Abstract
Background and objectives. Clinical trials of bone-marrow (BM)-derived cells for therapy after acute myocardial infarct (MI) have been controversial. The most commonly used cells for these trials have been mononuclear cells (MNC), obtained by fractionation of BM cells (BMCs) via different protocols. In this study, we performed a head-to-head comparison of: 1) whole BMC; 2) fractionated BM (fBM) using the commonly used Ficoll protocol; 3) the extract derived from the fBM (fBM extract) versus 4) saline (HBSS) control for treatment of acute MI. Methods. In total, 155 male C57BL/6J (10–12-week old) mice were included. Echocardiography was performed at baseline and 2 days after permanent ligation of the left anterior descending artery to induce MI. Echocardiography and histology were employed to measure outcome at 28 days post-MI. Results. Whole BMC therapy improved left ventricular ejection fraction (LVEF) post-MI, but fBM or fBM extract was not beneficial compared to control (change of LVEF of 4.9% ±4.6% (P = 0.02), –0.4% ±5.8% (P = 0.86), –2.0% ±6.2% (P = 0.97) versus −1.4% ±5.3%, respectively). The histological infarct size or numbers of arterioles or capillaries at infarct or border zone did not differ between the groups. Conclusions. Clinical studies should be performed to test whether whole BMC therapy translates into better outcome also after human MI.
Key messages
Clinical trials of bone-marrow-derived cell therapy for acute myocardial infarct have been controversial in terms of functional benefit.
Ficoll-fractionated bone-marrow cells, the most commonly used cell population in clinical trials, have not previously been compared head to head with whole bone-marrow cells and extract derived from fractionated bone-marrow cells (absence of live cells).
In this translational study, only whole bone-marrow cell-based therapy showed benefit after myocardial infarct, whereas fractionated bone-marrow cell populations were not better than saline. This underscores the importance of using different cell populations also in clinical trials.
Introduction
Coronary artery disease and its clinical outcomes such as myocardial infarction (MI), ischemic cardiomyopathy, and congestive heart failure remain prominent health challenges worldwide. Despite therapeutic advances, there are currently no approaches in clinical practice that replace infarct scar with functional myocardium (Citation1–3). Recently, cell-based treatments have raised hope for such a therapy. However, clinical trials of bone-marrow-derived cell therapy for acute MI have been controversial in terms of their cardiac functional benefit (Citation4–10). In addition, the mechanisms of any clinical benefit remain controversial, and many groups, including our own, support a paracrine theory (Citation11–13). In fact, we have recently shown that whole bone-marrow cell (BMC) extract has almost comparable beneficial effects on cardiac functional improvement in the absence of any live cells in a mouse model of acute MI (Citation13).
It is not known, however, which cell(s) obtained from the bone-marrow is/are ‘ideal’ for cardiac therapeutic purposes post-MI. Bone-marrow is an attractive source for cellular therapies as the stem and progenitor cells are easily accessible in high numbers without the need for prolonged ex-vivo manipulations. The majority of published clinical trials post-MI have reported gradient centrifugation of the bone-marrow as the main method for obtaining mononuclear cells (MNC) without red blood cells, granulocytes, and platelets. Interestingly, red blood cell contamination of the final cell product has been shown to impair its efficacy (Citation14). Unfortunately, at the same time, this method results in the loss of lobulated multinuclear cells such as granulocytes that have been shown to prevent cardiac remodeling when stimulated using granulocyte colony-stimulating factor (Citation15,Citation16). A recent animal study proposed that mononuclear BMCs survive better in an ischemic milieu compared to other cells (Citation17). Enhanced cell survival leads to more robust repopulation and also improves protective paracrine action of the cells. To our knowledge, there has been no head-to-head trial comparing the efficacy of whole BMC versus BM fraction (fBM) obtained by the commonly used Ficoll protocol versus the extract derived from fBM (in the absence of live cells) in a clinically relevant time point post-MI.
In this study, we utilized the ultrasound-guided injection technique in a clinically relevant time point post-MI to evaluate the efficacy of therapy with whole BMC versus fBM versus fBM extract versus saline control in preventing infarct expansion and post-MI cardiac remodeling.
Methods
Animals and study groups
In total, 155 male C57BL/6J (10–12 weeks old) mice were included in the study. Twenty-four mice served as donors for bone-marrow transplantation. All experimental procedures and handling of the animals were performed in accordance with Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health, and UCSF's Institutional Animal Care and Use Committee approved the protocol. A study flow chart is presented in .
Myocardial infarction and echocardiography
MI was surgically induced as previously described (Citation18). Briefly, the infarct was induced by permanent ligation of the left anterior descending artery at 50% of the length of the heart from the anterior-inferior edge of the left atrium to the apex. Echocardiography was accomplished under isoflurane anesthesia (isoflurane 1.5%/O2 1.0 L/min) with the use of a Vevo 660 (VisualSonics, Toronto, Canada) equipped with a 30 MHz transducer using maximal frame rate (Citation18–20). Echocardiograms were obtained at baseline, 2 days post-MI (before injection), and at day 28 post-MI. Two-dimensional echocardiography was used to determine left ventricular ejection fraction (LVEF) from end-diastolic and end-systolic volumes (EDV and ESV). Three cycles were measured for each assessment, and the average values were obtained. To confirm integrity of echocardiography data, two blinded, experienced clinicians (J.W.K. and Y.Z.) analyzed all echocardiographic studies independently, and consistent results were found (data not shown).
Ultrasound-guided injections
It has also been shown that the timing of the stem cell therapy post-MI is important (Citation21). A recent clinical trial suggests that the optimal time for cell therapy is 5 days or more after MI with little benefit derived from early treatment (Citation21). Our group has not only confirmed the importance of timing of cell-based therapy post-MI, but additionally we have shown that multiple injections of BMC did not have an additive effect on improving LVEF compared to monotherapy at day 3 (Citation22), which was therefore chosen to be the point of time for therapy in the current study. Further delaying the time of cell therapy post-MI resulted in no functional benefit (Citation22).
The animal table was set at a 30–40° angle, so that the animal head is up. The anterior myocardium with the target area was visualized using a modified parasternal long axis view. A transducer holder was used to confirm image stability. A 50-µL Hamilton syringe fitted with a sterile disposable 30-gauge needle was secured in a micromanipulator, and the needle was aligned before the injection procedure such that the needle was at 60° to the mouse surface. The needle was advanced with the use of the micromanipulator under echo guidance through the chest wall until the needle tip was in the desired location within the heart. All injections were documented as video clips on the Vevo 660 computer. Animals received ultrasound-guided injection of fBMs (n = 30), fBM extract (n = 25), whole BMCs (unfractionated) (n = 8), or Hanks’ buffered salt solution with 0.5% bovine serum albumin (HBSS/BSA) (n = 25) into myocardium as previously described (Citation18). Each heart was injected at day 3 post-MI with 10 µL of 1 × 106 cells, or the extract from 1 × 106 cells in 10 µL, or 10 µL HBSS/BSA, divided into two 5-µL injections into the peri-infarct zone of anterior wall.
Cell and extract preparation
Bone-marrow was harvested from 10–12-week-old C57BL/6 mice. Briefly, the mouse BMCs were flushed from tibias and femurs with cold HBSS/BSA and then strained through a 70-μm-nylon filter and washed twice with HBSS/BSA. Ficoll density gradient centrifugation was performed using Fico/Lite LM mouse® (Atlanta Biological Inc (Lawrenceville, GA, USA)) (density of 1.086±0.001 g/mL in 20°C and an osmolality of 280±10 mOs/kg H2O) to select fBM population from unfractionated BMC population. Fico/Lite LM® and HBSS +0.5% BSA was placed at room temperature before use. A 50-mL sterile centrifuge tube was filled with 15 mL of Fico/Lite solution before adding the cell suspension on it. Centrifugation was performed at 1000 g with brake off at 20°C. An upper layer containing plasma and platelets was carefully aspirated without disturbing the mononuclear cell layer at the interface. The mononuclear cell layer was carefully aspirated minimizing the amount of Fico/Lite in the sample and washed twice by HBSS +0.5% BSA. Concentrations of the BMCs and fBMs were adjusted to 105 cells/μL. Phycoerythrin or allophycocyanin-conjugated rat anti-mouse Sca-1, CD45, c-kit, CD133, CD34, Flk-1, and CD31 antibodies (eBioscience (San Diego, CA, USA) or BD Biosciences (San Jose, CA, USA)) were used for staining BMC and fBM cell populations and analyzed by fluorescence-activated cell sorter (FACS) (FACSCabilur with CellQuest software, BD Biosciences). The viability of BMCs and fBMs was checked using Trypan Blue staining with an average of 95% and 90% viability before injection. Cell-free fBM extract was prepared by subjecting the fBMs to three freeze–thaw cycles using an ethanol/dry ice bath, followed by microcentrifugation at 14,000 rpm to remove insoluble material (Citation13).
Tissue analysis
All animals were sacrificed at day 28, and eight animals from the fBM, fBM extract, and control groups were selected for histology including infarct size and vessel count analysis for arterioles and capillaries. Histology was not performed for the whole BMC group, since extensive histologic evaluation had already been reported previously (Citation13), and the group was included mostly to confirm previously published improved ejection fraction. Briefly, picrosirius-red staining was employed for infarct visualization, and the infarct size was measured as described previously (Citation19). Sections from the mid-ventricular level were stained using antibodies to CD31 (Biocare, Concord, CA, USA) and alpha-smooth muscle actin (SMA) (Sigma-Aldrich, St Louis, MO, USA) for capillaries and arterioles, respectively, to examine blood vessel densities. Blood vessel density was analyzed separately from the infarct zone (IZ), the border zone (BZ), and the remote zone (RZ). Border zone was defined as a high-power field containing one-third infarct scar and the other two-thirds normal myocardium. Adjusted capillary density counts were measured by calculating a ratio between IZ and BZ capillary density over RZ.
Statistical analysis
The results are expressed as mean ± SD. The Shapiro–Wilk procedure was applied to determine whether data are normally distributed. FACS data were compared using the chi-square test or Fisher's exact test as appropriate. Linear model of two-way repeated measures analysis of variance (ANOVA) with Dunnett's correction was used to test our hypothesis that experimental stem cell therapies have an effect on cardiac function and anatomy compared to control group. Statistical analysis was performed using either SPSS 16 (SSPP Inc., Chicago, IL, USA) or SAS (SAS Institute, Cary, NC, USA). A P-value less than 0.05 was considered significant.
Results
Bone-marrow-derived stem cell populations
The unfractionated mouse BMC population included the following: 2.13% Sca-1 + /CD45 +, 2.1% c-kit + /CD45 +, 0% CD133 +, 1.35% CD34 +, 0.18% Flk-1 +, 13.66% CD31 +, and 54.6% CD45 +. After Ficoll centrifugation, the composition of the cells was: 2.67% Sca-1 + /CD45 + (P = 0.02 for comparison to unfractionated cell population), 3.04% c-kit + /CD45 + (P < 0.001), 0% CD133 + (P = 1.00), 6.28% CD34 + (P < 0.001), 0.1% Flk-1 + (P = 0.18), 17.17% CD31 + (P < 0.001), and 81.9% CD45 + (P < 0.001).
Mortality and animal and heart weight
In our study, surgical inductions of MI led to 20.7% mortality. To avoid referral bias, we also excluded 12 additional animals (10.3%) before therapy when MI induction failure was detected or suspected by normal contraction at the left ventricular apex. Treatment had no influence on mortality (). The fBM, fBM extract, or whole BMC treatment had no effect over control treatment on animal weights (Δ animal weights after treatment: 4.3±1.5 g, 4.5±1.9 g, 3.9±1.4 g, and 4.0±1.9 g; P = 0.88, P = 0.66, and P = 1.00) or heart weights at sacrifice (117±17 mg, 118±20 mg, 109±9 mg, and 119±15 mg; P = 0.92, P = =1.00, and P = 0.46), respectively.
Echocardiography
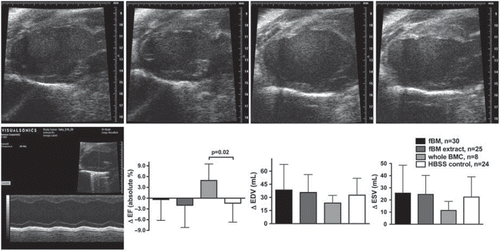
All animals had a similar preserved left ventricular function at baseline. At day 2 post-MI, EF decreased to a mean of 36% in all groups (P = ns). High-resolution B-mode echocardiography images at baseline and 28 days after left anterior descending coronary artery (LAD) ligation are presented in . Whole BMC therapy had a positive effect on LVEF, but fBM or fBM extract were not beneficial compared to HBSS control (4.9% ±4.6% (P = 0.02), –0.4% ±5.8% (P = 0.86), –2.0% ±6.2% (P = 0.97) versus –1.4% ±5.3%, respectively) (). Echocardiography data are presented in detail in the Supplemental Table I to be found online at http://informahealthcare.com/ann/doi.10.3109/07853890.2012.672026
Figure 2. Diastolic and systolic B-mode echocardiography are presented as pairs at baseline before MI (top) and 28 days post-MI after fractionated bone-marrow cell therapy (left to right in upper row) by using Vevo 660 (VisualSonics, Toronto, Canada) high-resolution 30 MHz ultrasound system. Bottom row shows M-mode echo at day 2 after MI and changes in left ventricular parameters after the therapies. Individual changes were calculated by subtracting day 28 values from day 2 values.
Histology
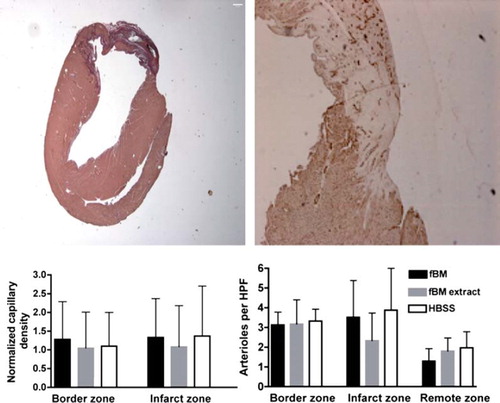
Histology was performed on the fBM, fBM extract, and HBSS groups and as noted in the Methods section. Infarct sizes were similar in fBM and fBM extract groups compared to the HBSS control group. Percentage infarct area were 12.1% ±11.2%, 5.8% ±3.0% versus 12.2% ±7.0%, P = 1.00 and P = 0.27, respectively. Vascularity with respect to arteriole and capillary count at IZ, BZ, or RZ did not differ between these three therapies (). Numbers of arterioles per high-power field in fBM and fBM extract groups compared to control group were at IZ 3.52±1.86 and 2.34±1.40 versus 3.88±2.12 (P = 0.97 and P = 0.25) and at BZ 3.13±0.65 and 3.17±1.24 versus 3.33±0.60 (P = 0.98 and P = 0.98). Capillary density at IZ or BZ did not differ significantly between treatment groups ().
Figure 3. Basal section at midventricular short axis orientation demonstrates basal extent of myocardial infarct including infarct, border, and remote zone at the same slice from an animal with fBM treatment. Infarct size was measured by applying picrosirius staining (left) and capillary density by CD31 staining (right). Capillary densities at border zone and infarct zone are normalized individually to remote zone capillary density (left). Mean arteriole density in border zone, infarct zone, and remote zone measured by fluorescent microscopy from five high-power (40 ×) fields (right). Data are expressed as mean ± SD. All P values are non-significant (>0.05).
Discussion
The main finding of this report is that fractionation of the mouse bone-marrow cells, as opposed to using the whole bone-marrow, limits cardiac functional improvement in a non-reperfused mouse model of MI. We performed cell therapy at a clinically relevant time period without a re-sternotomy by using ultrasound-guided cell delivery 3 days post-MI to mimic human clinical trials as closely as possible.
The regenerative capacity of different stem cells such as hematopoietic (CD34 +, Sca-1 +, c-Kit +), endothelial (CD133 + /KDR +, CD34/KDR +), mesenchymal and adipose tissue (CD34-, CD45-, c-Kit +, CD90 +, Sca-1 +), cardiac stem (c-Kit + /CD45-, Sca-1 + /CD45-, Isl-1 +, MDR-1 +), and embryonic stem cells (Citation23,Citation24) have been tested in translational settings and/or in clinical trials, but which cell is the ‘ideal’ cell for cardiac therapeutic purposes is not known. BM-derived mononuclear cells have been used in most human clinical trials, but in this study we show for the first time that whole mouse BMC therapy is in fact superior to mouse fBM therapy with respect to cardiac functional improvement post-MI. In this study, Ficoll density gradient centrifugation was applied to separate red blood cells, granulocytes, and platelets from whole bone-marrow. Paracrine factors and cytokines, including but not limited to tumor necrosis factor-α, transforming growth factor-β, and basic fibroblast growth factor, might be differentially expressed with whole BMCs versus fBMs (Citation25). Although it is not known whether an early inflammatory reaction post-MI is a cause or consequence of the myocyte loss, it is associated with accumulation of neutrophil granulocytes and release of cytotoxic factors such as oxygen free radicals and arachidonic acid metabolites. On the other hand, neutrophil lineage cells produce high levels of serine proteases, collagenases, and gelatinases that prevent cardiac remodeling in the long term (Citation26).
Our results using fBMs and corresponding cell extract for cardiac repair are in line with some placebo-controlled, randomized trials in animals and humans (Citation8–10). The histological infarct size or numbers of arterioles and capillaries at infarct zone or border zone did not differ between the fBM, fBM extract, and control groups. However, our prior publication showed that treatment with whole BMC resulted in a smaller infarct size compared to saline control (Citation13). In addition, whole BMC therapy at an early time point (day 6) post-MI, but not at day 28, led to significantly higher capillary area and number of arterioles versus control (Citation13). It should be noted, however, that there are also clinical studies showing improved cardiac function and reduced infarct size after MI (Citation4–7). Previous studies have reported variable findings in regard to cardiac outcomes after cell therapy post-MI. In our study, the fBM cell population was not beneficial. There are potentially many reasons for the discrepancy in the reports—cell preparation, cell number, mouse strain, and injection methods might all influence outcome. For example, compared to the study by Swijnenburg and colleagues (Citation27), our study was carried out using C57BL/6J mice without using GFP-expressing donor cells, whereas they used FVB-NJ mice with GFP-expressing donor cells. Mouse-strain-dependent heterogeneity of angiogenesis capacity may also influence the different outcomes. C57BL/6J mice had three times lower angiogenic response compared to FVB-NJ mice (Citation28), and the low regenerative potential of the C57BL/6J strain versus other mouse strains has also been demonstrated by experimental wound repair study (Citation29). Moreover, there were substantial (>2-fold) inter-strain variation in 8 out of 118 tested genes involved in angiogenesis or vessel growth. Interestingly, some pro-angiogenic considered genes such as vascular endothelial growth factor and placental growth factor were expressed at higher levels in low vascular growth strains such as C57BL/6J that describes complexity of this issue. Cell isolation and preparation techniques appear to influence clinical outcome after therapy (Citation30). For example, the REPAIR-AMI and ASTAMI trials showed differences in left ventricular function after stem cell therapy for acute MI (Citation7,Citation31). Retrospectively, the different protocols for cell isolation and storage (REPAIR-AMI: Ficoll, storage in X-vivo 10 medium plus serum; ASTAMI: Lymphoprep, storage in NaCl plus plasma) were found to have profound impact on functional outcome (Citation30). The authors pointed out that the cell number and viability may not entirely reflect the functional capacity of cells in vivo. In our study, fractioning BMCs using the Ficoll method led to enrichment of CD34 + (1.35% to 6.3%), CD45 + (54.6% to 81.9%), and CD45/Sca-1 double-positive cells (2.13% to 2.67%), whereas a decreasing trend type was noted within Flk-1 + cells, from 0.18% to 0.10%. Notably, the proportion of CD45 + cells was comparable to an earlier animal study (Citation27), and the proportion of CD45 + /CD34 + cells were within the same level as in some human clinical studies (Citation7,Citation31). Clearly, it should be kept in mind that human and mouse stem cell markers are not fully comparable. Additionally, previous studies have shown that progenitor cells derived from patients with ischemic heart disease are dysfunctional and therefore may have limited regenerative capacity (Citation32,Citation33). The systemic inflammatory phase of acute MI may also potentially further reduce stem cells’ proliferative capacity and cytokine responses. In contrast to clinical studies with bone-marrow aspiration at the time of acute or sub-acute MI, we used healthy young mice with the same strain in donors and recipients.
In humans at least 50,000 cells per kg of weight has been suggested to be the minimum effective dose for cardiac repair (Citation34). In viable cell injection groups of this study, 106 cells were injected for animals with an average weight of 25 g, indicating that we injected an 800-times higher cell count compared to the recommended minimum. All cell injections were performed using echo-guided technique with a micromanipulator, allowing us to confirm successful intramyocardial injections. Survival of intramyocardially injected hematopoietic cells have been superior to mesenchymal cells and skeletal myoblasts, indicating that they have at least more time to mediate paracrine factors such as vascular endothelial growth factor and fibroblast growth factor, whose concentrations increase at border and infarct zones after hematopoietic stem cell injection therapy (Citation17,Citation35).
In a recent meta-analysis of cardiac stem cell therapy in humans, cell type (fBM versus mesenchymal or circulating peripheral progenitor) did not significantly influence the changes in EF or infarct size (Citation36). However, to our knowledge, there are no studies in human using unfractionated BMC. This report has clinical implications in that most human clinical trials are currently being performed using selected MNC from the bone-marrow rather than whole BMC. We propose that the assumption should not be made that, in humans, fBM is the ‘ideal’ therapeutic cell of choice over whole BMC injection.
Limitations
We recognize the limitations of simulating human coronary artery disease and MI using a mouse model of acute MI induced by permanent ligation of coronary artery. It does not reflect contemporary medical practice where patients with an acute MI undergo early reperfusion of the culprit artery. However, this animal model is reproducible and well-established, allowing direct comparison to earlier results. We did not use GFP or any other cell-tracking methods after treatment to evaluate cell fate in the myocardium or other organs. However, our prior report (Citation13) suggests, and growing consensus exists, that implanted stem cells are not retained or do not survive for long periods (Citation9,Citation37–41). Moreover, changes in apoptosis frequency were not evaluated, as determining apoptosis is preferred at an early time point, and this was outside of the design of this study. On-going work in our laboratory is currently evaluating the proteomic differences between whole BMC versus fBM extracts.
Our results elucidate existing controversy around cell therapy for acute myocardial infarct by showing differences between therapeutic potential of different bone-marrow-derived cell populations. There is a need for characterizing temporal changes in local myocardial metabolism after these therapies in relation to cardiac function and remodeling to define the key modulators of the process. Clinical studies should be performed to test whether whole BMC therapy versus bone-marrow fraction translates into better outcome after human MI.
Supplementary Table I.
Download PDF (420.7 KB)Acknowledgements
We thank Petros Minasi for his help in this project.
Declaration of interest: This work was supported in part by the UCSF Translational Cardiac Stem Cell Foundation and a grant from the Wayne and Gladys Valley Foundation to Y.Y.; and grants from the following to J.W.K.: Sigrid Juselius Foundation, Finnish Foundation for Cardiovascular Research, Paavo Nurmi Foundation, Varsinais-Suomi Regional Fund of Finnish Cultural Foundation and The Finnish Medical Foundation. The authors report no conflicts of interest.
References
- Losordo DW, Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease: part II: cell-based therapies. Circulation. 2004;109:2692–7.
- Rosenzweig A. Cardiac cell therapy—mixed results from mixed cells. N Engl J Med. 2006;355:1274–7.
- Wollert KC, Drexler H. Clinical applications of stem cells for the heart. Circ Res. 2005;96:151–63.
- Assmus B, Schachinger V, Teupe C, Britten M, Lehmann R, Dobert N, . Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI). Circulation. 2002;106:3009–17.
- Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, . Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–8.
- Fernandez-Aviles F, San Roman JA, Garcia-Frade J, Fernandez ME, Penarrubia MJ, de la Fuente L, . Experimental and clinical regenerative capability of human bone marrow cells after myocardial infarction. Circ Res. 2004;95:742–8.
- Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, . Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–21.
- Hashemi SM, Ghods S, Kolodgie FD, Parcham-Azad K, Keane M, Hamamdzic D, . A placebo controlled, dose-ranging, safety study of allogenic mesenchymal stem cells injected by endomyocardial delivery after an acute myocardial infarction. Eur Heart J. 2008;29:251–9.
- de Silva R, Raval AN, Hadi M, Gildea KM, Bonifacino AC, Yu ZX, . Intracoronary infusion of autologous mononuclear cells from bone marrow or granulocyte colony-stimulating factor-mobilized apheresis product may not improve remodelling, contractile function, perfusion, or infarct size in a swine model of large myocardial infarction. Eur Heart J. 2008;29:1772–82.
- Herbots L, D'hooge J, Eroglu E, Thijs D, Ganame J, Claus P, . Improved regional function after autologous bone marrow-derived stem cell transfer in patients with acute myocardial infarction: a randomized, double-blind strain rate imaging study. Eur Heart J. 2009;30: 662–70.
- Uemura R, Xu M, Ahmad N, Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res. 2006;98:1414–21.
- Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, . Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20:661–9.
- Yeghiazarians Y, Zhang Y, Prasad M, Shih H, Saini SA, Takagawa J, . Injection of bone marrow cell extract into infarcted hearts results in functional improvement comparable to intact cell therapy. Mol Ther. 2009;17:1250–6.
- Assmus B, Tonn T, Seeger FH, Yoon CH, Leistner D, Klotsche J, . Red blood cell contamination of the final cell product impairs the efficacy of autologous bone marrow mononuclear cell therapy. J Am Coll Cardiol. 2010;55:1385–94.
- Deindl E, Zaruba MM, Brunner S, Huber B, Mehl U, Assmann G, . G-CSF administration after myocardial infarction in mice attenuates late ischemic cardiomyopathy by enhanced arteriogenesis. FASEB J. 2006;20:956–8.
- Yeghiazarians Y, Khan M, Angeli FS, Zhang Y, Jahn S, Prasad M, . Cytokine combination therapy with long-acting erythropoietin and granulocyte colony stimulating factor improves cardiac function but is not superior than monotherapy in a mouse model of acute myocardial infarction. J Card Fail. 2010;16:669–78.
- van der Bogt KE, Sheikh AY, Schrepfer S, Hoyt G, Cao F, Ransohoff KJ, . Comparison of different adult stem cell types for treatment of myocardial ischemia. Circulation. 2008;118:S121–9.
- Springer ML, Sievers RE, Viswanathan MN, Yee MS, Foster E, Grossman W, . Closed-chest cell injections into mouse myocardium guided by high-resolution echocardiography. Am J Physiol Heart Circ Physiol. 2005;289:H1307–14.
- Takagawa J, Zhang Y, Wong ML, Sievers RE, Kapasi NK, Wang Y, . Myocardial infarct size measurement in the mouse chronic infarction model: comparison of area- and length-based approaches. J Appl Physiol. 2007;102:2104–11.
- Zhang Y, Takagawa J, Sievers RE, Khan MF, Viswanathan MN, Springer ML, . Validation of the wall motion score and myocardial performance indexes as novel techniques to assess cardiac function in mice after myocardial infarction. Am J Physiol Heart Circ Physiol. 2007;292:H1187–92.
- Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, . Improved clinical outcome after intracoronary administration of bone-marrow-derived progenitor cells in acute myocardial infarction: final 1-year results of the REPAIR-AMI trial. Eur Heart J. 2006;27:2775–83.
- Zhang Y, Sievers RE, Prasad M, Mirsky R, Shih H, Wong ML, . Timing of bone marrow cell therapy is more important than repeated injections after myocardial infarction. Cardiovasc Pathol. 2011;20:204–12.
- van Laake LW, Passier R, Doevendans PA, Mummery CL. Human embryonic stem cell-derived cardiomyocytes and cardiac repair in rodents. Circ Res. 2008;102:1008–10.
- Caspi O, Huber I, Kehat I, Habib M, Arbel G, Gepstein A, . Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts. J Am Coll Cardiol. 2007;50:1884–93.
- Sun J, Li SH, Liu SM, Wu J, Weisel RD, Zhuo YF, . Improvement in cardiac function after bone marrow cell therapy is associated with an increase in myocardial inflammation. Am J Physiol Heart Circ Physiol. 2009;296:H43–50.
- Kolpakov MA, Seqqat R, Rafiq K, Xi H, Margulies KB, Libonati JR, . Pleiotropic effects of neutrophils on myocyte apoptosis and left ventricular remodeling during early volume overload. J Mol Cell Cardiol. 2009;47:634–45.
- Swijnenburg RJ, Govaert JA, van der Bogt KE, Pearl JI, Huang M, Stein W, . Timing of bone marrow cell delivery has minimal effects on cell viability and cardiac recovery after myocardial infarction. Circ Cardiovasc Imaging. 2010;3:77–85.
- Liu F, Smith J, Zhang Z, Cole R, Herron BJ. Genetic heterogeneity of skin microvasculature. Dev Biol. 2010;340:480–9.
- Clark LD, Clark RK, Heber-Katz E. A new murine model for mammalian wound repair and regeneration. Clin Immunol Immunopathol. 1998;88:35–45.
- Seeger FH, Tonn T, Krzossok N, Zeiher AM, Dimmeler S. Cell isolation procedures matter: a comparison of different isolation protocols of bone marrow mononuclear cells used for cell therapy in patients with acute myocardial infarction. Eur Heart J. 2007;28:766–72.
- Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, . Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355:1199–209.
- Heeschen C, Lehmann R, Honold J, Assmus B, Aicher A, Walter DH, . Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation. 2004;109:1615–22.
- Heiss C, Keymel S, Niesler U, Ziemann J, Kelm M, Kalka C. Impaired progenitor cell activity in age-related endothelial dysfunction. J Am Coll Cardiol. 2005;45:1441–8.
- Hill JM, Bartunek J. The end of granulocyte colony-stimulating factor in acute myocardial infarction? Reaping the benefits beyond cytokine mobilization. Circulation. 2006;113:1926–8.
- Norol F, Bonnet N, Peinnequin A, Chretien F, Legrand R, Isnard R, . GFP-transduced CD34 and Lin- CD34- hematopoietic stem cells did not adopt a cardiac phenotype in a nonhuman primate model of myocardial infarct. Exp Hematol. 2007;35:653–61.
- Abdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, . Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007;167:989–97.
- Reffelmann T, Kloner RA. Cellular cardiomyoplasty—cardiomyocytes, skeletal myoblasts, or stem cells for regenerating myocardium and treatment of heart failure? Cardiovasc Res. 2003;58:358–68.
- Hofmann M, Wollert KC, Meyer GP, Menke A, Arseniev L, Hertenstein B, . Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation. 2005;111:2198–202.
- Sheikh AY, Lin SA, Cao F, Cao Y, van der Bogt KE, Chu P, . Molecular imaging of bone marrow mononuclear cell homing and engraftment in ischemic myocardium. Stem Cells. 2007;25:2677–84.
- Cao F, Wagner RA, Wilson KD, Xie X, Fu JD, Drukker M, . Transcriptional and functional profiling of human embryonic stem cell-derived cardiomyocytes. PLoS ONE. 2008;3:e3474.
- Li Z, Lee A, Huang M, Chun H, Chung J, Chu P, . Imaging survival and function of transplanted cardiac resident stem cells. J Am Coll Cardiol. 2009;53:1229–40.