Abstract
Type 2 diabetes mellitus is a highly prevalent disease characterized by insulin resistance, hyperglycemia, and diminished pancreatic β-cell function. Conventional insulin products used to manage this disease include regular human insulin and intermediate-acting human insulin. However, due to several limitations imposed by human insulins, such as onset and duration of action that do not coincide with physiologic needs and increased risk of hypoglycemia, insulin analogs were developed. Because they more closely mimic the physiologic action of endogenous insulin, insulin analogs are associated with more effective glucose control, a lower risk of hypoglycemia, greater convenience, and, in some instances, less weight gain. Switching from human insulin to insulin analogs is easily accomplished. Several studies have demonstrated a high rate of success with patient-initiated, self-adjusted dosing algorithms compared to investigator/clinician-initiated dose adjustments. These studies and several other published guidelines on insulin analogs provide patients and clinicians with information pertaining to better treatment options and can help increase overall patient satisfaction.
Key messages
Absorption and onset of action of regular human insulin is poorly matched to the postprandial glucose spike, often resulting in postprandial hyperglycemia or late hypoglycemia, even when dosed properly.
Dose conversion from once- or twice-daily NPH (neutral protamine Hagedorn insulin) to basal insulin analogs is relatively simple but may require individualized adjustment due to the longer duration of action of basal insulin analogs. Studies of patient-initiated, self-adjusted dosing algorithms have shown better glycemic control compared to investigator/clinician-initiated dose adjustments, demonstrating their value in a conversion plan.
| Abbreviations | ||
| A1C | = | glycosylated hemoglobin A1c |
| AACE | = | American Association of Clinical Endocrinologists |
| ACCORD | = | Action to Control Cardiovascular Risk in Diabetes Study |
| ACE | = | American College of Endocrinology |
| ADA | = | American Diabetes Association |
| BIAsp | = | biphasic insulin aspart (70% insulin aspart suspension/30% insulin aspart) |
| EASD | = | European Association for the Study of Diabetes |
| FPG | = | fasting plasma glucose |
| NPH | = | neutral protamine Hagedorn insulin |
| OAD | = | oral antidiabetes drug |
| RHI | = | regular human insulin |
| SMBG | = | self-monitored blood glucose |
| T2DM | = | type 2 diabetes mellitus |
| TDD | = | total daily dose |
Introduction: prevalence of diabetes
The estimated global prevalence of diabetes in 2010 among adults was 285 million, equating to 6.4% of the entire population throughout the world (Citation1). For developed countries, it has been predicted that there will be a 69% increase in the number of adults with diabetes by the year 2030, at which time there will be an estimated 439 million patients (7.7% of the world population). In the United States, an estimated 26 million people (8.3% of the population) have diabetes, another 79 million may have prediabetes (Citation2), and the number of people with diabetes is projected to rise to more than 48 million by the year 2050 (Citation3). Diabetes poses a huge economic burden; in the United States alone, total costs due to diabetes were estimated to be $174 billion in 2007, of which $116 billion was attributed to direct medical costs (Citation2).
Type 2 diabetes mellitus (T2DM), a metabolic disorder characterized by insulin resistance, hyperglycemia, and loss of pancreatic β-cell function resulting in relative insulin deficiency (Citation4), often preceded by obesity, has reached epidemic proportions, accounting for 90%–95% of all diagnosed cases of diabetes (Citation2). It is most commonly found in middle-aged and older adult populations; due to an increase in childhood obesity and inactivity, however, the frequency of T2DM among adolescents and young adults, while still rare, is also increasing, especially among high-risk racial/ethnic groups (Citation2).
Glycemic goals and the role of insulin
Treatment goals and guidelines for the proper management of hyperglycemia and diabetes have been established by several national and international diabetes associations. A consensus algorithm published by the American Diabetes Association (ADA) in collaboration with the European Association for the Study of Diabetes (EASD) recommends a glycosylated hemoglobin A1c (A1C) level of < 7%, a fasting and preprandial glucose level of 70–130 mg/dL (3.9–7.2 mmol/L), and a postprandial (90–120 min) level of < 180 mg/dL (< 10 mmol/L) (Citation5). The American Association of Clinical Endocrinologists (AACE) recommend a target A1C of ≤ 6.5%, a fasting plasma glucose (FPG) level of < 110 mg/dL (< 6.1 mmol/L), and a 2-hour postprandial glucose of < 140 mg/dL (< 7.8 mmol/L) (Citation6). A1C testing should be performed approximately every 2–3 months in order to determine if target levels have been reached and to ensure they are maintained (Citation7).
The pathophysiology of T2DM historically involves both insulin resistance, in which cells do not respond appropriately to insulin, and progressive failure of pancreatic β-cells to secrete insulin. Recent research has shown that T2DM also involves an increase in glucagon secretion, a decrease in the production/secretion of gastrointestinal incretin hormones, and an increase in hepatic glucose production, along with other hormonal abnormalities (Citation8–10). Studies indicate that most patients with T2DM will ultimately require insulin in order to achieve and maintain specific glycemic goals (Citation9,Citation11). Insulin therapy has commonly been initiated as a last resort when oral antidiabetes drugs (OADs) fail to enable patients to achieve glycemic goals; however, because of these recent findings, the paradigm of T2DM treatment is shifting. Earlier initiation of insulin therapy, especially for patients with A1C levels of > 8.5% who do not respond to monotherapy, is recommended by the ADA/EASD consensus algorithm (Citation5).
When patients with T2DM cannot reach or maintain glycemic targets through diet and exercise or when combination therapy with antidiabetes medications does not meet therapeutic goals, ADA/EASD as well as AACE (in collaboration with the American College of Endocrinology (ACE)) recommend initiating insulin therapy (Citation5,Citation12). Clinical inertia is often the culprit when prescribers do not initiate insulin therapy in a timely fashion (Citation13), despite ADA/EASD and AACE/ACE recommendations that strongly support the initiation of insulin therapy to manage patients with poor glycemic control (Citation5,Citation12). In addition, the AACE and ACE state the benefits of maintaining focus on reasonable glycemic goals and adjusting and/or adding medications frequently (every 2 to 3 months) (Citation12).
As noted above, T2DM is a complex disease. Uncontrolled hyperglycemia is only one concern surrounding T2DM treatment; hypoglycemia is another. Multiple studies have shown that episodes of hypoglycemia may be associated with increased cardiovascular risk (Citation14,Citation15), although other studies have produced data that conflict with these reports (Citation16,Citation17). The Action to Control Cardiovascular Risk in Diabetes (ACCORD) Study included 10,251 patients with T2DM that had a mean age of 62 years, median duration of diabetes for 10 years, and median A1C of 8.1% who were randomized to either intensive (A1C goal < 6.0%) or standard treatment strategies (A1C goal 7.0%–7.9%) (Citation18). The ACCORD study ended early because significantly more patients in the intensive therapy group died compared with standard therapy (257 versus 203 patients, respectively; hazard ratio 1.22, 95% CI 1.01–1.46, P = 0.04) (Citation19). In addition, hypoglycemia requiring treatment and 10 kg or more weight gain were significantly more common in the intensive therapy group (P < 0.001). Data obtained over 3.4 years of follow-up, before cessation of treatment, confirm that the increased risk of mortality was associated with the intensive glycemic treatment strategy. However, these sub-analyses also revealed that factors associated with persisting higher A1C levels rather than low A1C were the likely contributors to the increased mortality risk associated with intensive glycemic treatment. From the results of the ACCORD trial came the realization that there exists a subpopulation of patients who are unable to achieve glycemic targets with intensive therapy and that aggressive intensification of treatment in this population may increase mortality risk secondary to cardiovascular disease as well as other causes (Citation20).
The challenge of insulin therapy is to achieve optimal glycemic levels by approximate replication of the time course action profile of endogenous insulin found in healthy individuals (Citation21). The endogenous pattern of insulin secretion among normal individuals entails three major peaks, called bolus or meal-time insulin, which correspond to the three standard meals during the day (Citation22). In contrast to bolus insulin, basal is the background insulin that is secreted over a 24-hour period. Basal insulin suppresses glucose production between meals and overnight, while bolus insulin limits hyperglycemia after meals. Basal and bolus insulin each account for about 50% of the total insulin in the body (Citation22).
It is essential for patients with diabetes to measure their own blood glucose levels, especially those who are treated with insulin. Self-monitored blood glucose (SMBG) provides information for patients and clinicians that enables them to assess the impact of several factors on blood glucose control, as well as patterns of blood glucose levels including episodes of hyper- and hypoglycemia (Citation7). Patients should be taught the correct frequency of testing and how to use the data to adjust food intake, exercise, or pharmacologic therapy to achieve specific glycemic goals, with these skills evaluated periodically (Citation7). In addition to antidiabetes medications, factors that can affect blood glucose levels include, but are not limited to:
Food choices/portion size
Activity levels (duration/intensity/timing with medication)
Other medications, such as antihistamines, antipsychotics, corticosteroids, estrogen, progesterone, etc. (doses/timing)
Emotional stress
Illness
Hormonal fluctuations
Dosing regimens for insulin therapy
The ADA and EASD have developed a consensus approach to managing hyperglycemia in patients with T2DM and published an algorithm () for initiating and advancing insulin therapy (Citation5). Several insulin formulation options are currently available in the US ( and ) (Citation23–35). Initial insulin therapy is aimed at controlling FPG levels, usually with intermediate- or long-acting insulin, although it is recognized that patients may also need prandial therapy with short- or rapid-acting insulin. Additionally, many advances have been made to improve insulin injections by using different techniques and offering options such as insulin pumps and insulin pen devices. All insulin available for use in pen devices currently in the US are noted in and (Citation36); use of such devices allows for greater patient convenience and improved dosing accuracy that may help avoid errors (Citation37).
Figure 1. Initiation and adjustment of insulin regimens. Insulin regimens should be designed taking lifestyle and meal schedule into account. The algorithm can only provide basic guidelines for initiation and adjustment of insulin. See reference (Citation76) for more detailed instructions. aPremixed insulins not recommended during adjustment of doses; however, they can be used conveniently, usually before breakfast and/or dinner, if proportion of rapid- and intermediate-acting insulins is similar to the fixed proportions available.
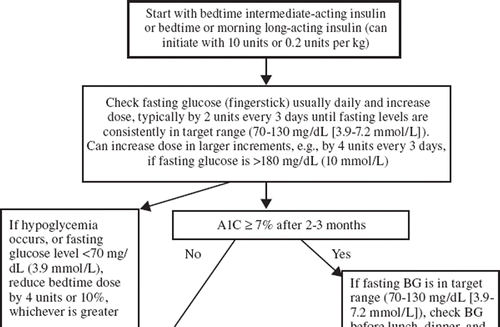
Table I. Insulins available in the US.
Table II. Insulin premixes available in the US.
As per the AACE/ACE algorithm, a common, although arbitrary, starting dose for basal insulin therapy in patients with T2DM is 10 units subcutaneously at bedtime (Citation12). A basal–bolus insulin regimen can involve four or more injections per day but offers greater flexibility for those patients with postprandial hyperglycemia, variable meal-times, and differing carbohydrate content. The before-meal insulin dose can be initiated either as a fixed dose regimen titrated as needed or based upon the current and target blood glucose as well as the anticipated carbohydrate intake. The dosing regimen should be based upon the patient's glycemic control as well as their willingness and ability to comprehend the regimen in order to control effectively the levels of A1C as well as the preprandial and postprandial levels of glycemia (Citation12).
Initiation of insulin can be conducted with a basal insulin analog or an insulin analog premix. Advantages of starting with basal insulin include the following: usually one injection daily, simple titration of dose, effective improvement in glycemic control, limited weight gain, and insulin pen device for ease of administration. Per AACE recommendations, advancement to a basal–bolus insulin regimen is indicated when A1C is > 8.5% despite maximum combination therapy (either combinations of OADs or OAD plus exenatide) (Citation6). Basal insulin (glargine or detemir) is usually given once daily and is converted to basal–bolus regimen with the addition of meal-time insulin (Citation12). Insulin premixes using a rapid-acting analog are usually given twice daily with breakfast and dinner but may occasionally be given once daily with the largest meal. The choice will often depend upon the individual patient's needs and abilities as well as willingness to self-monitor blood glucose for optimal control (Citation7). When insulin therapy is initiated, it is recommended to discontinue or taper insulin secretagogues (e.g. sulfonylureas) because they are not considered synergistic and the combination may increase the risk of hypoglycemia (Citation5). Studies have shown that encouraging patients to attend diabetes education classes will result in improved diabetes regimen adherence resulting in improved glycemic control (Citation7).
Pharmacokinetic/pharmacodynamic properties of insulin and insulin analogs: why insulin analogs may be a better choice
Regular human insulin
For many years, regular human insulin (RHI) had been used as a meal-time or prandial insulin treatment because it had been proven to be an effective form of insulin therapy (Citation38). Regular human insulin has limitations, however, since its pharmacokinetic/pharmacodynamic profile does not match that of endogenous insulin production in individuals without diabetes (Citation21). For example, RHI is more slowly absorbed and has a slower onset of action than endogenous insulin, and therefore it has been recommended that it is injected 30 minutes before meals (Citation21). This may cause postprandial hyperglycemia or late hypoglycemia, especially while dining out or for patients with inconsistent meal schedules. If the dose is not appropriately timed it can adversely affect blood glucose control, since the patient may experience a high blood glucose spike before the insulin begins to act (Citation21). Moreover, RHI is cleared slowly from the blood, with a duration of action up to 12 hours () (Citation24,Citation28,Citation29). It produces a peak effect that often does not coincide with the peak of glucose absorption, even with proper timing of administration, especially when patients are using insulin supplements between meals. However, RHI is less expensive than rapid-acting analogs and may be preferred for patients without insurance since a prescription is not required.
Long-acting/basal insulin therapy: choice between NPH and analogs
Neutral protamine Hagedorn insulin (NPH) had been the mainstay for maintaining basal insulin levels for many years; however, in terms of its pharmacokinetic profile (duration of action up to 24 hours, a distinct peak effect, and variable rates of absorption) (Citation21,Citation24,Citation37), AACE/ACE does not recommend the use of NPH as a basal insulin and has stated that NPH has been superseded by the synthetic analogs insulin glargine and insulin detemir because they mimic the basal physiologic insulin profile by providing a relatively peakless profile for approximately 24 hours (Citation12). AACE/ACE has also stated that glargine and detemir provide both better reproducibility and consistency than NPH, with a corresponding reduction in the risk of hypoglycemia.
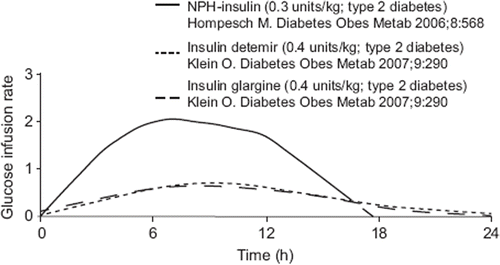
A recent paper reported that for insulin therapy, pharmacodynamic parameters have more clinical relevance than pharmacokinetic parameters (Citation39). The glucose clamp technique has proven to be an effective method to determine insulin pharmacodynamic parameters because it allows assessment of the onset, peak, and duration of action. Clinical trials that employed the glucose clamp technique concluded that once-daily administration of insulins glargine or detemir provides similar or more effective blood glucose control compared with NPH insulin, with a reduced risk of hypoglycemia (Citation39). Compared to NPH, with an action that peaks at 4–12 hours and can last up to 24 (), glargine and detemir show little to no peak activity and result in insulin absorption over a period of 24 hours () (Citation39–41), thereby reducing the risk of nocturnal hypoglycemia. Moreover, glargine does not have differential rates of absorption at different sites (Citation42). In comparison with other insulins, long-acting detemir has an excellent reproducibility of absorption profile within individuals and causes less weight gain (Citation12,Citation43,Citation44) if only one dose per 24 hours is required. Patients with type 1 diabetes mellitus (T1DM) have minimal endogenous insulin production and therefore are a much better model for assessing biological effects of exogenous insulin types, including insulin analogs. One study that included patients with T1DM found that 1 unit of detemir (24 nmol insulin) was approximately 30% less active than 1 unit of glargine (6 nmol insulin) (Citation44). The authors theorized that this difference could help to explain the shorter duration of action of detemir. Studies that included patients with T2DM have found similar differences in duration of detemir and glargine, resulting in patients often requiring a second dose of detemir. One study by Rosenstock et al. reported that 55% of patients treated with insulin detemir required a second dose per day in order to achieve reductions in A1C that were comparable to those achieved by patients treated with insulin glargine once daily (mean daily detemir doses: 0.78 U/kg (0.52 U/kg with once daily dosing, 1.00 U/kg with twice daily dosing); mean daily glargine dose: 0.44 IU/kg) (Citation45). Another trial also demonstrated a need of higher doses of insulin detemir (76.5 ± 50.5 units/day) than insulin glargine (43.5 ± 29.0 units/day, P < 0.001) (Citation46). However, because both of those trials were designed to use on-label treatment regimens, the option to add a second injection was allowed in the protocol for detemir, but not for glargine, confounding the ability to make a direct comparison between the two agents. Moreover, Rosenstock et al. concluded that since that study was not powered to compare once-daily and twice-daily dosing, post-hoc analysis led them to theorize that once-daily dosing of insulin detemir may be an appropriate starting regimen when initiating insulin therapy in patients on OADs, but a greater portion of patients may eventually require a twice-daily regimen (Citation45).
Figure 2. Glucose infusion rates in glucose-clamp experiments on long-acting insulins (Citation39–41) (from reference (Citation39) with permission).
The Treat-to-Target trial enrolled 756 overweight patients with an A1C > 7.5% in order to assess the efficacy of glargine compared to NPH (Citation47). After 24 weeks of treatment, reductions in A1C were similar in patients treated with glargine compared with NPH. The major difference observed between the two treatment groups was that almost 25% more patients treated with glargine achieved goal A1C reductions without any nocturnal hypoglycemia. The rate of overall symptomatic hypoglycemia was also lower in the glargine group (Citation47).
Another treat-to-target study comparing the insulin detemir and insulin glargine regimens among patients with T2DM found similar efficacy in A1C reduction and comparable hypoglycemia risk at 26 weeks (Citation48). However, patients treated with insulin detemir had significantly less weight gain than those who received insulin glargine (1.2 ± 3.96 kg versus 2.7 ± 3.94 kg, P = 0.001) (Citation48). A summary of treat-to-target studies that included insulin-naive patients with T2DM who were treated with biphasic insulin as- part (70% insulin aspart suspension/30% insulin aspart) (BIAsp 30), a basal insulin analog (insulin glargine or insulin detemir), or NPH insulin once or twice daily plus OADs reported that among older patients with higher bedtime plasma glucose, BIAsp 30 appeared to be more beneficial (Citation49). However, basal insulin appeared to achieve better FPG in patients with lower BMI and higher post-breakfast plasma glucose. The authors concluded that insulin analog premixes may be more appropriate than basal insulin to target A1C values in older individuals and those with higher bedtime plasma glucose (Citation49).
Detemir has a 14-carbon fatty acid chain that works by binding to albumin in the blood through a fatty acid molecule and then slowly dissociating from that complex, thereby delaying insulin absorption over time and resulting in a longer duration of action (Citation50). In contrast, insulin glargine forms a microcrystalline precipitate in subcutaneous tissue that is not readily absorbed. The microcrystals slowly dissociate and are then released into circulation, and therefore glargine is found to have significantly less interpatient variability than NPH, although the subsequent dissolution may contribute to variability in its duration of action (Citation51). Like glargine, detemir also has a relatively peakless action profile (Citation21). In a study that included 475 patients with hyperglycemia, detemir was found to be as effective as NPH in lowering A1C levels. Moreover, treatment with detemir was associated with significantly less weight gain (1.2 versus 2.8 kg, P < 0.001) and a 47% reduction in the risk of hypoglycemia (Citation52). Results from other studies support the findings of weight loss and decreased incidence of hypoglycemia associated with detemir therapy (Citation53,Citation54). The peakless pharmacokinetic action profiles of glargine and detemir are highly desirable for basal insulin coverage because this is associated with a decreased risk of hypoglycemia. For example, the rates of hypoglycemic events per patient per year decreased significantly from baseline among patients transitioned to once-daily or add-on therapy with insulin detemir for 6 months (from 9.05 to 6.44 events per patient per year, P = 0.0039) (Citation55).
Pooled data from two randomized, parallel-group trials were analyzed to determine if detemir's effects on body mass index (BMI) were advantageous as part of a basal–bolus regimen compared with NPH insulin. The trials took place over 22 and 24 weeks, respectively, and included 900 patients with T2DM treated with insulin that was intensified to either once- or twice-daily insulin detemir or NPH insulin in conjunction with insulin aspart or regular human insulin at meal-times (Citation53). Patients treated with insulin detemir had minimal weight gain (mean < 1 kg), regardless of their BMI at baseline, while patients treated with NPH insulin gained weight (estimated slope 0.075, P = 0.025). Notably, NPH insulin-treated patients with a BMI > 35 kg/m2 at baseline gained the most weight (mean of ∼2.4 kg), but insulin detemir-treated patients with a comparable BMI lost a mean of 0.5 kg (Citation53).
Although insulin glargine was initially recommended for once-daily dosing at bedtime, revised recommendations state that insulin glargine may be administered once daily at the same time every day. Morning administration of insulin glargine is associated with reduced nocturnal hypoglycemia and similar or improved A1C lowering when compared with bedtime insulin glargine or evening NPH (Citation56,Citation57). Because the long-acting insulin doses are typically dosed once daily with the potential for large doses of insulin (> 100 units), poor absorption, decreased efficacy, or lipodystrophy may occur. Alternating injection sites, splitting the dose into two separate sites, or twice daily administration may be beneficial (Citation57).
Rapid acting/bolus insulin analog therapy
Insulin analogs are manufactured synthetically with modifications to the amino-acid sequence in human insulin, with the goal of mimicking normal endogenous insulin secretion and action as closely as possible. The pharmacokinetic and pharmacodynamic properties of the rapid-acting insulin analogs are preferable to RHI because they exhibit faster absorption rates, more rapid onset, and a shorter duration of action () (Citation26). These properties result in better glycemic control with improved reductions in postprandial glucose excursions, especially involving meal-time injections in T2DM patients compared to RHI, thereby reducing the risk of hypoglycemia (Citation21,Citation58,Citation59).
In comparison to RHI, rapid-acting insulin analogs can be administered closer to meal-times or even immediately after a meal (Citation21). For most patients, lispro can be given 15 minutes before or immediately after a meal, while aspart can be given within 5–10 minutes before a meal, and glulisine can be given within 15 minutes before or 20 minutes after a meal, thereby making analog dosing highly convenient and offering greater flexibility to patients (Citation23,Citation25,Citation27). Meal-time dosing allows the patient to vary the amount of insulin injected based upon their SMBG as well as anticipated activity level and carbohydrate intake (Citation7). Rapid-acting insulin analogs have been shown to exhibit less variability between and within patients (Citation24,Citation59,Citation60). Despite this, the onset of action ranges from 5 to 15 minutes and the duration of action from 3 to 6 hours (), and thus therapy must be tailored to the individual patient until experience with the effects of the rapid-acting insulin is known.
Dosage conversion when switching
Initiation of dosage conversion from once-daily NPH to basal insulin analogs is generally done on a direct unit-to-unit basis, while transitioning from twice-daily NPH the dose is reduced by 20% (Citation32,Citation34). However, due to interpatient variability with human insulin and pharmacokinetic differences between human insulins and insulin analogs, the dose of any insulin analog should be adjusted based on the needs of individuals to achieve glycemic targets. In some cases, a higher dose of a rapid-acting insulin analog may be required for some patients transitioning from RHI therapy (Citation25,Citation27). Typically, equivalent doses of rapid-acting insulin analogs result in twice the maximal concentration and take half the time to achieve the maximal concentration as RHI (Citation24).
Based on data from Sreenan et al. (Citation61), when transitioning from NPH to detemir it is generally recommended to start with a dose that is a 20% reduction of the total daily NPH dose and titrate detemir therapy as needed. However, a comparative trial of detemir and NPH insulin administered in the evening revealed that after 20 weeks of treatment the mean doses were similar (37 and 33 units, respectively, or 0.4 units/kg for both) (Citation62). Similar results were observed in a comparative trial of NPH and glargine insulins. In the Treat-to-Target trial (Citation47), patients with T2DM were given NPH or glargine 10 units once daily at bedtime to assess glycemic control and the incidence of hypoglycemia with each regimen; the dose was titrated to meet glycemic goals. Although the dose increased for both drugs, the mean daily dose for glargine was significantly higher than for NPH (47.2 ± 1.3 units versus 41.8 ± 1.3 units, respectively, P < 0.005, between-treatment difference of 5.3 units). Both insulin regimens achieved similar A1C levels; however, glargine was associated with much less symptomatic hypoglycemia. This means that the equivalent success in glycemic control with NPH comes with more risk of hypoglycemia. With respect to dose adjustments, particularly of meal-time bolus insulins, it is very important to avoid insulin stacking. This suggests that the more predictable time action profiles of insulin analogs may help patients avoid insulin stacking, therefore reducing risk for hypoglycemia (Citation24,Citation37).
With regards to bolus insulin, one option for the preprandial (before-meal) insulin dose is to start at 5 units per meal (7% of daily dose of basal insulin) and titrate upward as needed by 2–3 units every 2–3 days based on measurement of the 2-hour postprandial glucose levels and taking into account the before-meal blood glucose level in anticipation of the subsequent meal. The dose should be titrated in order to control effectively the levels of A1C as well as the preprandial and postprandial levels of glycemia (Citation12).
Another option is to calculate the patient's meal-time insulin dose based upon pre-meal blood glucose readings and counting the anticipated carbohydrate content of the meal. This involves determination of the insulin sensitivity factor (or correction factor) and insulin-to-carbohydrate ratio as well as a target blood glucose (Citation63). With the use of insulin aspart, glulisine, or lispro, calculate the patient's total daily dose (TDD) of insulin. Divide 1800 by the TDD to equal the mg/dL drop in blood glucose that one unit of rapid-acting insulin would provide. Using the 500 rule for rapid insulin analogs to calculate the patient's carbohydrate ratio, divide 500 by the TDD to equal the amount of carbohydrates covered by one unit of insulin.
Example: Patient takes 90 units of insulin daily (TDD = 90 units); 1800 divided by 90 equals 20, therefore one unit of insulin decreases the patient's blood glucose 20 mg/dL. So 500 divided by 90 equals 5.6; therefore, one unit of insulin will cover approximately 6 g of carbohydrate. If the target blood glucose is 120 and her SMBG is 220, the patient would need 5 units of insulin to bring it down to 120 mg/dL. If the patient anticipated eating 36 g of carbohydrates, that would require 6 units of insulin, which means the patient would need 11 units of rapid-acting insulin for this meal.
Self-titration by patients may produce better efficacy in insulin therapy
Several treatment algorithms have been developed for insulin detemir and insulin glargine () (Citation39,Citation47,Citation55,Citation64–66). In the 26-week PREDICTIVE 303 Study, more than 5,600 patients with T2DM were randomized to detemir therapy dosed using the 303 algorithm or standard of care (doses adjusted by investigators according to standard of care practice) (Citation55). Patients were enrolled if they were likely to benefit from: 1) initiation of insulin detemir, 2) addition of insulin detemir to other glucose-lowering therapy, 3) a change from another glucose-lowering therapy, including insulin or an OAD, to insulin detemir, or 4) continuation of insulin detemir (Citation55). Patients adjusted their doses of insulin detemir every 3 days based on the mean of three adjusted FPG values calculated from capillary blood glucose calibrated to equivalent plasma glucose values. For half the patients, detemir doses were determined by using a simple algorithm as follows: reduce insulin dose by 3 units when mean adjusted FPG < 80 mg/dL (< 4.4 mmol/L); no change when adjusted FPG 80–110 mg/dL (4.4–6.1 mmol/L); and increase dose by 3 units when adjusted FPG > 110 mg/dL (> 6.1 mmol/L). Dose adjustments for the other half of patients were made by the investigators. At baseline mean A1C levels were 8.5%, but after 26 weeks of treatment with insulin detemir they decreased to 7.9% in the 303 algorithm group (–0.6% change from baseline, P < 0.0001) and decreased from 8.5% to 8.0% in the standard of care group (–0.5% change from baseline, P < 0.0001). The difference in A1C reduction between the two groups had a P-value of 0.0106. FPG decreased from 175 mg/dL (9.7 mmol/L) at baseline to 141 mg/dL (7.8 mmol/L) at the end of the study (P < 0.0001) among the 303 algorithm group, compared to 174 mg/dL (9.7 mmol/L) to 152 mg/dL (8.4 mmol/L) for the standard of care group (P < 0.0001 versus baseline). The 303 algorithm titration group had a significantly greater FPG reduction at week 26 than the standard of care group (least squares mean –32 mg/dL (1.8 mmol/L) versus –21 mg/dL (1.2 mmol/L), respectively, P < 0.0001) (Citation55).
Table III. Treatment algorithms for insulin glargine and insulin detemir for patients with T2DM.
In the 20-week TITRATE trial, the effectiveness of patient-directed titration of once-daily detemir based on an algorithm targeting FPG was also successfully demonstrated when subjects self-titrated insulin doses every 3 days according to self-measured FPG values according to the methods described in the TITRATE study () (Citation66).
Table IV. Insulin detemir titration algorithm used in the TITRATE trial.
The AT.LANTUS study included 4,961 patients with T2DM whose doses of insulin glargine were either self-titrated every 3 days or titrated by the investigators at every visit () (Citation64). At the end of that 24-week trial, patients in the self-titrating group had better glycemic control (A1C –1.22%) than those whose doses were adjusted by the investigators (–1.08%, P < 0.001), and significantly greater reductions in FPG (–62 mg/dL (–3.4 mmol/L) versus –57 mg/dL (–3.2 mmol/L), P < 0.001). In addition, although there was a significantly greater increase in the mean dose of insulin among the self-titrating (21.6 units) than the investigator-titrating (18.7 units) group (P < 0.003), there was no significant between-group difference in the incidence of severe hypoglycemia.
Table V. Summary of the two treatment algorithms for glargine in the AT.LANTUS trial.
Potential objectives for future clinical trials on insulin safety
Although the potential risk of cancer among patients treated with insulin glargine has recently generated concern (Citation67), currently there is no evidence to confirm that insulin analogs are associated with a greater risk of cancer than human insulin. As a result, unless long-term, randomized, prospective trials confirm there is a correlation between specific types of cancer and use of insulin analogs, there is no reason to stop such treatment that provides advantages over human insulin, including a reduction in the risk of hypoglycemia (Citation12). Another topic that has not received sufficient attention in clinical trials is the potential for increased risk of complications among patients treated with analogs compared to human insulin. Patients with T2DM often have multiple metabolic abnormalities in addition to hyperglycemia, including dyslipidemia, insulin resistance, and prothombotic/proinflammatory state, and currently there is controversy surrounding an increased risk of coronary heart disease among these patients (Citation68–72). Some studies have shown that improved glucose control with either human insulin or insulin analogs may reduce cardiovascular risk factors (Citation73–75). Therefore, it should be determined if the risk for cardiovascular disease, which is the leading cause of death among patients with T2DM, is greater for patients treated with analogs, especially since such assessments have been conducted for other diabetes treatments, based on head-to-head comparisons of human insulin with insulin analogs.
Conclusions
ADA, EASD, AACE, and ACE have published T2DM treatment guidelines recommending the use of insulin analogs to achieve glycemic goals, particularly for patients who are unable to meet target A1C levels with OAD therapy. AACE/ACE specifically recommends against the use of RHI and NPH insulin. Treatment with insulin analogs is relatively easy to initiate, adjust, and intensify according to the needs of patients and potentially reduces the risk of hypoglycemia compared with RHI and NPH insulin. For patients on NPH insulin, conversion to a long-acting insulin analog improves glycemic control, and self-titration of doses has the potential to provide further improvements without increasing the risk for hypoglycemia.
Acknowledgements
The author would like to thank Aric Fader, PhD, of MedVal Scientific Information Services, LLC, for providing writing and editorial assistance. This manuscript was prepared according to the International Society for Medical Publication Professionals’ Good Publication Practice for Communicating Company-Sponsored Medical Research: the GPP2 Guidelines. Funding to support the preparation of this manuscript was provided by Novo Nordisk Inc.
Declaration of interest: The author is a Certified Insulin Pump Trainer for Medtronic.
References
- Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14.
- Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Available at: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf (accessed 22 March 2011).
- Narayan KM, Boyle JP, Geiss LS, Saaddine JB, Thompson TJ. Impact of recent increase in incidence on future diabetes burden: U.S., 2005–2050. Diabetes Care. 2006;29:2114–6.
- American Diabetes Association. Economic costs of diabetes in the U.S. in 2007. Diabetes Care. 2008;31:596–615.
- Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, . Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy. A consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203.
- American Association of Clinical Endocrinologists. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract. 2007;13(suppl 1):1–68.
- American Diabetes Association. Standards of medical care in diabetes—2012. Diabetes Care. 2012;35(suppl 1):S11–63.
- Campbell RK. Clarifying the role of incretin-based therapies in the treatment of type 2 diabetes mellitus. Clin Ther. 2011;33:511–27.
- DeFronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773–95.
- Wajchenberg BL. β-cell failure in diabetes and preservation by clinical treatment. Endocr Rev. 2007;28:187–218.
- Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). JAMA. 1999;281:2005–12.
- Rodbard HW, Jellinger PS, Davidson JA, Einhorn D, Garber AJ, Grunberger G, . Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract. 2009;15:540–59.
- Ziemer DC, Miller CD, Rhee MK, Doyle JP, Watkins C Jr, Cook CB, . Clinical inertia contributes to poor diabetes control in a primary care setting. Diabetes Educ. 2005;31: 564–71.
- DeSouza C, Salazar H, Cheong B, Murgo J, Fonseca V. Association of hypoglycemia and cardiac ischemia: a study based on continuous monitoring. Diabetes Care. 2003;26: 1485–9.
- Desouza CV, Bolli GB, Fonseca V. Hypoglycemia, diabetes, and cardiovascular events. Diabetes Care. 2010;33:1389–94.
- Skyler JS, Bergenstal R, Bonow RO, Buse J, Deedwania P, Gale EA, . Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA Diabetes Trials. A position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Circulation. 2009;119: 351–7.
- Riddle MC, Ambrosius WT, Brillon DJ, Buse JB, Byington RP, Cohen RM, . Epidemiologic relationships between A1C and all-cause mortality during a median 3.4-year follow-up of glycemic treatment in the ACCORD trial. Diabetes Care. 2010;33:983–90.
- Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB, . Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–59.
- Gerstein HC, Miller ME, Genuth S, Ismail-Beigi F, Buse JB, Goff DC Jr, . Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med. 2011;364:818–28.
- Dailey G. Overall mortality in diabetes mellitus: where do we stand today? Diabetes Technol Ther. 2011;13(suppl 1):S65–74.
- Vazquez-Carrera M, Silvestre JS. Insulin analogues in the management of diabetes. Methods Find Exp Clin Pharmacol. 2004;26:445–61.
- Polonsky KS, Given BD, van Cauter E. Twenty-four-hour profiles and pulsatile patterns of insulin secretion in normal and obese subjects. J Clin Invest. 1988;81:442–8.
- NovoLog® (insulin aspart [rDNA origin] injection) [prescribing information]. Princeton, NJ: Novo Nordisk Inc.; July 2011.
- Hirsch IB. Insulin analogues. N Engl J Med. 2005;352: 174–83.
- Apidra® (insulin glulisine [rDNA origin] injection) [prescribing information]. Bridgewater, NJ: sanofi-aventis U.S. LLC; February 2009.
- Levy P. Insulin analogs or premixed insulin analogs in combination with oral agents for treatment of type 2 diabetes. Med Gen Med. 2007;9:12.
- Humalog® (insulin lispro [rDNA origin] injection) [prescribing information]. Indianapolis, IN: Eli Lilly and Company; 18 May 2011.
- Humulin® R (regular insulin human injection [rDNA origin]) 100 units per mL (U-100) [prescribing information]. Indianapolis, IN: Eli Lilly and Company; 25 March 2011.
- Novolin® R (regular human insulin [rDNA origin]) [prescribing information]. Princeton, NJ: Novo Nordisk Inc.; 14 May 2010.
- Humulin® N (NPH human insulin [rDNA origin]) [prescribing information]. Indianapolis, IN: Eli Lilly and Company; 2 September 2009.
- Novolin® N (NPH human insulin [rDNA origin]) [prescribing information]. Princeton, NJ: Novo Nordisk Inc.; 14 May 2010.
- Levemir® (insulin detemir [rDNA origin] injection) [prescribing information]. Princeton, NJ: Novo Nordisk, Inc.; January 2012.
- Plank J, Bodenlenz M, Sinner F, Magnes C, Gorzer E, Regittnig W, . A double-blind, randomized, dose-response study investigating the pharmacodynamic and pharmacokinetic properties of the long-acting insulin analog detemir. Diabetes Care. 2005;28:1107–12.
- Lantus® (insulin glargine [rDNA origin] injection) [prescribing information]. Bridgewater, NJ: Sanofi-Aventis US, LLC. April 2010.
- Cupp M. Comparison of insulins and injectable diabetes meds. Pharmacist's Letter/Prescriber's Letter. 2010;26:260304.
- Pearson TL. Practical aspects of insulin pen devices. J Diabetes Sci Technol. 2010;4:522–31.
- DeWitt DE, Hirsch IB. Outpatient insulin therapy in type 1 and type 2 diabetes mellitus: scientific review. JAMA. 2003;289:2254–64.
- Hall DL, Drab SR, Havrilla PL. Advances in diabetes therapy: rapid- and long-acting insulin analogs. Drug Topics. 2006;150:e1–15.
- Arnolds S, Kuglin B, Kapitza C, Heise T. How pharmacokinetic and pharmacodynamic principles pave the way for optimal basal insulin therapy in type 2 diabetes. Int J Clin Pract. 2010;64:1415–34.
- Hompesch M, Troupin B, Heise T, Elbroend B, Endahl L, Haahr H, . Time-action profile of insulin detemir and NPH insulin in patients with type 2 diabetes from different ethnic groups. Diabetes Obes Metab. 2006;8:568–73.
- Klein O, Lynge J, Endahl L, Damholt B, Nosek L, Heise T. Albumin-bound basal insulin analogues (insulin detemir and NN344): comparable time-action profiles but less variability than insulin glargine in type 2 diabetes. Diabetes Obes Metab. 2007;9:290–9.
- Bolli GB, Owens DR. Insulin glargine. Lancet. 2000;356: 443–5.
- Meneghini L, Liebl A, Abrahamson MJ. Insulin detemir: a historical perspective on a modern basal insulin analogue. Prim Care Diabetes. 2010;4(suppl 1):S31–42.
- Porcellati F, Rossetti P, Ricci BN, Marzotti S, Lucidi P, Luzio S, . Comparison of pharmacokinetics and dynamics of the long-acting insulin analogs glargine and detemir at steady state in type 1 diabetes mellitus: a double-blind, randomized, cross-over study. Diabetes Care. 2007; 30:2447–51.
- Rosenstock J, Davies M, Home PD, Larsen J, Koenen C, Schernthaner G. A randomised, 52-week, treat-to-target trial comparing insulin detemir with insulin glargine when administered as add-on to glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetologia. 2008;51: 408–16.
- Swinnen SG, Dain MP, Aronson R, Davies M, Gerstein HC, Pfeiffer AF, . A 24-week, randomized, treat-to-target trial comparing initiation of insulin glargine once-daily with insulin detemir twice-daily in patients with type 2 diabetes inadequately controlled on oral glucose-lowering drugs. Diabetes Care. 2010;33:1176–8.
- Riddle MC, Rosenstock J, Gerich J. The Treat-to-Target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26:3080–6.
- Raskin P, Gylvin T, Weng W, Chaykin L. Comparison of insulin detemir and insulin glargine using a basal-bolus regimen in a randomized, controlled clinical study in patients with type 2 diabetes. Diabetes Metab Res Rev. 2009;25:542–8.
- Fonseca V, Davidson J, Home P, Snyder J, Jellinger P, Dyhr TA, . Starting insulin therapy with basal insulin analog or premix insulin analog in T2DM: a pooled analysis of treat-to-target trials. Curr Med Res Opin. 2010;26:1621–8.
- Morales J. Defining the role of insulin detemir in basal insulin therapy. Drugs. 2007;67:2557–84.
- Lepore M, Pampanelli S, Fanelli C, Porcellati F, Bartocci L, Di Vincenzo A, . Pharmacokinetics and pharmacodynamics of subcutaneous injection of long-acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes. 2000;49:2142–8.
- Hermansen K, Davies M, Derezinski T, Ravn GM, Clauson P, Home P. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetes Care. 2006;29:1269–74.
- Raslova K, Tamer SC, Clauson P, Karl D. Insulin detemir results in less weight gain than NPH insulin when used in basal-bolus therapy for type 2 diabetes mellitus, and this advantage increases with baseline body mass index. Clin Drug Invest. 2007;27:279–85.
- Meneghini LF, Rosenberg KH, Koenen C, Merilainen MJ, Luddeke H-J. Insulin detemir improves glycaemic control with less hypoglycaemia and no weight gain in patients with type 2 diabetes who were insulin naive or treated with NPH or insulin glargine: clinical practice experience from a German subgroup of the PREDICTIVE study. Diabetes Obes Metab. 2007;9:418–27.
- Meneghini L, Koenen C, Weng W, Selam JL. The usage of a simplified self-titration dosing guideline (303 algorithm) for insulin detemir in patients with type 2 diabetes—results of the randomized, controlled PREDICTIVE™ 303 study. Diabetes Obes Metab. 2007;9:902–13.
- Hamann A, Matthaei S, Rosak C, Silvestre L. A randomized clinical trial comparing breakfast, dinner, or bedtime administration of insulin glargine in patients with type 1 diabetes. Diabetes Care. 2003;26:1738–44.
- Fritsche A, Schweitzer MA, Haring HU. Glimepiride combined with morning insulin glargine, bedtime neutral protamine hagedorn insulin, or bedtime insulin glargine in patients with type 2 diabetes. A randomized, controlled trial. Ann Intern Med. 2003;138:952–9.
- Rosenfalck AM, Thorsby P, Kjems L, Birkeland K, Dejgaard A, Hanssen KF, . Improved postprandial glycaemic control with insulin aspart in type 2 diabetic patients treated with insulin. Acta Diabetol. 2000;37:41–6.
- Mudaliar SR, Lindberg FA, Joyce M, Beerdsen P, Strange P, Lin A, . Insulin aspart (B28 asp-insulin): a fast-acting analog of human insulin: absorption kinetics and action profile compared with regular human insulin in healthy nondiabetic subjects. Diabetes Care. 1999;22:1501–6.
- Howey DC, Bowsher RR, Brunelle RL, Woodworth JR. [Lys(B28), Pro(B29)]-human insulin. A rapidly absorbed analogue of human insulin. Diabetes. 1994;43:396–402.
- Sreenan S, Virkamaki A, Zhang K, Hansen JB. Switching from NPH insulin to once-daily insulin detemir in basal-bolus-treated patients with diabetes mellitus: data from the European cohort of the PREDICTIVE study. Int J Clin Pract. 2008;62:1971–80.
- Philis-Tsimikas A, Charpentier G, Clauson P, Martinez Ravn G, Roberts VL, Thorsteinsson B. Comparison of once-daily insulin detemir with NPH insulin added to a regimen of oral antidiabetic drugs in poorly controlled type 2 diabetes. Clin Ther. 2006;28:1569–81.
- Diabetes Education Online. Calculating insulin dose. Available at: http://dtc.ucsf.edu/types-of-diabetes/type2/treatment-of-type-2-diabetes/medications-and-therapies/type-2-insulin- rx/calculating-insulin-dose/ (accessed 20 February 2012).
- Davies M, Storms F, Shutler S, Bianchi-Biscay M, Gomis R. Improvement of glycemic control in subjects with poorly controlled type 2 diabetes: comparison of two treatment algorithms using insulin glargine. Diabetes Care. 2005;28: 1282–8.
- Yki-Jarvinen H, Kauppinen-Makelin R, Tiikkainen M, Vahatalo M, Virtamo H, Nikkila K, . Insulin glargine or NPH combined with metformin in type 2 diabetes: the LANMET study. Diabetologia. 2006;49:442–51.
- Blonde L, Merilainen M, Karwe V, Raskin P. Patient-directed titration for achieving glycaemic goals using a once-daily basal insulin analogue: an assessment of two different fasting plasma glucose targets—the TITRATE study. Diabetes Obes Metab. 2009;11:623–31.
- McFarland MS, Cripps R. Diabetes mellitus and increased risk of cancer: focus on metformin and the insulin analogs. Pharmacotherapy. 2010;30:1159–78.
- Buyken AE, von Eckardstein A, Schulte H, Cullen P, Assmann G. Type 2 diabetes mellitus and risk of coronary heart disease: results of the 10-year follow-up of the PROCAM study. Eur J Cardiovasc Prev Rehabil. 2007;14:230–6.
- National Cholesterol Education Program Expert Panel on Detection EAToHBCIA. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–97.
- Juutilainen A, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Type 2 diabetes as a “coronary heart disease equivalent”: an 18-year prospective population-based study in Finnish subjects. Diabetes Care. 2005;28:2901–7.
- Whiteley L, Padmanabhan S, Hole D, Isles C. Should diabetes be considered a coronary heart disease risk equivalent? Results from 25 years of follow-up in the Renfrew and Paisley survey. Diabetes Care. 2005;28:1588–93.
- Opie LH, Yellon DM, Gersh BJ. Controversies in the cardiovascular management of type 2 diabetes. Heart. 2011;97: 6–14.
- Gerstein HC, Riddle MC, Kendall DM, Cohen RM, Goland R, Feinglos MN, . Glycemia treatment strategies in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol. 2007;99:34i–43i.
- Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, . Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009; 360:129–39.
- Eeg-Olofsson K, Cederholm J, Nilsson PM, Zethelius B, Svensson AM, Gudbjornsdottir S, . New aspects of HbA1c as a risk factor for cardiovascular diseases in type 2 diabetes: an observational study from the Swedish National Diabetes Register (NDR). J Intern Med. 2010;268: 471–82. Hirsch IB, Bergenstal RM, Parkin CG, et al. A real-world approach to insulin therapy in primary care practice. Clinical Diabetes. 2005;23:78–86.
- Hirsch IB, Bergenstal RM, Parkin CG, . A real-world approach to insulin therapy in primary care practice. Clinical Diabetes. 2005;23:78–86.