Abstract
Telomeres are located at the end of chromosomes. They are composed of repetitive TTAGGG tandem repeats and associated proteins of crucial importance for telomere function. Telomeric DNA is shortened by each cell division until a critical length is achieved and the cell enters senescence and eventually apoptosis. Telomeres are therefore considered a ‘biological clock’ of the cell. Telomerase adds nucleotides to telomeric DNA thereby contributing to telomere maintenance, genomic stability, functions, and proliferative capacity of the cell. In certain rare forms of progeria, point mutations within the telomere lead to accelerated telomere attrition and premature aging.
Endogenous factors causing telomere shortening are aging, inflammation, and oxidative stress. Leukocyte telomere length (LTL) shortening is inhibited by estrogen and endogenous antioxidants. Accelerated telomere attrition is associated with cardiovascular risk factors such as age, gender, obesity, smoking, sedentary life-style, excess alcohol intake, and even mental stress. Cardiovascular (CV) diseases and CV aging are usually but not invariably associated with shorter telomeres than in healthy subjects. LTL appears to be a biomarker of CV aging, reflecting the cumulative burden of endogenous and exogenous factors negatively affecting LTL. Whether accelerated telomere shortening is cause or consequence of CV aging and disease is not clear.
Key words::
Key messages
Telomere attrition is associated with aging, cardiovascular risk, and disease.
Leukocyte telomere length may be considered a biomarker of cardiovascular aging.
Introduction
Telomeres are located at the ends of chromosomal DNA (). They are made up of tandem repeats of the TTAGGG sequence and associated proteins. Telomeres participate in the maintenance of genomic and cellular stability and replication (Citation1,Citation2). Telomeres shorten with repeated cell division, and when a critical telomere length is reached the cell enters senescence followed by apoptosis. Therefore, telomeres are considered a potential biological clock (Citation1–3). Telomerase is a reverse transcriptase adding nucleotides to telomeric DNA (), thereby maintaining telomere length and functions (Citation1,Citation2). Elisabeth Blackburn, Carol Greider, and Jack Szostak, who all received the Nobel prize for telomere research in 2009, have published exciting comments on major steps on ‘the path from maize, Tetrahymena and yeast to human cancer and aging’ (Citation3) which offers interesting historical and personal perspectives on milestones in telomere research. An excellent review by Abraham Aviv (Citation4) deals with dynamics of telomere length from birth to old age, the heritability, and association of telomere length with cardiovascular disease and longevity. Calado and Young (Citation5), in another excellent review, focus on telomere diseases such as dyskeratosis congenita, aplastic anemia, pulmonary fibrosis, and telomeres in cancer, with critical comments on the role of telomeres in aging and associated cardiovascular diseases.
Figure 1. A: Left, telomeres (yellow) at the ends of chromosomes (blue). Right, tandem repeat of human telomeric DNA. B: Left, telomerase ribonucleoprotein complex, the telomerase reverse transcriptase (TERT), and, (right) telomerase-associated proteins. C: High telomerase activity of germ cells and tumors ensures the maintenance of telomere length and extended proliferation. Somatic cells have low or absent telomerase activity, and telomere shortening occurs in these cells with each cell division during normal aging. Low telomerase activity leads to telomere dysfunction and proliferative arrest. Accelerated telomere attrition is observed in premature aging. With permission from (Citation41).
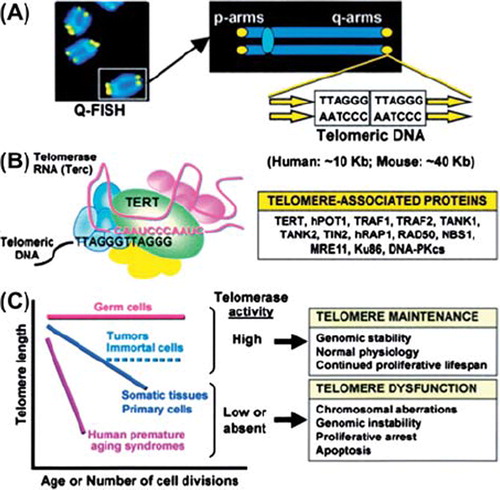
In all populations studied telomeres are shorter in age-matched males than in females, except in the very old (Citation6). In hematopoietic stem cells (HSCs) telomerase activity is high, resulting in improved maintenance of telomere length and normal cell function, including genomic stability and continued proliferative lifespan (). Human leukocyte telomere length (LTL) mirrors HSC telomere length (Citation4). In malignant tumors telomerase activity is also high, telomere length is maintained, and unwanted continued proliferation goes on (Citation2). In normal somatic tissue in which telomerase activity is low or absent, telomeres shorten by each cell division, eventually resulting in proliferative arrest and apoptosis. Some but not all mutations in the telomere complex are associated with premature aging and atherosclerosis. Mutations in the DKC1 gene which encodes a protein telomerase complex (Citation7) causes an inherited form of bone-marrow failure, dyskeratosis congenita (Citation5), which is not associated with premature aging or atherosclerosis. Symptoms of the Hutchinson–Gilford progeria syndrome (HGPS) include premature loss of hair and subcutaneous fat, early atherosclerosis, coronary artery disease, and stroke at early teenage. HGPS is caused by a mutation in the lamin A gene, resulting in a mutant lamin A protein called progerin. The average telomere length of fibroblasts is greatly reduced in HGPS (Citation8). A recent report (Citation9) suggested a synergistic relationship between telomere dysfunction and progerin during induction of cell senescence, providing a possible clue to how short telomeres might contribute to premature aging in HGPS.
Measurement of telomere length
Leukocyte telomere length (LTL) measured in DNA from blood leukocytes is by far the most commonly used measure of telomere length. It is convenient but does not reflect telomere attrition in poorly proliferating tissues such as brain, fat, or liver. For the determination of LTL in large samples Southern blot or quantitative polymerase chain reaction (qPCR) are widely used. Comparison of Southern blot with qPCR has shown Southern blot to be the more accurate method (Citation10), but it is more time-consuming and laborious than qPCR. Flow-fluorescence in situ hybridization (flow-FISH) technique is not feasible for large sample numbers and epidemiologic studies, because the FISH method requires intact nuclei and prompt processing of samples (Citation10).
Dynamics of telomere length
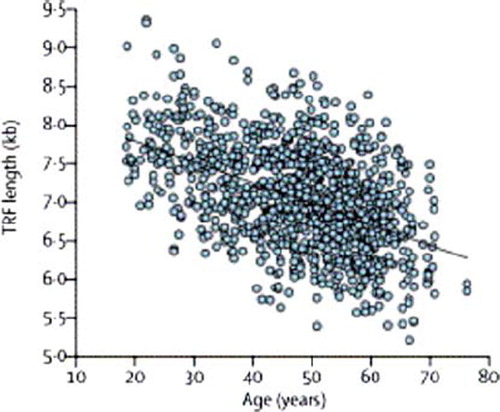
Telomere dynamics are complex and strongly influenced by genetic factors but also by a large number of environmental circumstances. LTL is highly variable between individuals both at birth and throughout life. LTL at birth and telomere attrition during aging are major determinants of LTL dynamics. LTL is at its longest at birth, shortens rapidly until adolescence, and then shortens at a slower rate until old age () (Citation11). LTL at birth is inheritable (Citation4) and inversely related to birth weight. LTL at birth is likely to reflect LTL in adult age (Citation4,Citation11) and might thus predict an association with diseases associated with aging later in life. Telomere length is equivalent in different organs and blood leukocytes from the same individual at birth and later in life (Citation12). However, in tissues with low mitotic activity (e.g. brain, fat, muscle, heart), telomeres are longer than in leukocytes (Citation13).
Figure 2. Leukocyte telomere length in 1122 women aged 18–72 years. TRF = telomere restriction fragment. With permission from (Citation17).
Physiologic (endogenous) factors affecting LTL are listed in . As human telomeres in somatic cells are shortened at each cell division (Citation1,Citation2), it follows that aging is the main factor causing telomere attrition. Aging is also a major cardiovascular (CV) risk factor. Inflammation and oxidative stress (Citation14) are important causes of LTL shortening, and they have central roles in aging processes.
Table I. Physiological (endogenous) factors affecting telomere length.
Telomerase inhibits telomere shortening in stem cells, but also in cancer cells, varying with cell type (Citation15). Estrogen inhibits telomere shortening (Citation16), possibly explaining why females have longer LTL than age-matched males. Endogenous antioxidants such as sodium oxygen dismutase are believed to inhibit aging processes and LTL shortening as well (Citation14).
Exogenous factors affecting LTL
Exogenous factors affecting LTL are mostly related to life-style and CV risk factors (). Among factors associated with accelerated LTL shortening, smoking and obesity are commonly reported (Citation17,Citation18). Accelerated LTL shortening has been linked to decreased telomerase activity in mental stress (Citation19). Association between intake of alcohol and LTL has been studied in relatively few studies. We observed a linear inverse relation between LTL at old age (mean age 78 years) and alcohol consumption during follow-up for 38 years in 622 male participants in the Helsinki Businessman study (unpublished). Self-reported years of healthy life was positively associated with LTL in a recent population-based study (Citation20). In patients with coronary artery disease, marine omega-3 fatty acid levels were positively associated with change in LTL over 5 years (Citation21). A particular strength of that study was that LTL was measured twice, both at the start and at the end of the study.
Table II. Exogenous factors associated with telomere length.
LTL in CV disease
In a recent study on 84 subjects with atherosclerotic disease, shorter telomere length was shown in the wall of saphenous vein and mammary artery than in aortic samples, and in arterial wall samples with atherosclerotic lesions compared with samples without atherosclerosis (Citation22). The results suggest that local factors associated with hemodynamics and atherosclerosis regulate telomere length in the vascular wall. Shorter LTL than in age- and sex-matched healthy control subjects has been reported in age-related CV diseases () such as atherosclerosis (Citation23–25), coronary artery disease, myocardial infarction (Citation26,Citation27), and transient ischemic attack in women (Citation28). However, a recent study failed to show an association of LTL with stroke in men (Citation29). Not all studies have reported associations of LTL with CV disease (), possibly depending on limited number, old age, and high number of risk factors of study participants. A contributing factor is probably misdiagnosing of CV diseases. In support of this assumption, the Cardiovascular Health Study (Citation30) showed that LTL was strongly associated with a physiologic index of disease burden but less strongly or not at all with disease in individual systems (CV disease, stroke, diabetes, arthritis). Adding to the complex scenario of LTL associations, a recent study reported an association between short LTL and cumulative inflammatory load as indexed by combined high levels of IL-6 and TNF-α (Citation31). Intensive lipid-lowering treatment with statins, which are known to exert anti-inflammatory effects, was reported to prevent telomere attrition in endothelial progenitor cells from 100 patients with coronary artery disease (Citation32).
Table III. Association of short LTL with cardiovascular disease.
As an example of microvascular disease, we recently observed that short LTL is predictive of progression of renal disease in patients with type 1 diabetes (Citation33). In that study we also observed that treatment with ACE inhibitors or angiotensin receptor blockers was associated with longer LTL than treatment with other antihypertensive drugs (calcium channel blockers, beta-adrenergic blockers, or diuretics).
Conclusions
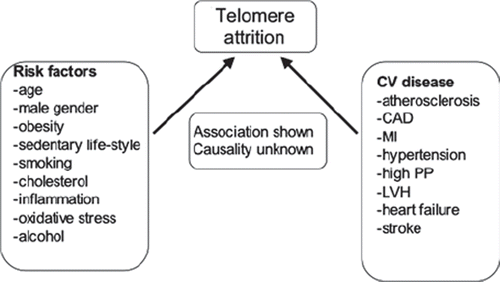
Although accelerated LTL shortening is associated with CV risk factors and disease, no cause and effect relationship between telomere length and CV disease has been established (). This may be explained by the larger number of factors affecting telomere length (–III). Rare exceptions are monogenic diseases with mutations within the telomere complex (Citation8) and premature CV aging. LTL appears to be a biomarker of CV risk and CV aging, reflecting the complex cumulative burden of telomere shortening factors and disease, modulated by factors slowing down telomere attrition. Prospective longitudinal studies are needed to shed light on the relation between telomere length and cardiovascular aging.
Figure 3. Several cardiovascular risk factors and diseases are associated with accelerated telomere attrition, but no cause–effect relation between telomere attrition and CV disease has been shown. LTL may therefore be considered a biomarker of cardiovascular aging. CAD = coronary artery disease; MI = myocardial infarction; PP = pulse pressure; LVH = left ventricular hypertrophy.
Declaration of interest: We received grants from the Else and Wilhelm Stockmann Foundation, and from the Liv och Hälsa Foundation, Helsinki, Finland. We declare no relevant conflict of interest.
References
- Blackburn EH. Switching and signaling at the telomere. Cell. 2001;106:661–7.
- Blasco MA. Telomeres and human disease: ageing, cancer, and beyond. Nat Rev Genet. 2005;6:611–22.
- Blackburn EH, Greider CW, Szostak JW. Telomeres and telomerase: the path from maize, Tetrahymena and yeast to human cancer and aging. Nature Med. 2006;12:1133–8.
- Aviv A. Genetics of leukocyte telomere length and its role in atherosclerosis. Mutat Res. 2012;730:68–74.
- Calado RT, Young NS. Telomere diseases. N Engl J Med. 2009;361:2353–65.
- Martin-Ruiz CM, Gussekloo J, van Heemst D, von Zglinicki T, Westendorp RGJ. Telomere length in white blood cells is not associated with morbidity or mortality in the oldest old: a population-based study. Aging Cell. 2005;4:287–90.
- Mitchell JR,Wood E, Collins K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature. 1999;402:551–5.
- Decker ML, Chavez E, Vulto I, Lansdorp PM. Telomere length in Hutchinson-Gilford progeria syndrome. Mech Ageing Dev. 2009;130:377–83.
- Cao K, Blair CD, Faddah DA, Kiekhaefer J, Olive M, Erdos MR, . Progerin and telomere dysfunction collaborate to trigger cellular senescence in normal human fibroblasts. J Clin Invest. 2011;121:2833–44.
- Aviv A, Hunt SC, Lin J, Cao X, Kimura M, Blackburn E. Impartial comparative analysis of measurement of leukocyte telomere length/DNA content by Southern blots and qPCR. Nucleic Acids Res. 2011;39:e134.
- Sidorov I, Kimura M, Yashin A, Aviv A. Leukocyte telomere dynamics and human hematopoietic stem cell kinetics during somatic growth. Exp Hematol. 2009;37:514–24.
- Kimura M, Gazitt Y, Cao X, Shao X, Lansdorp A, Synchrony of telomere length among hematopoietic stem cells. Exp Hematol. 2010;38:854–59.
- Gardner JP, Kimura M, Chai W, Durrani JF, Tchakmakjian L, Cao X, . Telomere dynamics in macaques and humans. J Gerontol A Biol Sci Med Sci. 2007;62:367–74.
- von Zglinicki T. Oxidative stress shortens telomeres. Trends in Biochem Sci. 2002;27:339–44.
- Kuhn E, Meeker AK, Visvanathan K, Gross AL, Wang T-L, Kurman RJ, . Telomere length in different histologic types of ovarian carcinoma with emphasis on clear cell carcinoma. Mod Pathol. 2011;24:1139–45.
- Lin J, Kroenke CA, Epel E, Kenna HA, Wolkowitz OM, Blackburn EH, . Greater endogenous estrogen exposure is associated with longer telomeres in postmenopausal women at risk for cognitive decline. Brain Res. 2011;1379:224–31.
- Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, . Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366:662–4.
- Strandberg TE, Saijonmaa O, Tilvis RS, Pitkälä KH, Strandberg AY, Miettinen TA, . Association of telomere length in older men with mortality and midlife body mass index and smoking. J Gerontol A Biol Sci Med Sci. 2011;66:815–20.
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, . Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci USA. 2004;101:17323–4.
- Njajou O, Hsueh W-C, Blackburn EH, Newman AB, Wu S-H, Li R, . Association between telomere length, specific causes of death, and years of healthy life in health, aging, and body composition, a population-based cohort study. J Gerontol A Biol Sci Med Sci. 2009;64A;860–64.
- Farzaneh-Far R, Lin J, Epel ES, Harris WS, Blackburn EH, Whooley MA. Association of marine omega-3 fatty acid levels with telomeric aging in patients with coronary heart disease. JAMA. 2010;303:250–7.
- Nzietchueng R, Elfarra M, Nloga J, Labat C, Carteaux JP, Maureira P, . Telomere length in vascular tissues from patients with atherosclerotic disease. J Nutr Health Aging. 2011;15:153–6.
- Samani NJ, Boultby R, Butler R, Thompson JR, Goodall AH. Telomere shortening in atherosclerosis. Lancet. 2001; 358:472–3.
- Benetos A, Gardner JP, Zureik M, Labat C, Xiaobin L Adamopoulus C. Short telomeres are associated with increased carotid atherosclerosis in hypertensive subjects. Hypertension. 2004;43:182–5.
- Panayiotou AG, Nicolaides AN, Griffin M, Tyllis T, Georgiou N, Bond D, . Leukocyte telomere length is associated with measures of subclinical atherosclerosis. Atherosclerosis. 2010;211:176–81.
- Brouilette S, Singh RK, Thompson JR Goodall AH, Samani NJ. White cell telomere length and risk of premature myocardial infarction. Arterioscler Thromb Vasc Biol. 2003;23:842–6.
- Fitzpatrick AL, Kronmal RA, Gardner JP, Psaty BM, Jenny NS, Tracy RP, . Leukocyte telomere length and cardiovascular disease in the Cardiovascular Health Study. Am J Epidemiol. 2007;165:14–21.
- Fyhrquist F, Silventoinen K, Saijonmaa O, Kontula K, de Faire U, Os I, . Telomere length and cardiovascular risk in hypertensive patients with left ventricular hypertrophy, the LIFE study. J Hum Hypertens. 2011;25:711–8.
- Zee RYL, Castonguay AJ, Barton NS, Ridker PM. Relative leukocyte telomere length and risk of incident ischemic stroke in men: a prospective nested case-control approach. Rejuven Res. 2010;13:411–4.
- Sanders J, Fitzpatrick AL, Boudreau RM, Arnold AM, Aviv A, Kimura M, . Leukocyte telomere length is associated with noninvasively measured age-related disease; the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2011 Sep 20. [Epub ahead of print].
- O'Donovan A, Pantell MS, Puterman E, Dhabhar FS, Blackburn EH, Yaffe K, . Cumulative inflammatory load is associated with short leukocyte telomere length in the Health, Aging and Body Composition study. PLoS One. 2011;6:e19687.
- Satoh M, Minami Y, Takahashi Y, Tabuchi T, Itoh T, Nakamura M. Effect of intensive lipid-lowering therapy on telomere erosion in endothelial progenitor cells obtained from patients with coronary artery disease. Clin Sci. 2009;116:827–35.
- Fyhrquist F, Tiitu A, Saijonmaa O, Forsblom C, Groop PH; FinnDiane Study Group. Telomere length and progression of diabetic nephropathy in patients with type 1 diabetes. J Intern Med. 2010;267:278–6.
- Chen W, Kimura M, Kim S, Cao X, Srinivasan SR, Berenson GS, . Longitudinal versus cross-sectional evaluations of leukocyte telomere length dynamics: age-dependent telomere shortening is the rule. J Gerontol A Biol Sci Med Sci. 2011;66A:312–9.
- Serra V, von Zglinicki T, Lorenz M, Saretzki G. Extracellular superoxide dismutase is a major antioxidant in human fibroblasts and slow down telomere shortening. J Biol Chem. 2003;278:6824–30.
- Pavanello S, Hoxha M, Dioni L, Bertazzi PA, Snenghi R, Nalesso A, . Shortened telomeres in individuals with abuse in alcohol consumption. Int J Cancer. 2011;129: 983–92.
- Lee D-C, Im J-A, Kim J-H, Lee H-R, Shim J-Y. Effect of long-term hormone therapy on telomere length in postmenopausal women. Yonsei Med J. 2005;46:471–9.
- Benetos A, Okuda K, Lajemi M, Kimura M, Thomas F, Skurnick J, . Telomere length as an indicator of biological aging: the gender effect and relation with pulse pressure and pulse wave velocity. Hypertension. 2001;37:381–5.
- Nordfjäll K, Eliasson M, Stegmayr B, Melander O, Nilsson P, Roos G. Telomere length is associated with obesity parameters but with a gender difference. Obesity. 2008;16: 2682–9.
- Kuznetsova T, Codd V, Brouilette S, Thijs L, Gonzalez A, Jin Y, . Association between left ventricular mass and telomere length in a population study Am J Epidemiol. 2010;172:440–50.
- Serrano AL, Andres V. Telomeres and cardiovascular disease: does size matter? Circ Res. 2004;94:575–84.