indicates that aortic stiffness has a better predictive value than each of classical risk factors. An illustration is given by which individualizes patients who died during a 9.3-year follow-up in a cohort of 1980 hypertensive patients (Citation2), according to their value of aortic stiffness at baseline. Among the 33 cardiovascular deaths, 20 occurred in patients with aortic stiffness in the highest tertile, and 9 deaths occurred in patients with aortic stiffness in the medium tertile. The predictive value of aortic stiffness contrasts with the random distribution of deaths among SCORE values. More quantitatively than the previous illustration, the additive value of PWV above and beyond traditional risk factors has been demonstrated by two separate studies (Citation21,Citation22). The first was performed in 1045 hypertensive patients, with a longitudinal follow-up of 5.9 years for coronary heart disease (CHD) events (Citation21). The increase in CHD with tertiles of PWV was steeper for patients belonging to the first and second tertiles of the Framingham risk score (FRS). In the group of low-to-medium-risk patients, FRS and PWV had similar predictive value (area under the receiver-operating characteristic (ROC) curve: AUC = 0.65 ± 0.07and0.63 ± 0.08, respectively), and, when combined, the predictive value increased since the AUC rose to0.76 ± 0.09, indicating that PWV improved the prediction of CV events beyond FRS. This improved ability of aortic stiffness to predict CV mortality was more recently confirmed by Mattace-Raso et al. in the elderly subjects from a general population (Citation22).
Figure 3. Individualization of patients who died during the 9.3-year follow-up in a cohort of 1980 hypertensive patients, according to their value of aortic stiffness at baseline (Citation2,Citation3). Among the 33 cardiovascular deaths, 20 occurred in patients with aortic stiffness in the highest tertile, and 9 deaths occurred in patients with aortic stiffness in the medium tertile. The predictive value of aortic stiffness contrasts with the random distribution of deaths among SCORE values.
Step 4. Clinical utility: Does the novel risk marker change predicted risk sufficiently to change recommended therapy? Yes, since three studies showed that patients at intermediate risk could be reclassified into a higher or a lower CV risk, when arterial stiffness was measured (Citation23–25). For instance, in the Framingham study, 15.7% of patients at intermediate risk could be reclassified into a higher (14.3%) or lower (1.4%) risk (Citation22). In a recent unpublished meta-analysis, 19% and 22% of intermediate-risk individuals were reclassified into higher or lower quartiles of risk for coronary heart disease and stroke outcomes, respectively (Citation26).
Step 5. Clinical outcomes: Does use of the novel risk marker improve clinical outcomes, especially when tested in a randomized clinical trial? No study has yet been done, although it is crucial to determine whether a reduction in arterial stiffness is a desirable therapeutic goal in terms of hard clinical end-points such as morbidity and mortality. Although this has been done in patients with end-stage renal disease (Citation27), it remains to be shown in a population of hypertensive patients at lower CV risk that a therapeutic strategy aiming at normalizing arterial stiffness proves to be more effective in preventing CV events than usual care. Such a study requires a large number of patients, benefiting from a long-term follow-up.
Step 6. Cost-effectiveness: Does use of the novel risk marker improve clinical outcomes sufficiently to justify the additional costs? Indeed, the ‘aortic stiffness’-based approach should be compared to other approaches currently used to stratify and reduce CV risk, including risk charts, guidelines, and other biomarkers. Here, the cost-effectiveness ratio describes the ratio between the additional cost associated with the measurement of aortic stiffness, and the higher ability of aortic stiffness to predict clinical outcomes, compared with risk charts, guidelines, and other biomarkers. There is thus a need for several multicenter end-point-driven studies using aortic stiffness as the therapeutic target and comparison of this approach with more classical ones.
Conclusion
These data highlight the importance of arterial stiffness for providing direct evidence of target organ damage and determining the overall CV risk of asymptomatic hypertensive subjects. Arterial stiffening is also able to predict CV outcomes, beyond classical CV risk factors. Thus, measurement of aortic stiffness may avoid patients being mistakenly classified as at low or moderate risk, when they actually have an abnormally high aortic stiffness placing them within a higher risk group.
Declaration of interest: Prs Laurent and Boutouyrie received research grants from manufacturers marketing apparatus measuring arterial stiffness, such as Esaote Pie Medical, AtCor and Omron.
References
- Mancia G, de Backer G, Cifkova R, Dominiczak A, Fagard R, Germano G, . Guidelines for the management of arterial hypertension. The Task Force for the Management of Arterial Hypertension of the European Society of Cardiology (ESC) and of the European Society of Hypertension (ESH). J Hypertens. 2007;25:1105–87.
- Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, . Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–41.
- Boutouyrie P, Vermersch S, Laurent S, Briet M. Cardiovascular risk assessment through target organ damage: role of carotid to femoral pulse wave velocity. Clin Exp Pharmacol Physiol. 2008;35:530–3.
- Cavalcante JL, Lima JA, Redheuil A, Al-Mallah MH. Aortic stiffness: current understanding and future directions. J Am Coll Cardiol. 2011;57:1511–22.
- Adji A, O'Rourke MF, Namasivayam M. Arterial stiffness, its assessment, prognostic value, and implications for treatment. Am J Hypertens. 2011;24:5–17.
- Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47.
- Conroy RM, Pyörälä K, Fitzgerald AP, Sans S, Menotti A, De Backer G, .; SCORE project group. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987–1003.
- Wang TJ, Gona P, Larson MG, Tofler GH, Levy D, Newton-Cheh C, . Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006;355:2631–9.
- Zethelius B, Berglund L, Sundström J, Ingelsson E, Basu S, Larsson A, . Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med. 2008;358:2107–16.
- Laurent S, Boutouyrie P. Recent advances in arterial stiffness and wave reflection in human hypertension. Hypertension highlights. Hypertension. 2007;49:1202–6.
- Henskens LH, Kroon AA, van Oostenbrugge RJ, Gronenschild EH, Fuss-Lejeune MM, Hofman PA, . Increased aortic pulse wave velocity is associated with silent cerebral small-vessel disease in hypertensive patients. Hypertension. 2008;52:1120–6.
- Hanon O, Haulon S, Lenoir H, Seux ML, Rigaud AS, Safar M, . Relationship between arterial stiffness and cognitive function in elderly subjects with complaints of memory loss. Stroke. 2005;36:2193–7.
- Scuteri A, Tesauro M, Appolloni S, Preziosi F, Brancati AM, Volpe M. Arterial stiffness as an independent predictor of longitudinal changes in cognitive function in the older individual. J Hypertens. 2007;25:1035–40.
- Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, . Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006. 27:2588–605.
- Świerblewska E, Hering D, Kara T, Kunicka K, Krusze-wski P, Bieniaszewski L, . An independent relationship between muscle sympathetic nerve activity and pulse wave velocity in normal humans. J Hypertens. 2010;28: 979–84.
- Laurent S, Briet M, Boutouyrie P. Large/small artery cross-talk and recent morbidity-mortality trials in hypertension. Hypertension. 2009;54:388–92.
- Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness on survival in end-stage renal disease. Circulation. 1999;99:2434–9.
- Meaume S, Benetos A, Henry OF, Rudnichi A, Safar ME. Aortic pulse wave velocity predicts cardiovascular mortality in subjects > 70 years of age. Arterioscler Thromb Vasc Biol. 2001;21:2046–50.
- Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–27.
- Hlatky MA, Greenland P, Arnett DK, Ballantyne CM, Criqui MH, Elkind MS, .; American Heart Association Expert Panel on Subclinical Atherosclerotic Diseases and Emerging Risk Factors and the Stroke Council. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009;119:2408–16.
- Boutouyrie P, Tropeano AI, Asmar R, Gautier I, Benetos A, Lacolley P, . Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension. 2002;39:10–15.
- Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, . Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–63.
- Sehestedt T, Jeppesen J, Hansen TW, Rasmussen S, Wachtell K, Ibsen H, . Risk stratification with the risk chart from the European Society of Hypertension compared with SCORE in the general population. J Hypertens. 2009;27: 2351–7.
- Mitchell GF, Hwang SJ, Vasan RD, Larson MG, Pencina MJ, Hamburg NM, . Arterial stiffness and cardiovascular events. The Framingham Heart Study. Circulation. 2010;121: 505–11.
- Muiesan ML, Salvetti M, Paini A, Monteduro C, Rosei CA, Aggiusti C, . Pulse wave velocity and cardiovascular risk stratification in a general population: the Vobarno study. J Hypertens. 2010;28:1935–43.
- Ben-Shlomo Y, McEniery C, Boutouyrie P, Cameron, J, Chen CH, Cruickshank, K, Predictive value of pulse wave velocity for cardiovascular events in 15220 subjects: an individual participant meta-analysis on behalf of the PWV collaborative group. J Hypertens. 2010;28 (suppl. A):E446.
- Guerin AP, Blacher J, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness attenuation on survival of patients in end-stage renal failure. Circulation. 2001;20:987–92.