Abstract
A number of target-specific oral anticoagulants (TSOAs) have been developed in recent years, and some have shown considerable promise in large-scale, randomized clinical trials in the prevention and treatment of thromboembolism. Unlike traditional anticoagulants, such as vitamin K antagonists, these TSOAs exhibit predictable pharmacokinetics and pharmacodynamics. Among these agents, rivaroxaban, a direct Factor Xa inhibitor, has been approved for clinical use in many countries for the management of several thromboembolic disorders. As with the other TSOAs, rivaroxaban is given at fixed doses without routine coagulation monitoring. However, in certain patient populations or special clinical circumstances, measurement of drug exposure may be useful, such as in suspected overdose, in patients with a haemorrhagic or thromboembolic event during treatment with an anticoagulant, in those with acute renal failure, or in patients who require urgent surgery. This article summarizes the influence of rivaroxaban on commonly used coagulation assays and provides practical guidance on laboratory testing of rivaroxaban in routine practice. Both quantitative measurement (using the anti-Factor Xa method) and qualitative measurement (using prothrombin time, expressed in seconds) are discussed, together with some practical considerations when performing these tests and interpreting the test results.
Key words::
Key messages
Results of the prothrombin time (PT) assay vary markedly with different reagents; the conventional international normalized ratio does not correct for the variations and must not be used for rivaroxaban. Normal values of PT measured in seconds using a reagent sensitive to rivaroxaban may indicate no clinically relevant residual anticoagulant effect of rivaroxaban.
Anti-Factor Xa chromogenic assays are specific and sensitive for quantitative measurements of rivaroxaban exposure when used in conjunction with rivaroxaban calibrators; the results should be expressed as rivaroxaban concentrations.
For both qualitative and quantitative measurements the results need to be interpreted in relation to the timing of tablet intake in accordance with the pharmacokinetic profile of rivaroxaban.
Introduction
Thromboembolic disorders remain one of the major causes of morbidity and mortality, and antithrombotic therapy has been the cornerstone for the management of these disorders. Vitamin K antagonists (VKAs), unfractionated heparin (UFH), low-molecular-weight heparins (LMWHs), and fondaparinux are widely used anticoagulants. Parenterally administered UFH, LMWH, and fondaparinux are commonly used in the short-term prevention and treatment of thromboembolism; oral VKAs are used for long-term anticoagulation in various indications. All these agents have an indirect mode of action, either by requiring co-factors such as antithrombin in the case of the heparins and fondaparinux, or by interfering with the hepatic synthesis of vitamin K-dependent coagulation factors for the VKAs (Citation1,Citation2). However, these agents are associated with limitations, including the parenteral route of administration (Citation1), or the need for routine coagulation monitoring and dose titration (Citation2). In an attempt to overcome some of the limitations associated with the traditional anticoagulants, target-specific oral anticoagulants (TSOAs) have been developed. The TSOAs directly target a single key factor of the coagulation cascade (i.e. Factor Xa or thrombin), without the need for any co-factors.
Among these TSOAs, the direct Factor Xa inhibitors rivaroxaban and apixaban and the direct thrombin inhibitor dabigatran etexilate (hereafter referred to as dabigatran), are the most investigated in large-scale phase III trials and have been licensed for use in specific indications. Rivaroxaban is approved in many countries for the prevention of venous thromboembolism (VTE) after elective hip or knee replacement surgery; treatment of deep vein thrombosis (DVT) and/or pulmonary embolism (PE), prevention of recurrent DVT and PE; and for the prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation (AF). In addition, the European Medicines Agency's Committee for Medicinal Products for Human Use has recommended that rivaroxaban, combined with acetylsalicylic acid with or without clopidogrel (or ticlopidine), be approved for the prevention of atherothrombotic events in adult patients with acute coronary syndrome who have elevated cardiac biomarkers.
As with the parenteral anticoagulants (such as LMWH), rivaroxaban has a fast onset of action. After oral administration, it is absorbed rapidly, reaching maximum plasma concentration at 2–4 hours after tablet intake. The half-life of rivaroxaban (5–13 hours) (Citation3,Citation4) is much shorter than that of the VKAs (Citation2). In human plasma, rivaroxaban inhibits free Factor Xa and prothrombinase activity as well as clot-bound Factor Xa, thus effectively blocking thrombin generation (Citation5,Citation6). Rivaroxaban does not directly affect the activity of platelets but potently inhibits tissue-factor-induced platelet aggregation indirectly via the inhibition of thrombin generation (Citation5,Citation7). Inhibition of Factor Xa activity by rivaroxaban is closely correlated to its plasma concentration (Citation3). Owing to its predictable pharmacokinetics and pharmacodynamics, rivaroxaban does not require routine coagulation or drug monitoring and is given at fixed doses. However, laboratory testing of rivaroxaban plasma concentrations may be of assistance in specific situations, such as in suspected overdose, in patients with a haemorrhagic or thromboembolic event during treatment with rivaroxaban, in patients with acute renal failure, or in patients who require urgent surgery ().
Table I. Guidance on laboratory assays to measure rivaroxaban in plasma.
Although physicians are familiar with laboratory monitoring of the VKAs, there is a need for practical information on laboratory testing of the TSOAs, including rivaroxaban. The aim of this article is to provide guidance on the laboratory tests available in routine clinical practice that may be used for measuring rivaroxaban directly or indirectly, and to outline some important considerations when performing such laboratory tests.
Prothrombin time
The prothrombin time (PT) assay is a readily available and widely used global clotting test that can be used to measure the integrity of the extrinsic and final common pathways of coagulation. Inhibitors or deficiencies of clotting factors within these pathways cause prolongation of PT (Citation8). PT is measured in seconds and is most commonly used to monitor VKAs, with the result expressed as an international normalized ratio (INR). Because commercially available PT reagents (thromboplastins) vary in their sensitivity to the anticoagulant effects of VKAs, the INR monitoring system was developed and has been used since the 1980s to adjust for the different sensitivities of the thromboplastins used in a given laboratory. Physicians are familiar with INR monitoring of patients who are receiving VKA therapy, such as for stroke prevention in patients with AF or for prevention of recurrent VTE (Citation2,Citation9,Citation10), and INR testing in these patients is essential to ensure the appropriate anticoagulation intensity of VKA therapy.
During VKA therapy, the patient's INR remains stable throughout the day because of the long half-life of the drugs (e.g. warfarin, ˜2 days; phenprocoumon, 5–7 days) (Citation2,Citation11). The INR reflects the indirect, long-lasting effects of the VKA on the decarboxylation of vitamin K-dependent coagulation factors and, therefore, does not directly correlate with the pharmacokinetics of the VKA in question.
Because of its mode of action, rivaroxaban also prolongs PT when reagents that are sensitive to rivaroxaban are used, such as Neoplastin® CI Plus (Diagnostica Stago, Asnières-sur-Seine, France), and RecombiPlasTin (Instrumentation Laboratory, Bedford, MA, USA) (Citation12). In contrast to the VKAs, these prolongations directly correlate with the pharmacokinetics of the drug, and, because of the relatively short half-life of rivaroxaban, PT prolongation is short-lived and changes during the course of the day. For example, rivaroxaban has no significant influence on PT 24 hours after tablet intake (Citation3,Citation13).
The PT assay (expressed in seconds) using the reagent Neoplastin CI Plus is influenced by rivaroxaban in a dose-dependent manner, closely correlating to plasma rivaroxaban concentration (Citation3). However, PT varies markedly with different thromboplastins because of their different response sensitivities to rivaroxaban (Citation12,Citation14,Citation15). For example, in plasma samples from patients receiving rivaroxaban for the prevention of VTE in routine clinical practice, PT prolongation was greatest when Neoplastin CI Plus reagents were used for the assay, whereas Innovin® (Siemens HealthCare Diagnostics, Marburg, Germany) showed the least sensitivity to rivaroxaban () (Citation13). The conventional INR system originally designed and still used for monitoring VKAs does not correct for these variations (Citation12,Citation14). Therefore, INR values of the same plasma sample from patients treated with rivaroxaban will differ widely depending on PT reagent used. Moreover, the values change during the day depending on the time of tablet intake in relation to blood sampling time (). This phenomenon has also been observed with other direct Factor Xa inhibitors such as apixaban (Citation14). In plasma samples spiked with apixaban and other Factor Xa inhibitors (including rivaroxaban), to achieve a doubling of PT, the concentration of each Factor Xa inhibitor varied 2.6- to 8-fold depending on the thromboplastin reagent used, and conversion of the PT result to INR increased the variability instead of decreasing it (Citation14).
Figure 1. Prothrombin time (PT) measured A: in seconds (Citation13); and B: as INR (Lindhoff-Last, unpublished data) in patients undergoing hip or knee replacement surgery taking rivaroxaban 10 mg once daily. Results are median values (n = 47). INR = international normalized ratio.
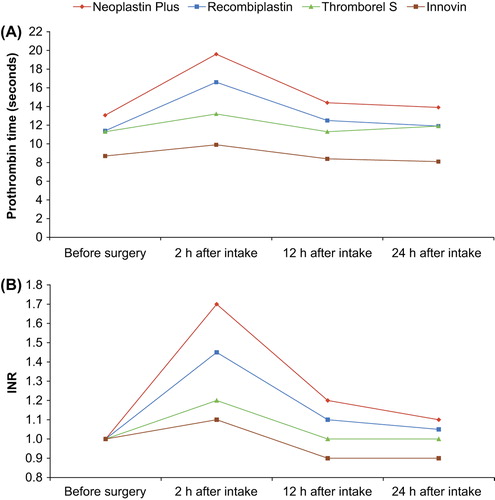
In addition to the variability between reagents, the PT assay is a global clotting test that can be influenced by a number of factors, including hepatic impairment, sepsis, acute trauma with significant blood loss, and vitamin K deficiency (Citation16). Therefore, baseline PT values of patients without anticoagulation differ widely, and prolongation of PT during treatment with rivaroxaban depends not only on the concentration of the drug but also on the clinical situation of the patient.
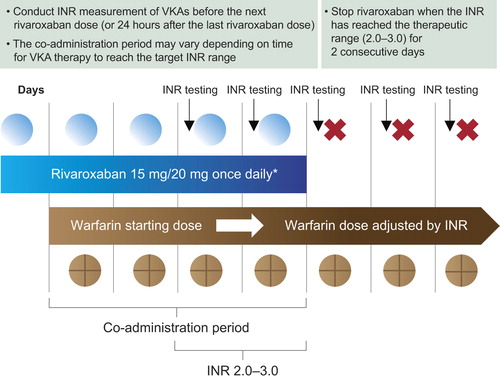
Rivaroxaban is now available for long-term anticoagulation therapy, such as for stroke prevention in patients with AF, and for the treatment of DVT and/or PE (Citation17). Therefore, some patients who require long-term anticoagulation and receive warfarin may be transitioned to rivaroxaban therapy. Because both drugs affect the PT/INR, understanding of INR levels during the transitioning period is crucial to ensure adequate anticoagulation. After therapy transition from VKAs (e.g. warfarin, at an INR level of 2.0–3.0) to rivaroxaban, INR values will be elevated (over-additive effect) in the first few days after transitioning. This is because the residual effects of the VKA will still exist, particularly at peak rivaroxaban plasma levels (e.g. 2–4 hours after dosing measured with Neoplastin CI Plus reagents; Kubitza et al. unpublished data). However, this does not reflect the anticoagulation intensity of rivaroxaban, so INR testing should be stopped when rivaroxaban is initiated. Rivaroxaban has minimal influence on PT/INR at trough plasma concentrations (i.e. 24 hours after dosing; ), which suggests that if transitioning from rivaroxaban to a VKA (e.g. warfarin) is required under certain circumstances, INR monitoring of the warfarin effect during the co-administration period should be performed at the trough concentration of rivaroxaban, to avoid any influence of rivaroxaban on the INR (Citation17), and rivaroxaban can be discontinued when the target INR level is achieved. This co-administration period is vital to ensure an adequate level of anticoagulation during transitioning from rivaroxaban to warfarin and other VKAs because of their slow onset of action (). Because rivaroxaban has a rapid onset of action, if transitioning from a parenteral anticoagulant to rivaroxaban is indicated, rivaroxaban should be initiated at the time of the next scheduled dose of the parenteral anticoagulant, such as 12 or 24 hours after the last dose of subcutaneous LMWH (Citation18), or at the time of discontinuation of continuous intravenously administered UFH. Conversely, if patients receiving rivaroxaban require switching to a parenteral anticoagulant, the first dose of parenteral anticoagulant should be administered when the next rivaroxaban dose is due.
Figure 2. Transitioning from rivaroxaban to a vitamin K antagonist (VKA). *Approved rivaroxaban dose for treatment of deep vein thrombosis (DVT), and prevention of recurrent DVT and pulmonary embolism following an acute DVT, and for the prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation. INR = international normalized ratio.
Activated partial thromboplastin time
The activated partial thromboplastin time (aPTT) assay measures fibrin clot formation, typically via activation of the intrinsic pathway of the coagulation cascade, and the results are expressed in seconds. Inhibitors or deficiencies of clotting factors within this pathway result in the prolongation of aPTT (Citation16). Because rivaroxaban directly inhibits Factor Xa activity (and consequently thrombin generation), it prolongs the aPTT clotting time; however, rivaroxaban has a weaker effect on aPTT than on PT. Similarly to PT, the prolongation of aPTT induced by rivaroxaban is short-lived and varies significantly among different aPTT reagents (Citation13,Citation15,Citation16). Therefore, aPTT is not a suitable assay for measuring rivaroxaban. However, aPTT measurement may provide a qualitative indication of the anticoagulant activity of dabigatran (Citation19), although the assay has limited sensitivity and is not suitable for precise quantification of the anticoagulant effect of dabigatran (Citation20,Citation21).
Thrombin time
Thrombin time (TT) is a clotting assay that specifically measures the time it takes for a clot to form in the plasma after an excess of thrombin has been added. The TT assay is most useful as a sensitive method to assess the effects of thrombin inhibitors (Citation19). The Hemoclot® Thrombin Inhibitor assay (Hyphen BioMed, Neuville-sur-Oise, France) is a sensitive diluted TT assay that allows for quantitative measurement of direct thrombin inhibitor activity in plasma and can be used for the direct thrombin inhibitors, including dabigatran.
Because rivaroxaban does not directly affect the activity of thrombin, TT is not influenced by rivaroxaban. In plasma samples of patients receiving rivaroxaban for VTE prevention after elective hip or knee surgery, no significant effect of rivaroxaban on TT was found (Citation13). Therefore, although this test is suitable for measuring dabigatran (Citation19), it should not be used for rivaroxaban.
Anti-Factor Xa chromogenic assays
Rivaroxaban directly inhibits Factor Xa activity, and the inhibition is closely correlated with rivaroxaban plasma concentrations (Citation3). Data from in vitro studies using human plasma showed that anti-Factor Xa chromogenic assays, when used with rivaroxaban calibrators and controls, are able specifically to measure a wide range of rivaroxaban plasma concentrations (i.e. 20–660 μg/L), covering the expected levels after therapeutic dosing () (Citation22). It has been shown recently that, in plasma samples of patients receiving rivaroxaban for VTE prevention after elective hip or knee surgery, rivaroxaban plasma levels could be measured accurately using anti-Factor Xa chromogenic assays in conjunction with rivaroxaban calibrators at different concentrations, compared with the concentrations measured by high-performance liquid chromatography–tandem mass spectrometry (HPLC MS/MS) (Citation23).
Table II. Plasma concentrations of rivaroxaban at steady state in different patient populations.
In addition, compared with clot-based assays (such as PT), anti-Factor Xa chromogenic assays are less sensitive to sample collection conditions and variations in the amount of intrinsic clotting factors among patients, because of pre-dilution of the plasma samples (Citation24). However, addition of antithrombin should be avoided because it causes falsely high measured concentrations (Citation23). A rapid assay has been developed that is specific for direct Factor Xa inhibitors without interference from plasma factors and indirect Factor Xa inhibitors. The assay is based on inhibition of a constant and excess concentration of human Factor Xa with the use of a high ionic strength pH 7.90 buffer that inhibits the catalytic effect of heparins/fondaparinux (Citation25). Standardized assay kits are now commercially available (e.g. from Technoclone, Hyphen Biomed, and Diagnostica Stago); however, anti-Factor Xa chromogenic assays are not yet routinely used in many hospitals and clinics.
Other assays
Rivaroxaban also prolongs HepTest and prothrombinase-induced clotting time, but these assays are not suitable for measuring rivaroxaban for several reasons, as reported previously (Citation12,Citation16). In addition, rivaroxaban has been shown to affect the various parameters of the thrombin generation test; however, the assay is not suitable for measuring rivaroxaban in routine practice because it does not have the sensitivity for measuring low plasma concentrations of rivaroxaban and it requires a high technical standard and laboratory skills (Citation16).
Practical considerations
Qualitative measurement of rivaroxaban using prothrombin time
Owing to the limitations of the PT assay (Citation16), an absence of PT prolongation does not necessarily mean that the plasma rivaroxaban level is low. For example, this could be caused by the low sensitivity of reagents to rivaroxaban and/or if the blood sample is taken later than 8 hours after rivaroxaban dosing. Importantly, the conventional PT/INR monitoring that is used for VKAs cannot be used for rivaroxaban. In addition, it is not recommended to use point-of-care devices with insensitive reagents (such as Innovin) for assessing levels of anticoagulation with rivaroxaban.
As with other anticoagulants, it may be useful to measure the baseline PT values in patients who require long-term anticoagulation, as well as their hepatic and renal status. Thereafter the PT assay using a reagent sensitive to rivaroxaban (such as Neoplastin CI Plus or RecombiPlasTin) may be used to confirm qualitatively the presence or absence of the anticoagulant effect of rivaroxaban (provided that the baseline PT of the patient is normal), but not to measure rivaroxaban plasma levels. For the measurement of peak activity (i.e. at the time of maximum plasma concentration), blood should be taken 2–4 hours after dosing. However, clinicians should not rely on PT results before commencing invasive procedures or surgery because of the limitations of the PT assay; decisions should be based on clinical assessment of the specific situation.
Quantitative measurement of rivaroxaban using anti-Factor Xa chromogenic assays
Anti-Factor Xa chromogenic assays have been shown to be specific and sensitive for measuring a wide range of rivaroxaban plasma concentrations that cover the expected rivaroxaban concentrations after therapeutic doses. The measurements showed acceptable accuracy and precision (Citation16,Citation26) and are recommended for quantitative measurements of rivaroxaban exposure, using rivaroxaban calibrators with results expressed as μg/L of rivaroxaban. Assessment of rivaroxaban concentrations must be interpreted in relation to the timing of drug administration in accordance with the pharmacokinetic profile of rivaroxaban (e.g. peak and trough levels). For example, in patients undergoing hip replacement surgery, the median rivaroxaban plasma concentration after a 10 mg once daily dose was 125 μg/L at 2–4 hours (peak level) after dosing, but at 24 hours (trough level) it was 9 μg/L (Citation27). In patients receiving rivaroxaban 20 mg once daily for the treatment of acute DVT, the estimated mean peak and trough rivaroxaban concentrations were 270 μg/L and 26 μg/L, respectively () (Citation28). It should be noted that variability in the pharmacokinetics of rivaroxaban was moderate (i.e. 30%–40% coefficient of variation) in the studied populations (Citation27,Citation28); this should be taken into consideration when interpreting test results, together with patient characteristics and the clinical situation.
For the measurement of peak plasma concentration of rivaroxaban, blood samples should be taken 2–4 hours after dosing. Trough levels could be measured to indicate if there is drug accumulation, and blood samples should be taken prior to the next dose of rivaroxaban (or 24 hours after dosing).
Recommendations—prior to urgent surgery or in the event of bleeding complications during therapy
The timing of the last rivaroxaban dose should be confirmed
Plasma rivaroxaban levels could be measured using an anti-Factor Xa assay if available, noting the blood sampling time in relation to the last rivaroxaban tablet and checking the results against the expected levels
If anti-Factor Xa assays are not available in emergency situations, the PT test may be performed using a rivaroxaban-sensitive reagent (e.g. Neoplastin CI Plus or RecombiPlasTin) and the results expressed in seconds (but not as INR); a normal value could indicate that a clinically relevant residual anticoagulant effect of rivaroxaban is unlikely
Potential drug accumulation may be suspected if rivaroxaban plasma concentrations are > 200 μg/L when measured at ≥ 12 hours after the last rivaroxaban tablet
Physicians should assess the urgency of surgery against the risk of bleeding, and delay the surgery till 24 to 48 hours after the last rivaroxaban dose depending on the type of surgery
In case of bleeding events during therapy, a general assessment of the patient's bleeding risk and clinical circumstance should be carried out (including renal and hepatic functions, co-medications), in addition to testing rivaroxaban plasma levels.
Potential influence of rivaroxaban on coagulation function testing
Because of their modes of action, the TSOAs (including rivaroxaban) may influence routine coagulation function testing. Recent studies have shown that the presence of clinically relevant concentrations of rivaroxaban in plasma can interfere with PT- and aPTT-based assays for the measurement of clotting factor activity in plasma, leading to the underestimation of clotting factor activity (Citation29,Citation30). False-positive lupus anticoagulant testing was reported by one participating centre of the phase III EINSTEIN DVT study (Citation31) in patients receiving rivaroxaban for the treatment of acute DVT and prevention of recurrent VTE (Citation32). The influence of rivaroxaban on specific coagulation assays and coagulation factor activities has also been reported recently in an ex vivo study in patients receiving rivaroxaban for VTE prevention in routine clinical practice (Citation33). Clinicians should be aware of the potential influence of rivaroxaban (as of other TSOAs) when performing specific coagulation assays. To minimize the potential influence of rivaroxaban in these situations, blood samples should be taken 24 hours (or longer) after the last rivaroxaban dose.
Discussion and conclusions
As with other TSOAs, rivaroxaban influences global coagulation assays, such as PT and aPTT, and this has been further demonstrated in recent in vitro and ex vivo studies (Citation13,Citation26,Citation29,Citation34). However, these routine coagulation tests do not accurately reflect the circulating levels of rivaroxaban and are not suitable for quantitative assessment of rivaroxaban exposure. The different sensitivities of assay reagents are not specific to rivaroxaban but also occur with other direct Factor Xa inhibitors (such as apixaban and edoxaban) (Citation16,Citation35,Citation36). In contrast, anti-Factor Xa chromogenic assays with substance-specific calibrators are able to provide a more precise, quantitative measurement of the concentrations of direct Factor Xa inhibitors in plasma (Citation14). Moderate interindividual variability in rivaroxaban plasma concentrations has been observed in previous studies (Citation3,Citation37), which should be taken into account when interpreting the test results. A higher than expected plasma level of rivaroxaban does not necessarily indicate an increased risk of bleeding complications. Importantly, when interpreting the test results and assessing bleeding risks prior to urgent major surgery, or when patients experience bleeding complications during rivaroxaban therapy, the patient's renal and hepatic functions, co-morbidities, and co-medications, as well as previous history of bleeding and other relevant clinical information should be considered.
No correlation has been observed in phase II and III studies between rivaroxaban plasma levels (i.e. peak and trough) and risk of bleeding. This might be because blood was not taken during the bleeding event but only at trough or maximum levels of the drug. Prospective studies addressing this issue would be helpful, but would be challenging to accomplish. As with other TSOAs, there is currently no specific antidote for rivaroxaban. In case of a life-threatening bleeding event, prothrombin complex concentrate is recommended as the first-choice haemostatic agent (over activated prothrombin complex concentrate and recombinant Factor VIIa) based on existing information, in addition to other routine support measures (Citation38,Citation39). Further information on the management of bleeding in patients receiving rivaroxaban can be found in a previous publication by Turpie et al. (Citation38).
In summary, it is strongly recommended to use anti-Factor Xa assays for quantitative measurement of rivaroxaban exposure when required (over qualitative assessment with the PT assay). Although assays are available for laboratory testing of rivaroxaban, routine measurement of its anticoagulant effect or plasma concentration are not required or recommended. Any assessment of rivaroxaban exposure should only be carried out in special clinical circumstances. Clinicians should adhere to the recommendations outlined in the labelling for the approved indications for optimal effectiveness and safety in patients with thromboembolic disorders.
Acknowledgements
The authors would like to acknowledge Yong-Ling Liu, who provided editorial support with funding from Bayer HealthCare Pharmaceuticals and Janssen Scientific Affairs, LLC.
Declaration of interest: E. Lindhoff-Last has acted as a consultant and is a member of the national advisory board of Bayer Schering Pharma AG, Boehringer Ingelheim, Daiichi Sankyo; a consultant for Pfizer and a consultant and member of the international advisory board of Instrumentation Laboratory. J. Ansell is a consultant for Bristol-Myers Squibb, Pfizer, Boehringer Ingelheim, Janssen, and Daiichi and serves on the Data Safety Monitoring Board of Bristol-Myers Squibb. T. Spiro is an employee of Bayer HealthCare Pharmaceuticals Inc. M. M. Samama is a consultant for Bayer, sanofi-aventis, Eli Lilly, and Daiichi Sankyo, and is a member of an advisory committee of Johnson & Johnson and Pfizer.
References
- Garcia DA, Baglin TP, Weitz JI, Samama MM. Parenteral anticoagulants: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e24S–e43S.
- Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G. Oral anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e44S–e88S.
- Kubitza D, Becka M, Voith B, Zuehlsdorf M, Wensing G. Safety, pharmacodynamics, and pharmacokinetics of single doses of BAY 59-7939, an oral, direct Factor Xa inhibitor. Clin Pharmacol Ther. 2005;78:412–21.
- Kubitza D, Becka M, Roth A, Mueck W. Dose-escalation study of the pharmacokinetics and pharmacodynamics of rivaroxaban in healthy elderly subjects. Curr Med Res Opin. 2008;24:2757–65.
- Perzborn E, Strassburger J, Wilmen A, Pohlmann J, Roehrig S, Schlemmer KH, et al. In vitro and in vivo studies of the novel antithrombotic agent BAY 59-7939 ‐ an oral, direct Factor Xa inhibitor. J Thromb Haemost. 2005;3:514–21.
- Depasse F, Busson J, Mnich J, Le Flem L, Gerotziafas GT, Samama MM. Effect of BAY 59-7939 - a novel, oral, direct Factor Xa inhibitor - on clot-bound Factor Xa activity in vitro. J Thromb Haemost. 2005;3(Suppl 1): Abstract P1104.
- Perzborn E, Roehrig S, Straub A, Kubitza D, Misselwitz F. The discovery and development of rivaroxaban, an oral, direct Factor Xa inhibitor. Nat Rev Drug Discov. 2011;10:61–75.
- Bates SM, Weitz JI. Coagulation assays. Circulation. 2005;112: e53–60.
- You JJ, Singer DE, Howard PA, Lane DA, Eckman MH, Fang MC, et al. Antithrombotic therapy for atrial fibrillation: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012; 141:e531S–e575S.
- Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e419S–e494S.
- Heni N, Glogner P. Pharmacokinetics of phenprocoumon in man investigated using a gas chromatographic method of drug analysis. Naunyn-Schmiedeberg’s Arch Pharmacol. 1976;293:183–6.
- Samama MM, Martinoli JL, Le Flem L, Guinet C, Plu-Bureau G, Depasse F, et al. Assessment of laboratory assays to measure rivaroxaban – an oral, direct Factor Xa inhibitor. Thromb Haemost. 2010;103: 815–25.
- Mani H, Hesse C, Stratmann G, Lindhoff-Last E. Rivaroxaban differentially influences ex vivo global coagulation assays based on the administration time. Thromb Haemost. 2011;106:156–64.
- Barrett YC, Wang Z, Frost C, Shenker A. Clinical laboratory measurement of direct Factor Xa inhibitors: anti-Xa assay is preferable to prothrombin time assay. Thromb Haemost. 2010;104:1263–71.
- Hillarp A, Baghaei F, Fagerberg Blixter I, Gustafsson KM, Stigendal L, Sten-Linder M, et al. Effects of the oral, direct factor Xa inhibitor rivaroxaban on commonly used coagulation assays. J Thromb Haemost. 2011;9:133–9.
- Lindhoff-Last E, Samama MM, Ortel TL, Weitz JI, Spiro TE. Assays for measuring rivaroxaban: their suitability and limitations. Ther Drug Monit. 2010;32:673–9.
- Bayer Pharma AG. Xarelto® (rivaroxaban) Summary of Product Characteristics. 2013. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000944/WC500057108.pdf. Accessed 9 April 3013.
- Mills RM, Berkowitz RD, Damaraju CV, Jennings LK, Wildgoose P. Initiation of rivaroxaban following low molecular weight heparin for thromboprophylaxis after total joint replacement: The Safe, Simple Transitions (SST) study. Thromb Res. 2012;130:709–15.
- van Ryn J, Stangier J, Haertter S, Liesenfeld KH, Wienen W, Feuring M, et al. Dabigatran etexilate - a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost. 2010;103:1116–27.
- Boehringer Ingelheim International GmbH. Pradaxa® (dabigatran etexilate) Summary of Product Characteristics. 2013. Available at: www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000829/WC500041059.pdf. Accessed 9 April 2013.
- Douxfils J, Mullier F, Robert S, Chatelain C, Chatelain B, Dogne JM. Impact of dabigatran on a large panel of routine or specific coagulation assays. Laboratory recommendations for monitoring of dabigatran etexilate. Thromb Haemost. 2012;107:985–97.
- Samama MM, Contant G, Spiro TE, Perzborn E, Guinet C, Gourmelin Y, et al. Evaluation of the anti-Factor Xa chromogenic assay for the measurement of rivaroxaban plasma concentrations using calibrators and controls. Thromb Haemost. 2012;107:379–87.
- Mani H, Rohde G, Stratmann G, Hesse C, Herth N, Schwers S, et al. Accurate determination of rivaroxaban levels requires different calibrator sets but not addition of antithrombin. Thromb Haemost. 2012; 108:191–8.
- McGlasson DL, Kaczor DA, Krasuski RA, Campbell CL, Kostur MR, Adinaro JT. Effects of pre-analytical variables on the anti-activated factor X chromogenic assay when monitoring unfractionated heparin and low molecular weight heparin anticoagulation. Blood Coagul Fibrinolysis. 2005;16:173–6.
- Samama MM, Amiral J, Guinet C, Perzborn E, Depasse F. An optimised, rapid chromogenic assay, specific for measuring direct Factor Xa inhibitors (rivaroxaban) in plasma. Thromb Haemost. 2010;104:1078–9.
- Asmis LM, Alberio L, Angelillo-Scherrer A, Korte W, Mendez A, Reber G, et al. Rivaroxaban: quantification by anti-FXa assay and influence on coagulation tests: a study in 9 Swiss laboratories. Thromb Res. 2012;129:492–8.
- Mueck W, Borris LC, Dahl OE, Haas S, Huisman MV, Kakkar AK, et al. Population pharmacokinetics and pharmacodynamics of once- and twice-daily rivaroxaban for the prevention of venous thromboembolism in patients undergoing total hip replacement. Thromb Haemost. 2008;100:453–61.
- Mueck W, Lensing AW, Agnelli G, Decousus H, Prandoni P, Misselwitz F. Rivaroxaban: population pharmacokinetic analyses in patients treated for acute deep-vein thrombosis and exposure simulations in patients with atrial fibrillation treated for stroke prevention. Clin Pharmacokinet. 2011;50:675–86.
- Gerotziafas GT, Hatmi M, Samama MM, Elalamy I. Optimisation of the assays for the measurement of clotting factor activity in the presence of rivaroxaban. Thromb Res. 2012;129:101–3.
- Tichelaar V, de Jong H, Nijland H, Kluin-Nelemans H, Meijer K, Mulder A. Interference of rivaroxaban in one-stage and chromogenic factor VIII:C assays. Thromb Haemost. 2011;106:990–2.
- The EINSTEIN Investigators. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499–510.
- Merriman E, Kaplan Z, Butler J, Malan E, Gan E, Tran H. Rivaroxaban and false positive lupus anticoagulant testing. Thromb Haemost. 2011;105:385–6.
- Mani H, Hesse C, Stratmann G, Lindhoff-Last E. Ex vivo effects of low-dose rivaroxaban on specific coagulation assays and coagulation factor activities in patients under real life conditions. Thromb Haemost. 2013;109:127–36.
- Freyburger G, Macouillard G, Labrouche S, Sztark F. Coagulation parameters in patients receiving dabigatran etexilate or rivaroxaban: two observational studies in patients undergoing total hip or total knee replacement. Thromb Res. 2011;127:457–65.
- Haas S. Facts and artefacts of coagulation assays for Factor Xa inhibitors. Thromb Haemost. 2010;103:686–8.
- Samama MM, Guinet C. Laboratory assessment of new anticoagulants. Clin Chem Lab Med. 2011;49:761–72.
- Mueck W, Eriksson BI, Bauer KA, Borris L, Dahl OE, Fisher WD, et al. Population pharmacokinetics and pharmacodynamics of rivaroxaban – an oral, direct Factor Xa inhibitor – in patients undergoing major orthopaedic surgery. Clin Pharmacokinet. 2008;47:203–16.
- Turpie AGG, Kreutz R, Llau J, Norrving B, Haas S. Management consensus guidance for the use of rivaroxaban – an oral, direct factor Xa inhibitor. Thromb Haemost. 2012;108:876–86.
- Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011;124:1573–9.
