Abstract
Background. Whether rosiglitazone use in patients with type 2 diabetes may affect thyroid cancer risk has not been investigated.
Methods. The reimbursement databases of all diabetic patients under oral anti-diabetic agents or insulin from 1996 to 2009 were retrieved from the National Health Insurance of Taiwan. An entry date was set at 1 January 2006, and 887,665 patients with type 2 diabetes were followed for thyroid cancer incidence until the end of 2009 for ever-users, never-users, and subgroups of rosiglitazone exposure using tertile cut-offs for time since starting rosiglitazone, duration of therapy, and cumulative dose. Hazard ratios were estimated by Cox regression.
Results. There were 103,224 ever-users and 784,441 never-users, with respective numbers of incident thyroid cancer of 84 (0.08%) and 764 (0.10%), and respective incidence of 23.12 and 28.09 per 100,000 person-years. The overall multivariable-adjusted hazard ratio was not significant. However, in dose-response analyses, the adjusted hazard ratios (95% confidence intervals) were significant for the third tertile of duration of therapy (≥ 14 months) and cumulative dose (≥ 1,800 mg) for age ≥ 50 years: 0.53 (0.31–0.89) and 0.50 (0.29–0.87), respectively.
Conclusions. This study suggests that rosiglitazone use in patients with type 2 diabetes may reduce the risk of thyroid cancer.
Key messages
The incidence of thyroid cancer has been increasing worldwide, which may be related to insulin resistance.
This study suggests that use of rosiglitazone, an insulin sensitizer, may reduce the risk of thyroid cancer by approximately 50% if the duration of therapy is ≥ 14 months or the cumulative dose is ≥ 1,800 mg.
Introduction
The incidence of thyroid cancer has been increasing worldwide (Citation1,Citation2). In the United States, although the trend of mortality from thyroid cancer has been steady during the period from 1973 to 2002, the incidence of thyroid cancer has increased 2.4-fold during the period (Citation3). It is believed that the cause of the increase in the incidence of thyroid cancer in recent years may be partly due to the enhanced detection of early-stage cancer by the use of thyroid ultrasound and ultrasound-guided fine-needle aspiration cytology examination (Citation4). However, this may not wholly explain the increasing incidence around the world, and some other contributing factors are probably in play (Citation4). Gursoy hypothesized that the rising thyroid cancer incidence around the world might be related to insulin resistance (Citation2). Therefore, medications targeting insulin resistance, like the thiazolidinediones, are theoretically preventive for thyroid cancer.
The ever-approved thiazolidinediones include troglitazone, rosiglitazone, and pioglitazone (Citation5,Citation6). Troglitazone has been withdrawn from the world market since 2000 because of its risk of hepatic failure (Citation5), and rosiglitazone was taken off the European market in 2010 because of the concern of a higher risk of myocardial infarction (Citation5). Recently pioglitazone has been challenged with a possible risk of bladder cancer (Citation5,Citation7).
Some recent studies described below provide a rationale for further in-depth analyses on whether rosiglitazone may protect against thyroid cancer or affect the risk of its incidence. An in vitro study using normal porcine thyrocytes and follicular carcinoma cell lines suggests an anti-tumor effect of thiazolidinediones in thyroid cells (Citation8). Another in vitro study evaluating the antiproliferative effects of rosiglitazone, pioglitazone, or antiblastics (bleomycin, cisplatin, gemcitabine) using primary cultured human anaplastic thyroid cancer cells suggests that both thiazolidinediones exert similar antiproliferative effects as the antiblastics (Citation9). However, preliminary studies conducted in humans suggest that differential effects of pioglitazone and rosiglitazone on thyroid cancer can be observed. In an earlier study conducted in 20 patients with differentiated thyroid cancer, rosiglitazone may have induced radioiodine uptake in 5 patients and reduced serum thyroglobulin levels in 3 patients, even though none of them had a partial or complete response at 3 months’ follow-up by RECIST criteria (Citation10). In a later study, treatment with rosiglitazone (4 mg/day for 2 weeks followed by 8 mg/day for up to 6 months) in nine radioiodine-negative patients with progressive differentiated thyroid cancer suggested that rosiglitazone may be effective in reducing cancer size (antiproliferative effect) and increasing iodine uptake (redifferentiative effect) as assessed by positron emission tomography and computed tomography (Citation11). On the other hand, these antiproliferative and redifferentiative effects were not similarly demonstrated in five patients treated with pioglitazone (30 mg/day for 2 weeks followed by 45 mg/day for up to 6 months) (Citation12).
Therefore, the purpose of the present study was to evaluate whether rosiglitazone use in patients with type 2 diabetes mellitus would protect against or affect the risk of thyroid cancer, by using the National Health Insurance (NHI) reimbursement databases of Taiwan.
Materials and methods
The study was approved by an ethic review board of the National Health Research Institutes with registered approval number 99274.
Since March 1995 a compulsory and universal system of health insurance (the so-called NHI) has been implemented in Taiwan. All contracted medical institutes must submit computerized and standard claim documents for reimbursement. More than 99% of citizens are enrolled in the NHI, and > 98% of the hospitals nationwide are under contract with the NHI. The average number of annual physician visits in Taiwan is one of the highest around the world, at approximately 15 visits per year per capita in 2009.
The National Health Research Institutes is the only organization approved, as per local regulations, for handling the NHI reimbursement databases for academic research. The databases contain detailed records on every visit for each patient, including outpatient visits, emergency department visits, and hospital admission. The databases also include principal and secondary diagnostic codes, prescription orders, and claimed expenses.
The identification information of the individuals was scrambled for the protection of privacy. Diabetes was coded 250.1–250.9 and thyroid cancer 193, based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM).
shows the process of selecting patients for inclusion in the study. The selected entry date was 1 January 2006. The databases of all patients who had been diagnosed as having diabetes and under treatment with either oral anti-diabetic agents or insulin during the period of 1996–2009 from the whole nation and who remained in the insurance program after the entry date were first retrieved (n = 1,554,030). A total of 235,287 patients who had ever been treated with pioglitazone were excluded to avoid the possible contamination by its use. After excluding patients who had a diagnosis of diabetes after the year 2006 (n = 342,351), patients who held a Severe Morbidity Card as having type 1 diabetes (n = 7120; in Taiwan, patients with type 1 diabetes were issued a so-called ‘Severe Morbidity Card’ after certified diagnosis, and they were exempt from many of the co-payments), patients having a diagnosis of thyroid cancer before 2006 (n = 3640), those who died (n = 96,320) or withdrew from the NHI (n = 12,502) before entry date, duplicated identification number (n = 106), and unclear information on date of birth or sex (n = 5122), a total of 887,665 patients with a diagnosis of type 2 diabetes mellitus and under therapy with oral anti-diabetic agents or insulin were recruited.
Figure 1. Flow chart showing the process of selecting patients for inclusion in the study from the National Health Insurance (NHI) databases of Taiwan covering the period of 1996–2009. Selected entry date was set on 1 January 2006 and follow-up ended on 31 December 2009.
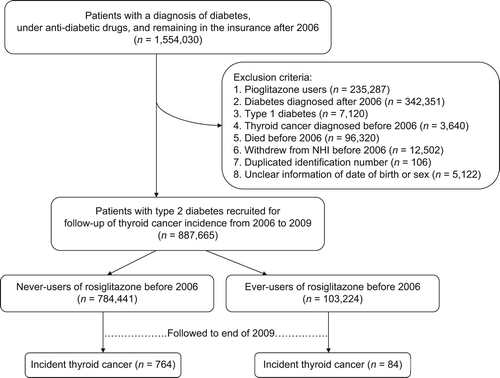
Those who had ever been prescribed with rosiglitazone before entry date were defined as ever-users; and never-users were defined as those who had never been prescribed with rosiglitazone before entry date. To evaluate whether a dose-responsive relationship could be seen between rosiglitazone and thyroid cancer, the tertile cut-offs for the following parameters used by the Kaiser Permanente Northern California study for evaluating the association between pioglitazone and bladder cancer (Citation7) were applied: 1) time since starting rosiglitazone in months; 2) duration of therapy in months; and 3) cumulative dose in mg.
An entry date at the beginning of 2006 was used based on the following reasons: 1) Because rosiglitazone was marketed in 2001 in Taiwan, this entry date, being in the middle of the marketing date of rosiglitazone in Taiwan and the ending date of the available NHI databases in 2009, provided a longest exposure of 4 to 5 years at entry and at the same time a longest follow-up duration of 4 years; and 2) The issue of bladder cancer associated with pioglitazone noted in the PROspective pioglitAzone Clinical Trial In macroVascular Events (PROactive) was published in 2005 (Citation13), and in 2007, the safety of rosiglitazone had been challenged with a risk of acute myocardial infarction (Citation5). These had caused tremendous change of prescription behavior in the physicians to withdraw thiazolidinediones including rosiglitazone and pioglitazone (troglitazone has not been marketed in Taiwan), and the patients might have stopped taking the drugs even if they were prescribed after the year 2006. Therefore, the use of a later entry date would make the estimation of the duration of therapy and cumulative dose of rosiglitazone less reliable. In addition, this would also shorten the follow-up duration for cancer incidence.
All co-morbidities and covariates were determined as a status/diagnosis before the entry date. The ICD-9-CM codes for the co-morbidities were (Citation14–18): nephropathy 580–589, hypertension 401–405, chronic obstructive pulmonary disease (a surrogate for smoking) 490–496, cerebrovascular disease 430–438, ischemic heart disease 410–414, peripheral arterial disease 250.7, 785.4, 443.81, and 440–448, eye disease 250.5, 362.0, 369, 366.41, and 365.44, obesity 278, dyslipidemia 272.0–272.4, previous thyroid benign disease 240–246, and cancer other than thyroid cancer 140–208 (excluding 193). Medications included sulfonylurea, metformin, insulin, acarbose, statin, fibrate, angiotensin-converting enzyme inhibitor and/or angiotensin receptor blocker, calcium channel blocker, aspirin, ticlopidine, clopidogrel, dipyridamole, and non-steroidal anti-inflammatory drugs. To compare whether rosiglitazone users had a higher probability of receiving examinations that might potentially lead to the diagnosis of thyroid cancer than non-users of rosiglitazone, any of the following examinations were considered as ‘potential detection examinations’: 1) thyroid sonography; 2) thyroid aspiration; and/or 3) thyroid function test (T3, T4, and thyroid stimulating hormone). Baseline characteristics between ever-users and never-users of rosiglitazone were compared by chi-square test.
The incidence density of thyroid cancer was calculated for ever-users and never-users and for different subgroups of exposure. The numerator for the incidence was the number of patients with incident thyroid cancer during the 4-year follow-up, and the denominator was the person-years of follow-up. For ever-users, the follow-up duration was either censored at the date of thyroid cancer diagnosis or at the date of the last record of the available reimbursement databases in individuals without incident thyroid cancer. For never-users, the follow-up was censored at the date of rosiglitazone initiation or thyroid cancer diagnosis or the last reimbursement record, depending on whichever occurred first. This ensured no exposure to rosiglitazone throughout the whole follow-up period until censor in the referent group of never-users.
Cox proportional hazards regression was performed to estimate the hazard ratios for thyroid cancer for ever-users versus never-users, and for the various subgroups of dose-responsive parameters. The following three models were created: 1) unadjusted; 2) adjusted for age and sex; and 3) adjusted for all variables compared previously as baseline characteristics between ever- users and never-users. Because diabetes and the use of rosiglitazone are both rare below the age of 50 years, the above Cox regression models were also created for patients aged 50 years or older.
Analyses were conducted using SAS statistical software, version 9.1 (SAS Institute, Cary, NC, USA). P < 0.05 was considered statistically significant.
Results
compares the baseline characteristics between ever-users (n = 103,224) and never-users (n = 784,441) of rosiglitazone. All variables differed significantly between the two groups. Ever-users are characterized by older age distribution, higher proportion with a diabetes duration ≥ 5 years, higher proportions of all co-morbidities and other cancer, higher proportions of using other medications, and a higher proportion of receiving potential detection examinations.
Table I. Baseline characteristics of never- and ever-users of rosiglitazone.
shows the incidences of thyroid cancer between ever-users and never-users of rosiglitazone, and among the different categories of the dose-responsive parameters for rosiglitazone exposure. The incidence rate in never- and ever-users of rosiglitazone was 28.09 and 23.12 per 100,000 person-years, respectively.
Table II. Exposure to rosiglitazone and incidences of thyroid cancer.
shows the hazard ratios with regard to different categories of rosiglitazone exposure in all ages and in age ≥ 50 years, respectively. In the models evaluating the overall hazard ratios for ever-users versus never-users, none of the hazard ratios was significant for the fully adjusted models. However, in the models evaluating the dose-responsive exposure to rosiglitazone, a lower risk could be seen in the third tertiles of duration of therapy and cumulative dose, especially when patients aged ≥ 50 years were analyzed.
Table III. Hazard ratios for thyroid cancer with regard to different levels of exposure to rosiglitazone.
Discussion
This is the first population-based study evaluating whether the use of rosiglitazone in patients with type 2 diabetes mellitus would affect the risk of thyroid cancer. The findings suggested a null association without increasing or decreasing the risk of thyroid cancer in overall analyses. However, when stratified by the dose-responsive parameters, patients in the highest exposure tertile of duration of therapy and cumulative dose showed significantly lower risk of thyroid cancer in those aged 50 years or older (). These findings may support the hypothesis of insulin resistance as an etiology of thyroid cancer (Citation2,Citation4). However, more studies are required to examine the usefulness and risk-benefit of rosiglitazone in the prevention and treatment of thyroid cancer.
As shown in , ever-users of rosiglitazone had a significantly higher probability of receiving insulin and other oral anti-diabetic agents. Because metformin may reduce (Citation17,Citation19,Citation20) but sulfonylureas or insulin may increase the risk of cancer (Citation20,Citation21), indication bias related to these therapies could not be excluded. In addition, smoking is associated with a reduced risk of thyroid cancer in a recent pooled analysis of five prospective studies in the USA (Citation22); but obesity (Citation23), exposure to radiation (Citation24), and iodine deficiency (Citation25) are important risk factors for thyroid cancer. In the present study, smoking was not available for adjustment, but the proportion of a surrogate for smoking, i.e. chronic obstructive pulmonary disease, was significantly higher in rosiglitazone users than in non-users (). Although this surrogate has been considered in the multivariable-adjusted models (), residual confounding from smoking could not be completely excluded. It should also be pointed out that the use of obesity diagnosis instead of real measurement of anthropometric parameters such as body mass index or waist circumference might have underestimated the impact of obesity. A potential bias due to the lack of information of radiation exposure and iodine deficiency should also be acknowledged.
Women always have a higher risk of thyroid cancer than men, and obesity and thyroid benign disease have been implicated as important risk factors for thyroid cancer (Citation4). The validity of the present study could be assured because the risk of thyroid cancer was significantly lower in men, higher in those with a diagnosis of obesity with borderline significance, and significantly higher in those with benign thyroid disease in the fully adjusted model. The respective adjusted hazard ratios (95% confidence interval, P value) were 0.59 (0.51–0.69, P < 0.0001), 1.45 (0.93–2.24, P = 0.0984), and 3.35 (2.80–4.01, P < 0.0001).
Although misclassification of thyroid cancer might occur, such a probability was low because labeled diagnoses should be printed out in all prescriptions handed to the patients. Mislabeling of a cancer diagnosis would not be acceptable to the patients when they saw the diagnosis. Because the databases were derived from the whole population, there was no concern of potential selection bias related to sampling error.
This study has several strengths. The databases included all claim records on outpatient visits, emergency department visits, and hospital admission, and we caught the diagnoses from all sources. Cancer is considered a severe morbidity by the NHI, and most medical co-payments can be waived. Furthermore, there is a low drug cost-sharing required by the NHI, and patients with certain conditions, such as low-income household, veterans, or patients with prescription refills for chronic disease, are exempted from the drug cost-sharing (Citation26). Therefore the detection rate of thyroid cancer would not tend to differ among different social classes. The use of medical records also reduced the potential bias related to self-reporting.
The study limitations included a lack of actual measurement data for confounders such as obesity, smoking, alcohol drinking, family history, lifestyle, diet, hormones, and genetic parameters. In addition, we did not have biochemical data such as levels of glucose, insulin, insulin-like growth factor-1, and thyroid stimulating hormone for evaluating their impact. Another limitation is the lack of information on the pathology, grading, and staging of thyroid cancer.
In summary, although the overall hazard ratios are not significant, the present study supports a protective effect of rosiglitazone against the risk of thyroid cancer when the duration of therapy is ≥ 14 months or the cumulative dose is ≥ 1,800 mg in patients with type 2 diabetes mellitus, especially in those aged ≥ 50 years. More in-depth studies for the potential usefulness of rosiglitazone in the prevention and treatment of thyroid cancer are warranted.
Acknowledgements
The study was supported by the Department of Health (DOH89-TD-1035) and the National Science Council (NSC 101–2314-B-002–117, NSC102–2314-B-002–067) of Taiwan. It is based in part on data from the National Health Insurance Research Database provided by the Bureau of National Health Insurance, Department of Health and managed by National Health Research Institutes (registered number 99274). The interpretation and conclusions contained herein do not represent those of Bureau of National Health Insurance, Department of Health or National Health Research Institutes. The guarantor of this study is Tseng C.-H.
Declaration of interest: The author reports no conflicts of interest.
References
- Kilfoy BA, Zheng T, Holford TR, Han X, Ward MH, Sjodin A, et al. International patterns and trends in thyroid cancer incidence, 1973–2002. Cancer Causes Control. 2009;20:525–31.
- Gursoy A. Rising thyroid cancer incidence in the world might be related to insulin resistance. Med Hypotheses. 2010;74:35–6.
- Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–7.
- Shih SR, Chiu WY, Chang TC, Tseng CH. Diabetes and thyroid cancer risk: literature review. Exp Diabetes Res. 2012;2012:578285.
- Tseng CH. Pioglitazone and bladder cancer in human studies: is it diabetes itself, diabetes drugs, flawed analyses or different ethnicities?J Formos Med Assoc. 2012;111:123–31.
- Tseng CH, Tseng FH. Peroxisome proliferator-activated receptor agonists and bladder cancer: lessons from animal studies. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2012;30:368–402.
- Lewis JD, Ferrara A, Peng T, Hedderson M, Bilker WB, Quesenberry CP Jr, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care. 2011;34:916–22.
- Fröhlich E, Machicao F, Wahl R. Action of thiazolidinediones on differentiation, proliferation and apoptosis of normal and transformed thyrocytes in culture. Endocr Relat Cancer. 2005;12:291–303.
- Antonelli A, Ferrari SM, Fallahi P, Berti P, Materazzi G, Minuto M, et al. Thiazolidinediones and antiblastics in primary human anaplastic thyroid cancer cells. Clin Endocrinol (Oxf). 2009;70:946–53.
- Kebebew E, Lindsay S, Clark OH, Woeber KA, Hawkins R, Greenspan FS. Results of rosiglitazone therapy in patients with thyroglobulin-positive and radioiodine-negative advanced differentiated thyroid cancer. Thyroid. 2009;19:953–6.
- Rosenbaum-Krumme SJ, Freudenberg LS, Jentzen W, Bockisch A, Nagarajah J. Effects of rosiglitazone on radioiodine negative and progressive differentiated thyroid carcinoma as assessed by 124I PET/CT imaging. Clin Nucl Med. 2012;37:e47–52.
- Rosenbaum-Krumme SJ, Bockisch A, Nagarajah J. Pioglitazone therapy in progressive differentiated thyroid carcinoma. Nuklearmedizin. 2012;51:111–15.
- Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, et al.; PROactive investigators. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–89.
- Tseng CH. Diabetes and risk of bladder cancer: a study using the National Health Insurance database in Taiwan. Diabetologia. 2011;54:2009–15.
- Tseng CH. Diabetes and risk of prostate cancer: a study using the National Health Insurance. Diabetes Care. 2011;34:616–21.
- Tseng CH. Pioglitazone and bladder cancer: a population-based study of Taiwanese. Diabetes Care. 2012;35:278–80.
- Tseng CH. Diabetes, metformin use, and colon cancer: a population-based cohort study in Taiwan. Eur J Endocrinol. 2012;167:409–16.
- Tseng CH. New-onset diabetes with a history of dyslipidemia predicts pancreatic cancer. Pancreas. 2013;42:42–8.
- Tseng CH. Benign prostatic hyperplasia is a significant risk factor for bladder cancer in diabetic patients: a population-based cohort study using the National Health Insurance in Taiwan. BMC Cancer. 2013;13:7.
- Hsieh MC, Lee TC, Cheng SM, Tu ST, Yen MH, Tseng CH. The influence of type 2 diabetes and glucose-lowering therapies on cancer risk in the Taiwanese. Exp Diabetes Res. 2012;2012:413782.
- Bowker SL, Yasui Y, Veugelers P, Johnson JA. Glucose-lowering agents and cancer mortality rates in type 2 diabetes: assessing effects of time-varying exposure. Diabetologia. 2010;53:1631–7.
- Kitahara CM, Linet MS, Beane Freeman LE, Check DP, Church TR, Park Y, et al. Cigarette smoking, alcohol intake, and thyroid cancer risk: a pooled analysis of five prospective studies in the United States. Cancer Causes Control. 2012;23:1615–24.
- Kim HJ, Kim NK, Choi JH, Sohn SY, Kim SW, Jin SM, et al. Associations between body mass index and clinico-pathological characteristics of papillary thyroid cancer. Clin Endocrinol (Oxf). 2013;78:134–40.
- Nikiforov YE. Radiation-induced thyroid cancer: what we have learned from chernobyl. Endocr Pathol. 2006;17:307–17.
- Belfiore A, La Rosa GL, La Porta GA, Giuffrida D, Milazzo G, Lupo L, et al. Cancer risk in patients with cold thyroid nodules: relevance of iodine intake, sex, age, and multinodularity. Am J Med. 1992;93:363–9.
- Cheng TM. Taiwan's new national health insurance program: genesis and experience so far. Health Aff (Millwood). 2003;22:61–76.