Abstract
Background. Human adenovirus-36 (Adv36) increases adiposity, but also upregulates distal insulin signaling in vitro in human adipose and muscle tissue and in vivo in the rodent independently of adiposity. Accordingly, healthy adults and children with antibodies against Adv36 had increased insulin sensitivity and reduced hepatic lipid accumulation. We hypothesized that Adv36 infection would be less frequent in individuals with type 2 diabetes or impaired glycemic control.
Methods. Presence of antibodies against Adv36 was analyzed for association to type 2 diabetes or impaired glycemic control in a two-wave population-based sample of well-characterized adults (n = 1734). Indices of impaired glycemic control included oral glucose tolerance, and circulating fasting levels of glucose, insulin, and insulin-like growth factor binding protein-1 (IGFBP-1).
Results. Adv36 seropositivity was more common in those with normal glucose tolerance (NGT) than in those with diabetes (females: OR 17.2, 95% CI 4.0–74.3; males: OR 3.5, 95% CI 1.8–6.7). Also, females with NGT had higher frequency of Adv36 seropositivity than females with prediabetes (impaired glucose tolerance and/or impaired fasting glucose; OR 1.8, 95% CI 1.1–3.1). Within the female prediabetes group Adv36 seropositivity was associated with higher insulin sensitivity reflected by reduced HOMA-IR and increased IGFBP-1.
Conclusion. Adv36 infection is associated with lower occurrence of type 2 diabetes and better insulin sensitivity in adults, particularly among females.
Key words::
Key messages
Presence of Adv36 antibodies was less common in type 2 diabetes and in females with impaired glycemic control.
Adv36 seropositivity associated with less impaired insulin sensitivity in females with impaired glycemic control.
Introduction
Human adenovirus-36 (Adv36), as it was first reported in 1980, and 53 other known human adenovirus serotypes, are generally associated with infection of the upper respiratory tract, gastrointestinal tract, or the conjunctiva (Citation1,Citation2). Dhurandhar et al. have shown that natural and experimental Adv36 infection of several animal species resulted in increased adiposity and an increasing proliferation and differentiation of preadipocytes and lipid accumulation in mature adipocytes (Citation3–11). In humans, the presence of Adv36 antibodies is evidence of prior Adv36 infection. The presence of Adv36 antibodies in children and adults has been associated with increased BMI or body fat in cohorts from the USA, China, South Korea, Italy, and Czechoslovakia (Citation12–20). Recently we reported that Adv36 antibodies were present in 15%–20% of lean healthy adults and children in a cohort of 1946 clinically well-characterized Swedes, and that Adv36 positivity was 1.5 to 2 times more common in obese children and severely obese adult females compared to lean individuals (Citation21). In some studies, but not all, Adv36-induced/associated obesity has been reported to have a favorable metabolic profile with low blood lipids in animals (Citation4) and adult humans (Citation12,Citation21). Also, Adv36 has been shown to enhance insulin sensitivity and glucose uptake (reviewed in 2) in vivo in rats (Citation5) and in vitro in human muscle and fat cells (Citation22,Citation23). Adv36 was associated with increased insulin sensitivity and reduced hepatic lipid accumulation in human subjects without diabetes regardless of adiposity (Citation24).
Insulin signaling is characterized by a proximal signaling module with binding of insulin to its receptor (IR), followed by activation of the IR substrates IRS1 and IRS2 and activation of Ras via Shc; and a distal signaling module involving activation (by IRS and Ras) of phosphatidylinositol 3-kinase (PI3K) pathway, and subsequent activation of AKT2 that upregulates glucose transporters (e.g. Glut4).
Adv36, like other adenoviruses (Citation25), reduces activation of IR, IRS1, and IRS2 (Citation22–24). However, recent studies show that Adv36 improves glucose uptake in skeletal muscle and adipose tissue (through Glut1 and Glut4) and reduces hepatic glucose release by activating the distal signaling components PI3K and AKT2 via upregulation of Ras and adiponectin signaling (Citation22,Citation24,Citation26). We hypothesized that Adv36 seropositivity would be less common in type 2 diabetes patients and in individuals with prediabetes, with impaired glucose tolerance and/or high fasting serum glucose level, compared to healthy controls. We also hypothesized that Adv36 seropositivity in these individuals would be associated with increased insulin sensitivity measured by the homeostasis model assessment of insulin resistance (HOMA-IR) index and level of circulating insulin-like growth factor binding protein-1 (IGFBP-1). IGFBP-1 correlates well to insulin sensitivity (Citation27–29). To test these hypotheses 1734 clinically well-characterized adults from a longitudinal population-based cohort in Stockholm, Sweden, were studied (Citation30).
Materials and methods
Study subjects
A selection of individuals within the large longitudinal population-based Stockholm Diabetes Prevention Program (SDPP) of females and males, living in Stockholm County, and aged 35–55 years at baseline, were studied. The baseline sampling, of 7949 adults, was performed in 1992–1998 and a follow-up was made in 2002–2006 (Citation30). Oral glucose tolerance tests (OGTT) (Citation31) were performed in all participants at both examinations. Of the 7949 adults, all those who developed type 2 diabetes between baseline and follow-up, and all those with prediabetes at follow-up, and matched lean and overweight/obese controls who had normal glucose tolerance (NGT) at both baseline and follow-up, were included in this study (; ). Prediabetes was defined as impaired fasting serum glucose (IFG; fasting glucose ≥ 6.1 to < 7.0 mM), impaired oral glucose tolerance (IGT; 2-h glucose ≥ 7.8 mM to < 11.1 mM), or the combination (IFG+ IGT). NGT was defined as fasting glucose < 6.1 mM and 2-h glucose < 7.8 mM. A total of 183 males and 106 females developed type 2 diabetes after baseline and were included in this study. Of the males, 99 were diagnosed with type 2 diabetes by oral glucose tolerance test (OGTT) at the follow-up examination, whereas 84 males were diagnosed with type 2 diabetes by a physician during the time period between baseline and follow-up. Corresponding numbers among females were 58 and 48, respectively. Also all individuals with prediabetes at follow-up including 290 males and 211 females were studied. Of the individuals with prediabetes, 254 males and 181 females had NGT at baseline; of the type 2 diabetes cases, 111 males and 60 females had NGT at baseline, and all the others had prediabetes at baseline. The control group consisted of males and females with NGT at both baseline and follow-up. Control individuals with body mass index (BMI) ≥ 28.0 kg/m2 and those with BMI 20.0–24.9 kg/m2 were selected by age- and sex-matching between the overweight/obesity group and the lean group. There were 218 males (age; mean ± SEM: 56.2 ± 0.33, range 46–66 years) and 252 females (age; mean ± SEM: 55.5 ± 0.32, range 44–63 years) in the overweight/obese group. In the lean group there were 219 males (age; mean ± SEM: 56.8 ± 0.34, range 46–65 years) and 255 females (age; mean ± SEM: 55.6 ± 0.32, range 44–63 years). Serum, collected after an overnight fast, and clinical data used in this study were from the follow-up collected in 2002–2006; however, fasting glucose level and OGTT data were used also from baseline to determine IFG and IGT status at baseline. Serum and clinical data on BMI, glucose, insulin, homeostatic model assessment of insulin resistance (HOMA-IR = glucose× insulin/22.5, higher level reflecting higher insulin resistance), HOMA-β (= (20 × insulin)/(glucose–3.5), higher level reflecting higher β-cell insulin secretion) were available from each individual studied. Serum triglyceride levels and insulin-like growth factor binding protein-1 (IGFBP-1) levels were available from all those with type 2 diabetes, and those with IGT or IFG+ IGT at follow-up, that had NGT at baseline, and from 297 controls with NGT at both baseline and follow-up (Supplementary Table 1 to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2014.935469). Serum and clinical data were collected at the same date for each individual. Concentrations of serum glucose were analyzed in duplicate by a glucose oxidase method using a Yellow Springs Glucose Analyzer (Yellow Springs, OH, USA). Serum insulin was assayed by radioimmunoassay, using in-house antibodies, human insulin as a standard, and charcoal addition to separate antibody-bound and free serum insulin (Citation32). Serum triglyceride levels (reference interval for age > 18 years: 0.45–2.6 mmol/L) were measured on a Beckman Coulter DXC800 (Beckman Coulter In., Brea, CA, USA) by the Karolinska University Hospital's accredited chemistry laboratory. Serum IGFBP-1 was measured using an in-house RIA with intra- and inter-assay (CV) values of 3% and 10%, respectively (Citation33). The ethical committee of Karolinska Institutet approved the study, and informed consent was obtained from all participants.
Figure 1. Study groups. The study groups were selected from the population-based Stockholm Diabetes Prevention Program (SDPP). BMI = body mass index kg/m2.
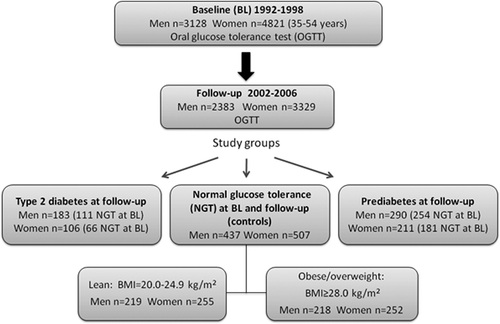
Table I. Clinical characteristics of the SDPP study groups. Data are from the follow-up if nothing else is specified.
ELISA for antibodies against Adv36
This Adv36 ELISA was first described in Almgren et al. (Citation21) and was used in Aldhoon-Hainerová et al. (Citation19). The analyses were performed by a researcher blinded to any phenotypic data. The 96-well ELISA microplates were coated overnight at 4°C with recombinant Adv36 fiber protein fragment fused with maltose binding protein (10 μg/mL; Obetech, Richmond, VA, USA). After a washing step, blocking with 20 mg/mL of bovine serum albumin for 1 h, and another washing step, a mixture of horse-radish peroxidase (HRP)-conjugated coating protein (Obetech, Richmond, VA) and the competitor protein recombinant maltose binding protein (MBP) (62.5 μg/mL; Obetech, Richmond, VA) as well as serum (study sample or adequate control) were added. After 1 h of incubation and subsequent washing, the HRP substrate TMB (3,3’,5,5’-tetramethylbenzidine; Thermo Scientific 34028, Rockford, IL, USA) was added, and reaction stopped after ∼25 min with 1M HCl. The plate was read at a wavelength of 450 nm. All samples were run in duplicate. ELISA cut-off for positive score was based on analysis of human serum samples with known Adv36-SNA status. Thereafter, the OD450 reading from the 1:1280 dilution of the positive rabbit serum was used as cut-off marker for positive ELISA score since it gave a signal corresponding to the defined ELISA cut-off (Citation21). An OD450 equal or greater than this value in both duplicates was scored as a positive assay for Adv36 antibodies. A reading of less than this value in both duplicates was scored negative. Disparate readings with one reading above and one below the cut-off value were considered equivocal, and the sample was reanalyzed in duplicate. A sample with repeat analyses was considered to be positive if > 50% of the replicates were positive with an OD450 higher than the cut-off value. Hence, data from a reanalyzed serum sample were used only if the reanalysis provided consistency between the duplicates.
Statistical analysis
Adv36 positivity in the ELISA was analyzed for statistically significant association to type 2 diabetes and prediabetes using the Pearson's chi-square test, along with estimation of odds ratio (OR), 95% confidence interval (CI) of OR, and using logistic regression with age and natural logarithm of BMI as non-categorical covariates. A prior study showed that the prevalence of Adv36 seropositivity in Sweden (Stockholm) was 7% in serum collected in the mid-1990s, and it approximately doubled during the following 10–15 years (Citation21). There is no information available on Adv36 existence in Stockholm before the mid-1990s. Thus, in the current study, for cases with prediabetes or type 2 diabetes at follow-up that had NGT at baseline (mid-1990s in Stockholm), there is a documented possibility for Adv36 infection to have occurred before the onset of the development of prediabetes. The following quantitative clinical outcome variables were analyzed with regard to Adv36 status among those with NGT at baseline (study group is presented in Supplementary Table 1 (to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2014.935469)): levels of fasting serum triglycerides, glucose, insulin, HOMA-IR, IGFBP-1, and HOMA-β. Non-normally distributed quantitative variables were transformed by the natural logarithm (ln) to normalize their distributions. Difference in quantitative clinical data between Adv36 positivity and Adv36 negativity was tested for using unpaired t test and univariate analysis of covariance (ANCOVA). In the ANCOVA, age and the natural logarithm of BMI was used as covariates. These tests were performed for females and males separately because of a statistically significant sex × Adv36 term (P < 0.0005) in ANCOVA for the outcomes ‘type 2 diabetes versus NGT’, ‘prediabetes versus NGT’, ‘IGFBP-1’, and ‘HOMA-IR’. The insulin resistance variable HOMA-IR was not analyzed in the combined type 2 diabetes and the prediabetes female group because of a statistically significant Adv36 × glucose tolerance status interaction on HOMA-IR (P < 0.0005). The distribution of the residuals from ANCOVA was checked for normality. All analyses were performed using IBM SPSS Statistics version 20.0 (IBM, Armonk, NY, USA). Reported P values are two-tailed. A two-tailed P ≤ 0.05 was regarded as significant in the tests of association between Adv36 and type 2 diabetes or prediabetes. In the analysis of relationship between Adv36 and insulin sensitivity a two-tailed P ≤ 0.025 (= 0.05/2 sex-groups) was regarded as significant. HOMA-IR and levels of IGFBP-1, triglycerides, and insulin correlated well with each other in each of the four subgroups in each sex (P ≤ 0.0005) and were thereby regarded as one ‘outcome’ in the attempt to correct for multiple testing according to Bonferroni.
Results
Less Adv36 seropositivity in type 2 diabetes or prediabetes
Infection with Adv36 (presence of antibodies against Adv36) was successfully scored in serum samples from 280 type 2 diabetes patients, 485 prediabetes cases, and 468 lean and 456 overweight/obese individuals with normal glucose tolerance (NGT). For each of these four groups in each sex, less than 4% of the samples failed to provide an Adv36 ELISA score (). In the NGT control group there was no significant difference in Adv36 seropositivity between lean and overweight/obese individuals (P > 0.25); therefore the NGT group was hereafter not subdivided by BMI.
Positive Adv36 serology was more common among the NGT controls than among those with type 2 diabetes both in females and males (females: OR 17.2, 95% CI 4.0–74.3, P = 0.0003; males: OR 3.5, 95% CI 1.8–6.7, P = 0.0003) (; ). This Adv36–type 2 diabetes association was not due to the increased BMI in the type 2 diabetes groups; prevalence of Adv36 positivity in NGT controls was significantly higher compared to the type 2 diabetes patients also when applying a logistic regression model with BMI and age as covariates (females: ORadj 15.7, 95% CI 2.1–112.7, Padj = 0.007; males: ORadj 3.1, 95% CI 1.5–6.2, Padj = 0.002). The prevalence of Adv36 positivity in female NGT controls was higher also than that among females with prediabetes at follow-up (OR 1.8, 95% CI 1.1–3.1, P = 0.043) (; ). However, the Adv36–prediabetes association in females did not reach statistical significance in a logistic regression model with BMI and age as covariates (ORadj 1.7, 95% CI 0.96–3.0, Padj = 0.070). Male NGT controls had a similar frequency of Adv36 positivity as males with prediabetes had (OR 1.1, 95% CI 0.62–1.4, P = 1.0). In the NGT control group there was no significant difference in Adv36 seropositivity between the sexes (P = 0.22). Together, females and males with NGT had a higher prevalence of Adv36 positivity compared to the females and males with type 2 diabetes and females with prediabetes at follow-up in an analysis adjusted for BMI, age, and sex × Adv36 interaction (ORadj 2.8, 95% CI 1.8–4.3, Padj ≤ 0.0005). A subgroup defined by NGT at baseline (Supplementary Table 1 to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2014.935469) was analyzed in the same way. The results from this subgroup were similar to the results from the whole study group; in the NGT at baseline subgroup the prevalence of Adv36 positivity was higher in the female and male NGT controls than among females and males with type 2 diabetes and females with prediabetes at follow-up adjusted for BMI and age (ORadj 2.4, 95% CI 1.5–4.0, Padj ≤ 0.0005).
Figure 2. Prevalence of seropositivity for human adenovirus-36 (Adv36) in serum samples from adults with normal glucose tolerance, prediabetes, and type 2 diabetes. Seropositivity was lower in females and males with type 2 diabetes (T2DM; black bars) and females with prediabetes (PreT2DM; impaired fasting glucose and/or impaired glucose tolerance; grey bars) compared to those with normal glucose tolerance (NGT; white bars). Error bars indicate standard error of proportion. Statistical significance of differences was tested using Pearson's chi-square test.
Table II. Unadjusted and adjusted relationships odds ratios for Adv36 seronegativity in females and males with prediabetes and type 2 diabetes compared to those with normal glucose tolerance (NGT).
Adv36 seropositivity in relation to insulin sensitivity
The subgroup of study participants with NGT at baseline, i.e. at the time when Adv36 was first measured in the Swedish population (Citation21), was studied further (Supplementary Table 1 to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2014.935469). In the present study, Adv36 was measured only at follow-up. Because of the low number of Adv36-positive type 2 diabetes patients, they were analyzed statistically in-group with those who had prediabetes at follow-up. In the female patients with type 2 diabetes or prediabetes at follow-up, Adv36 seropositivity was borderline statistically significantly related to triglyceride level (t = 2.1, P = 0.054, df = 187), but was related to higher IGFBP-1 levels (t = 2.1, P = 0.034, df = 187) (; ). In these Adv36-positive female patients, levels of IGFBP-1 were similar to those observed in individuals with NGT at follow-up (). However, the association between higher IGFBP-1 level and Adv36 seropositivity did not reach statistical significance when adjusting for age and BMI (P = 0.099). To study specifically the prediabetic group the type 2 diabetes cases were excluded. Thus, in female patients with NGT at baseline who had developed prediabetes at follow-up, there were statistically significant associations for Adv36 positivity both to higher IGFBP-1 and to lower HOMA-IR (IGFBP-1: t = 2.4, P = 0.018, df = 129; and HOMA-IR: t = 3.0, P = 0.003, df = 129) (; ), which were supported by ANCOVA adjusting for age and BMI (IGFBP-1: F(1) = 3.9, Padj = 0.049; and HOMA-IR: F(1) = 4.9, Padj = 0.028) (). This was also in accordance with lower fasting serum insulin levels in the Adv36-positive prediabetes females before and after adjustment for age and BMI (P = 0.003 and Padj = 0.020) (). In these Adv36-positive female prediabetes patients, levels of both IGFBP-1 and HOMA-IR were similar to those observed in individuals with NGT at follow-up ().
Figure 3. Relationship of Adv36 seropositivity to: (A) fasting serum triglyceride levels; (B) IGFBP-1; and (C) HOMA-IR levels in females with normal glucose tolerance (NGT) at baseline who had NGT at follow-up, or had developed prediabetes or type 2 diabetes at follow-up. Bars indicate the back-transformed mean of ln-transformed clinical parameter. Error bars indicate the back-transformed 95% confidence interval of ln-transformed clinical parameter. Statistical significance of differences was tested using the t test. White bars = Adv36-negative; black bars = Adv36-positive.
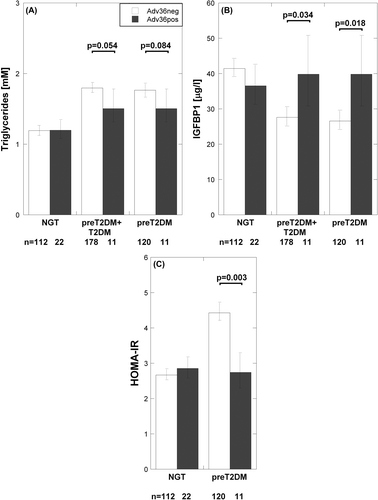
Table III. Unadjusted and adjusted relationships for Adv36 positivity to triglycerides, IGFBP-1, and measures of insulin resistance in persons with normal glucose tolerance (NGT) at baseline who had NGT at follow-up, or had developed prediabetes or type 2 diabetes at follow-up.
In female NGT controls there was no association between Adv36 and HOMA-IR, IGFBP-1, or triglyceride levels (P > 0.4). For males, neither the type 2 diabetes–prediabetes nor the NGT group had significant association between Adv36 and HOMA-IR, IGFBP-1, or triglyceride levels (P > 0.20). HOMA-β did not associate to Adv36 in any group (P > 0.20).
Discussion
This is the first report showing that antibodies against Adv36 are less common in adults with type 2 diabetes compared to healthy individuals with normal glucose tolerance (NGT). In fact, Adv36 seropositivity was on average more than 10-fold less common in females and three times less common in males with type 2 diabetes compared to NGT controls. Further, the data suggested that females with prediabetes, defined as impaired glucose tolerance and/or elevated fasting glucose level, had a ∼2-fold lower prevalence of Adv36 seropositivity compared to NGT controls. Prediabetic females with Adv36 seropositivity had higher insulin sensitivity measured by both HOMA-IR and IGFBP-1 than those being Adv36-seronegative.
These findings are in agreement with the previously reported association between Adv36 seropositivity and better glycemic control and lowered hepatic lipid content among 1507 white and African American adults and children apparently without diabetes, independent of adiposity (Citation24). Further, in a longitudinal study of 1400 Mexican American Hispanics without diabetes, Adv36 positivity was associated with lower fasting glucose levels at baseline in overweight adult females, and with lower fasting insulin level 10 years later in non-obese adults (Citation34). Among 1179 Czech adolescents, Adv36 positivity was associated with increased body fat and reduced blood glucose levels (Citation19). Also, as we recently reported, there was a tendency for lower HOMA-IR in 472 Swedish obese female and male adults without diabetes to be Adv36-positive (Citation21). Krishnapuram et al. (Citation26) reported an increased glucose disposal rate for those who were Adv36-positive among 181 obese and overweight postmenopausal American females without diabetes. Moreover, Adv36 caused increased adiposity, greater insulin sensitivity, and increased glucose uptake in rats (Citation5), increased insulin sensitivity in both chow-fed and high-fat-fed mice (Citation5,Citation24), reduced hepatic lipid content in high-fat-fed mice (Citation24), and increased glucose uptake in fat biopsy cells and skeletal muscle biopsy cells (Citation22,Citation23). Trovato et al. reported that Adv36 positivity was associated with an enhanced recovery of insulin sensitivity after nutritional intervention treatment in non-alcoholic fatty liver disease patients (Citation35). Thus, our current finding is supported by several lines of evidence that Adv36 infection induces an improvement of systemic glycemic control without requiring a reduction in adiposity. An intriguing speculation, based on the in vivo rodent studies (Citation24), is that Adv36 infection may reverse a pathological process towards type 2 diabetes. The fact that in this study we could not detect Adv36-associated better insulin sensitivity among those with NGT, while other studies have reported such an association among persons without diabetes (Citation24,Citation26,Citation34), might be due to our separation of subjects with prediabetes from the NGT group, but other studies did not, by definition, exclude such subjects from the groups without diabetes.
Experimental mechanistic studies have shown that Adv36, in adipose and skeletal muscle tissue, increases cellular glucose uptake by the glucose transporters Glut1 and Glut4, independent of insulin but through recruitment of the distal insulin signaling pathway (PI3K/AKT) via Ras activation (Citation22–24,Citation26). The upregulation of distal insulin signaling was not affected by knocking down proximal insulin signaling (IR knock-down) (Citation26). Further, adiponectin, important for insulin sensitization, is strongly upregulated by Adv36 infection in adipose tissue (Citation36). Experimental molecular data support an effect of Adv36 on the metabolism also in the liver. Hepatic glucose output was reduced in parallel with lowered level of Glut2 and an increased level of adiponectin receptors in Adv36-infected mice (Citation24). Adiponectin signaling activates 5’AMP-activated protein kinase (AMPK) which is a key target for the anti-diabetic drug metformin. Proximal insulin signaling is often impaired in type 2 diabetes. The fact that Adv36 upregulates distal insulin signaling resulting in improved disposal of circulating glucose despite elevated adiposity and systemic inflammation may suggest Adv36 as a model for development of alternative anti-diabetic drugs. The E4 open reading frame-1 gene product (E4orf1) of Adv36 is necessary and sufficient for enhancing glucose disposal (reviewed in 2).
Circulating IGFBP-1 levels correlate well with insulin sensitivity, and low circulating IGFBP-1 levels have been reported to predict the development of glucose intolerance and diabetes (Citation27,Citation28,Citation37). The increased insulin sensitivity, indicated by lowered HOMA-IR and elevated IGFBP-1, in Adv36-positive females with prediabetes, compared to those who were Adv36-negative, was supported by tendencies for reduced fasting serum glucose and insulin levels. The reduced level of circulating triglycerides among those who were Adv36-positive is also suggesting improved insulin sensitivity, since increased serum triglyceride levels are associated with higher insulin resistance (Citation38,Citation39).
In this study, the association between type 2 diabetes and Adv36 was evident for both females and males, whereas the association between prediabetes and Adv36 was found among females only. An improved insulin sensitivity by Adv36 among persons without diabetes has previously been reported for both sexes (Citation24,Citation34), although the findings have been stronger for females (Citation26,Citation34). The protective effect of Adv36 might be hidden sex-specifically by other risk factors for insulin resistance, such as increased visceral fat deposition among males but subcutaneously and more favorable among females (Citation38,Citation40). During the recent decades there has been a change in middle-age type 2 diabetes from a female to a male preponderance (Citation41), and the risk for type 2 diabetes given a family history of type 2 diabetes was stronger in Swedish males than females (Citation42). This may in part be explained by a protective effect of Adv36 being penetrant particularly in females. In this context, it is noteworthy that the prevalence of Adv36 seems to have increased in the Stockholm area of Sweden during the last decades (Citation21).
There was no difference in Adv36 seropositivity between lean and overweight/obese NGT individuals, which could be explained by relatively low BMI values for the overweight/obese group (BMI 25th–75th percentiles: 28.7–31.9 kg/m2), compared to the BMI values for obesity groups associated to Adv36 in whites (BMI mean ≥ 35 kg/m2) (Citation12,Citation14,Citation21).
There is some generalizability of our findings, at least to the Stockholm population. The findings derive from a sample representing those without diabetes aged 35–55 years at baseline in the Stockholm population, and age-, sex-, and area-matched persons without diabetes. All who had type 2 diabetes or prediabetes at follow-up 8–10 years later (8 years for females, 10 years for males), and controls with the same age, were included in the current study. Clinical characteristics and serum samples analyzed were from the follow-up.
Adv36 seropositivity was scored using an ELISA that was previously shown to provide very good precision and to detect eight times lower titers of Adv36 antibodies than the standard, and more time-consuming, serum neutralization assay (SNA) (Citation21). Accordingly, the ELISA scored positive for 36.9% of SNA-negative serum samples (Citation21). Both studies using this Adv36 ELISA (Citation19,Citation21) and previously reported SNA-based studies of human samples (Citation24,Citation26,Citation34) provide findings in agreement with the experimental animal or in vitro studies showing that Adv36 improves insulin sensitivity (Citation5,Citation23,Citation24).
The present study has some limitations. 1) Insulin sensitivity dependence on Adv36 status within the female type 2 diabetes group could not be studied due to few Adv36-positive type 2 diabetes cases. The few Adv36-positive type 2 diabetes cases also resulted in a wide confidence interval of OR for the association between Adv36 and type 2 diabetes in females. 2) Data on levels of circulating triglycerides and IGFBP-1 were not known for all study subjects. However, these data were available for all type 2 diabetes and almost all with prediabetes that had NGT at baseline, and controls with the same age. The age and BMI distributions of those with triglyceride and IGFBP-1 level data were similar to the age and BMI distributions in the full sample stratified for glucose tolerance and sex. 3) Our study population was limited to the age group 44–66 years. However, previous studies have demonstrated that Adv36 is associated with improved insulin sensitivity also in adolescents and young adults (Citation24,Citation34). 4) The NGT controls consisted of one lean group and one overweight–obese group that were sex- and age-matched to allow for comparison of Adv36 serology between those two BMI groups. There was no significant difference in Adv36 seropositivity between lean and overweight/obese individuals (P > 0.25); therefore the NGT controls were treated as one group in the analysis of Adv36 in relation to type 2 diabetes and prediabetes. Ideally, NGT controls should have been BMI-matched to the type 2 diabetes and the prediabetes groups. However, the latter was considered by adjusting the analyses for BMI and age. 5) The NGT controls had normal glucose tolerance at both baseline and follow-up examinations and may therefore be considered ‘super-normal’ and tend to accentuate differences between patients with diabetes or prediabetes and those with NGT. 6) Because the disorder-defined groups overlapped considerably the correction for multiple testing did not account for tests in more than one such group. 7) Time elapsed since Adv36 infection in this study is unknown, and it is unknown for how long a seropositive signal remains after infection. It was previously reported that obese individuals had a greater decline in antibody titer after antiviral vaccination than lean individuals had (Citation43), which may suggest an underestimation of the occurrence of past Adv36 infection in obese. However, no such finding—in any direction—has been reported for type 2 diabetes, and our results were adjusted for BMI. 8) Adv36 was measured only at follow-up, and the study is associative and does not demonstrate causation.
In conclusion, presence of antibodies against Adv36 in serum was dramatically reduced in Swedish adults with type 2 diabetes and adult females with prediabetes. The Adv36-positive females with prediabetes had a more normal glucose sensitivity than the female prediabetes cases that were negative for Adv36. These findings are supported by previous studies in healthy persons, and in experimental models, suggesting that Adv36 promotes glycemic control by upregulation of the distal insulin-signaling components.
Supplementary Table 1
Download PDF (36.9 KB)Acknowledgements
We thank Elisabeth Norén-Krog, Elvi Sandberg, Yvonne Strömberg, and Margareta Andersson at Karolinska University Hospital for their experimental support and the nurses of the health care centers who carried out the OGTTs. This work was supported by grants from the VINNOVA (2009-00223, www.vinnova.se), the Swedish Research Council (2010-3631, www.vr.se), the Karolinska Institutet Foundation (www.ki.se), the Swedish Council for Working Life and Social Research (2008-0375, www.forte.se), the Swedish Diabetes Association (www.diabetes.se), the Family Erling Persson Foundation, and the Obetech Obesity Research Center (www.obesityvirus.com).
C.L., M.A., R.L.A., M.S., C.-G.Ö., and A.H. conceived and designed the experiments. M.A. performed the experiments (guided by J.H.). C.L. analyzed the data. C.-G.Ö., A.H., J.H., and R.L.A. contributed reagents and materials. C.L. wrote the paper. M.A., A.H., R.L.A., C.-G.Ö., M.S., J.H., and K.B. revised the manuscript.
Declaration of interest: Richard L. Atkinson is the owner of Obetech, LLC which provided ELISA assay reagents to Karolinska Institutet for this study. All the analyses in this manuscript were performed at Karolinska Institutet independently of Obetech. Jia He was an employee of Obetech. The other authors report no conflicts of interest.
References
- Wigand R, Gelderblom H, Wadell G. New human adenovirus (candidate adenovirus 36), a novel member of subgroup D. Arch Virol. 1980;64:225–33.
- Dhurandhar NV. Insulin sparing action of Adenovirus 36 and its E4orf1 protein. J Diabetes Complications. 2013;27:191–9.
- Dhurandhar NV, Israel BA, Kolesar JM, Mayhew GF, Cook ME, Atkinson RL. Increased adiposity in animals due to a human virus. Int J Obes Relat Metab Disord. 2000;24:989–96.
- Dhurandhar NV, Whigham LD, Abbott DH, Schultz-Darken NJ, Israel BA, Bradley SM, et al. Human adenovirus Ad-36 promotes weight gain in male rhesus and marmoset monkeys. J Nutr. 2002;132:3155–60.
- Pasarica M, Shin AC, Yu M, Ou Yang HM, Rathod M, Jen KL et al. Human adenovirus 36 induces adiposity, increases insulin sensitivity, and alters hypothalamic monoamines in rats. Obesity (Silver Spring). 2006;14:1905–13.
- Pasarica M, Loiler S, Dhurandhar NV. Acute effect of infection by adipogenic human adenovirus Adv36. Arch Virol. 2008;153:2097–102.
- Pasarica M, Mashtalir N, McAllister EJ, Kilroy GE, Koska J, Permana P, et al. Adipogenic human adenovirus Ad-36 induces commitment, differentiation, and lipid accumulation in human adiposederived stem cells. Stem Cells. 2008;26:969–78.
- Rogers PM, Fusinski KA, Rathod MA, Dubuisson O, Wang Z, Dasuri K, et al. Human adenovirus Ad-36 induces adipogenesis via its E4 orf-1 gene. Int J Obes (Lond). 2008;32:397–406.
- Dhurandhar NV, Kulkarni P, Ajinkya SM, Sherikar A. Effect of adenovirus infection on adiposity in chicken. Vet Microbiol. 1992;31:101–7.
- Vangipuram SD, Yu M, Tian J, Stanhope KL, Pascarica M, Havel PJ, et al. Adipogenic human adenovirus-36 reduces leptin expression and secretion and increases glucose uptake by fat cells. Int J Obes (Lond). 2007;31:87–96.
- Vangipuram SD, Sheele J, Atkinson RL, Holland TC, Dhurandhar NV. A human adenovirus enhances preadipocyte differentiation. Obes Res. 2004;12:770–7.
- Atkinson RL, Dhurandhar NV, Allison DB, Bowen RL, Israel BA, Albu JB, et al. Human adenovirus-36 is associated with increased body weight and paradoxical reduction of serum lipids. Int J Obes (Lond). 2005;29:281–6.
- Na HN, Kim J, Lee HS, Shim KW, Kimm H, Jee SH, et al. Association of human adenovirus-36 in overweight Korean adults. Int J Obes (Lond). 2011;36:195–200.
- Trovato GM, Castro A, Tonzuso A, Garozzo A, Martines GF, Pirri C, et al. Human obesity relationship with Ad36 adenovirus and insulin resistance. Int J Obes. 2009;33:1402–9.
- Trovato GM, Martines GF, Garozzo A, Tonzuso A, Timpanaro R, Pirri C, et al. Ad36 adipogenic adenovirus in human non-alcoholic fatty liver disease. Liver Int. 2010;30:184–90.
- Na HN, Hong YM, Kim J, Kim HK, Jo I, Nam JH. Association between human adenovirus-36 and lipid disorders in Korean schoolchildren. Int J Obes (Lond). 2010;34:89–93.
- Gabbert C, Donohue M, Arnold J, Schwimmer JB. Adenovirus 36 and obesity in children and adolescents. Pediatrics. 2010;126:721–6.
- Atkinson RL, Lee I, Shin HJ, He J. Human adenovirus-36 antibody status is associated with obesity in children. Int J Pediatr Obes. 2010; 5:157–60.
- Aldhoon-Hainerová I, Zamrazilová H, Hlavatá K, Gojová M, Kunešová M, Hill M, et al. Clinical and laboratory characteristics of 1179 Czech adolescents evaluated for antibodies to human adenovirus 36. Int J Obes (Lond). 2014;38:285–91.
- Yamada T, Hara K, Kadowaki T. Association of adenovirus 36 infection with obesity and metabolic markers in humans: a meta-analysis of observational studies. PLoS One. 2012;7:e42031.
- Almgren M, Atkinson R, He J, Hilding A, Hagman E, Wolk A, et al. Adenovirus-36 is associated with obesity in children and adults in Sweden as determined by rapid ELISA. PLoS One. 2012;7:e41652.
- Wang ZQ, Cefalu WT, Zhang XH, Yu Y, Qin J, Son L, et al. Human adenovirus type 36 enhances glucose uptake in diabetic and nondiabetic human skeletal muscle cells independent of insulin signaling. Diabetes. 2008;57:1805–13.
- Rogers PM, Mashtalir N, Rathod MA, Dubuisson O, Wang Z, Dasuri K, et al. Metabolically favorable remodeling of human adipose tissue by human adenovirus type 36. Diabetes. 2008;57:2321–31.
- Krishnapuram R, Dhurandhar EJ, Dubuisson O, Kirk-Ballard H, Bajpeyi S, Butte N, et al. Template to improve glycemic control without reducing adiposity or dietary fat. Am J Physiol Endocrinol Metab. 2011;300:E779–89.
- Jiang S, Gavrikova TA, Pereboev A, Messina JL. Adenovirus infection results in alterations of insulin signaling and glucose homeostasis. Am J Physiol Endocrinol Metab. 2010;298:E1295–304.
- Krishnapuram R, Kirk-Ballard H, Dhurandhar EJ, Dubuisson O, Messier V, Rabasa-Lhoret R, et al. Insulin receptor-independent upregulation of cellular glucose uptake. Int J Obes (Lond). 2013;37:146–53.
- Heald AH, Cruickshank JK, Riste LK, Cade JE, Anderson S, Greenhalgh A, et al. Close relation of fasting insulin-like growth factor binding protein-1 (IGFBP-1) with glucose tolerance and cardiovascular risk in two populations. Diabetologia. 2001;44:333–9.
- Lewitt MS, Hilding A, Ostenson CG, Efendic S, Brismar K, Hall K. Insulinlike growth factor-binding protein-1 in the prediction and development of type 2 diabetes in middle-aged Swedish men. Diabetologia. 2008;51:1135–45.
- Kotronen A, Lewitt M, Hall K, Brismar K, Yki-Järvinen H. Insulin-like growth factor binding protein 1 as a novel specific marker of hepatic insulin sensitivity. J Clin Endocrinol Metabol. 2008;93:1536–40.
- Eriksson A-K, Ekbom A, Granath F, Hilding A, Efendic S, Östenson C-G. Psychological distress and risk of pre-diabetes and type 2 diabetes in a prospective study of Swedish middle-aged men and women. Diabetic Med. 2008;25:834–42.
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of WHO consultation. Diabetic Med. 1998;15:539–53.
- Herbert V, Lau KS, Gottlieb CW, Bleicher SJ. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965;25:1375–84.
- Povoa G, Roovete A, Hall K. Cross-reaction of serum somatomedin-binding protein in a radioimmunoassay developed for somatomedin-binding protein isolated from human amniotic fluid. Acta Endocrinol (Copenh). 1984;107:563–70.
- Lin WY, Dubuisson O, Rubicz R, Liu N, Allison DB, Curran JE, et al. Long-term changes in adiposity and glycemic control are associated with past adenovirus infection. Diabetes Care. 2013;36:701–7.
- Trovato GM, Martines GF, Trovato FM, Pirri C, Pace P, Garozzo A, et al. Adenovirus-36 seropositivity enhances effects of nutritional intervention on obesity, bright liver, and insulin resistance. Dig Dis Sci. 2012;57:535–44.
- Dubuisson O, Dhurandhar EJ, Krishnapuram R, Kirk-Ballard H, Gupta AK, Hegde V, et al. PPARgamma-independent increase in glucose uptake and adiponectin abundance in fat cells. Endocrinology. 2011;152:3648–60.
- Rajpathak SN, He M, Sun Q, Kaplan RC, Muzumdar R, Rohan TE, et al. Insulin-like growth factor axis and risk of type 2 diabetes in women. Diabetes. 2012;61:2248–54.
- Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2006;295:1288–99.
- Subramanian S, Chait A. Hypertriglyceridemia secondary to obesity and diabetes. Biochim Biophys Acta. 2012;1821:819–25.
- Lönnqvist F, Thörne A, Large V, Arner P. Sex differences in visceral fat lipolysis and metabolic complications of obesity. Arterioscler Thromb Vasc Biol. 1997;17:1472–80.
- Gale EA, Gillespie KM. Diabetes and gender. Diabetologia. 2001;44: 3–15.
- Hilding A, Eriksson AK, Agardh EE, Grill V, Ahlbom A, Efendic S, et al. The impact of family history of diabetes and lifestyle factors on abnormal glucose regulation in middle-aged Swedish men and women. Diabetologia. 2006;49:2589–98.
- Sheridan PA, Paich HA, Handy J, Karlsson EA, Hudgens MG, Sammon AB, et al. Obesity is associated with impaired immune response to influenza vaccination in humans. Int J Obes (Lond). 2012;36:1072–7.

