Abstract
Lipoglycopeptide, ketolide, and quinolone antibiotics are currently in clinical development, with specific advantages over available molecules within their respective classes. The lipoglycopeptide oritavancin is bactericidal against MRSA, vancomycin-resistant enterococci, and multiresistant Streptococcus pneumoniae, and proved effective and safe for the treatment of acute bacterial skin and skin structure infection (ABSSSI) upon administration of a single 1200 mg dose (two completed phase III trials). The ketolide solithromycin (two phase III studies recruiting for community-acquired pneumonia) shows a profile of activity similar to that of telithromycin, but in vitro data suggest a lower risk of hepatotoxicity, visual disturbance, and aggravation of myasthenia gravis due to reduced affinity for nicotinic receptors. Among quinolones, finafloxacin and delafloxacin share the unique property of an improved activity in acidic environments (found in many infection sites). Finafloxacin (phase II completed; activity profile similar to that of ciprofloxacin) is evaluated for complicated urinary tract and Helicobacter pylori infections. The other quinolones (directed towards Gram-positive pathogens) show improved activity on MRSA and multiresistant S. pneumoniae compared to current molecules. They are in clinical evaluation for ABSSSI (avarofloxacin (phase II completed), nemonoxacin and delafloxacin (ongoing phase III)), respiratory tract infections (zabofloxacin and nemonoxacin (ongoing phase III)), or gonorrhea (delafloxacin).
Key messages
New antibiotics in the classes of lipoglycopeptides, ketolides, and quinolones are in the late stages of clinical development, mainly for the treatment of acute bacterial infections of skin and skin structures and/or of the respiratory tract.
These molecules mainly address the problem of resistance in Gram-positive bacteria; their dose has been rationally established based on pharmacokinetic/pharmacodynamic concepts in order to optimize the chance of therapeutic success while at the same time avoiding the risk of selection of resistance.
They all presented a satisfactory safety profile in clinical trials, which should be further documented upon administration to larger patient populations.
Since the beginning of this century, only 14 new antibiotics have been approved for use in human medicine (). Still-unmet needs include mainly the so-called ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, Enterobacter spp.), which remain difficult to treat because they have accumulated resistance mechanisms to most of the antibiotics available so far. Global initiatives have been raised to try stimulating research and investment in the field of antibiotic development, like the ‘10 x ‘20 Initiative’ (Citation1) from the Infectious Disease Society of America (http://www.idsociety.org/10x20/) or the ‘Innovative Medicine Initiative’ (http://www.imi.europa.eu/) from the European Union. At this stage, a series of molecules are in the late stages of clinical development (Citation2,Citation3), many of them being essentially new molecules in existing classes, which display, however, better properties in terms of intrinsic activity, reduced susceptibility to resistance mechanisms, and improved pharmacokinetic or safety profiles. This paper will focus on those families that count molecules in phase III of clinical development, namely lipoglycopeptides (oritavancin), ketolides (solithromycin), and quinolones (nemonoxacin; delafloxacin). It will explain the prevailing rationale in the development of these antibiotics and examine their current pharmacological profile based on available clinical data.
Table I. Antibiotics approved by the FDA and the EMA since 2000.
Lipoglycopeptides: focus on oritavancin
Lipoglycopeptides are a subclass within the glycopeptide antibiotics, which all possess a lipophilic tail attached to the amino sugar substituting the cyclic heptapeptide core. Teicoplanin, introduced in the clinics in Europe in 1988, is a natural representative of this subclass. More recently, semi-synthetic derivatives were produced, among which telavancin has been on the market since 2009, dalbavancin has been approved by the FDA in May 2014, and oritavancin is in the late phase of development.
The rationale for the development of these drugs was to cope with vancomycin resistance, which spread in enterococci mainly in the USA at the end of the 1980s.
Vancomycin's mode of action consists in an inhibition of the late stages of peptidoglycan synthesis (4, and references cited herein). The cyclic heptapeptide core of the molecule establishes non-covalent interactions with the D-alanyl-D-alanine termini of the pentapeptide moiety of lipid II. The resulting steric hindrance around these termini prevents the access of enzymes that are needed for cross-linking peptidoglycan precursors via transglycosylation and transpeptidation reactions. As a result, vancomycin is slowly bactericidal, with a spectrum of activity limited to Gram-positive bacteria, because its large size prevents it from crossing the outer membrane of Gram-negative bacteria. Two resistance mechanisms have emerged over the years (4, and references cited herein). The first one, mainly found in enterococci, consists in the acquisition of genes allowing for the synthesis of alternative cell wall precursors ending in D-alanyl-D-lactate or in D-alanyl-D-serine, which show a reduced affinity for vancomycin. At the present time, the prevalence of this resistance mechanism in enterococci collected from infection sites culminates in the USA, reaching 14% (E. faecalis) to 88% (E. faecium) (Citation5) versus about 10% in Europe but with huge variations among countries (Citation6), 15% in Latin America (Citation7), 2% (E. faecalis) to 18% (E. faecium) in Canada (Citation8), and, quite surprisingly, low levels of resistance (< 5%) in Asia (Citation9). Worryingly, a few cases of gene transfer to multidrug-resistant MRSA (methicillin-resistant S. aureus) were reported (13 in the USA and a few in other countries (Citation10), including in important epidemic lineages like US100, US300, and US800). Fortunately, the biological cost of this resistance mechanism is high in MRSA, which may help preventing its spread (Citation11). Another mechanism of resistance emerged in staphylococci, which actually rather confers a moderate level of resistance (VISA phenotype; vancomycin-intermediate Staphylococcus aureus). The molecular mechanism thereof is still poorly understood, but it manifests itself by a thickening of the cell wall, in which vancomycin becomes unable to saturate the large number of free D-alanyl-D-alanine termini. These strains are usually cross-resistant to the lipopeptide daptomycin (Citation12), which needs to cross the cell wall to access its target in the bacterial membrane. Heteroresistance to vancomycin is also common in S. aureus and corresponds to the presence of subpopulations of bacteria with reduced susceptibility to vancomycin. Heteroresistance or intermediate resistance is associated with higher risk of therapeutic failure (Citation13). The prevalence of these strains is controversial because of the difficulty to detect them correctly.
In this context, early work from Eli Lilly demonstrated that chloroeremomycin, which differs from vancomycin by the stereochemistry of the sugar substituting the ring 4 amino-acid and by the presence of an additional L-4-epi-vancosamine, displayed enhanced activity, including against vancomycin-resistant strains, possibly due to dimerization facilitating a co-operative binding to the target (Citation14). In parallel, derivatives of vancomycin substituted by an alkyl side chain on their vancosamine sugar showed also enhanced activity on resistant strains (Citation15). Combining these two features, oritavancin (initially LY333328; Eli Lilly, Indianapolis, IN, USA) was first described in 1996 (Citation16) as the chlorobiphenylmethyl side chain analog of chloroeremomycin (). As compared to vancomycin, this antibiotic shows higher intrinsic activity (lower MICs) () against susceptible Gram-positive organisms, as well as against staphylococci displaying the VISA phenotype or even against VRE (vancomycin-resisitant enterococci) or VRSA (vancomycin-resistant S. aureus) (Citation17,Citation18). This can be explained by a dual mode of action (see for review 19), which combines an inhibition of transpeptidase and transglycosylase activity with an alteration of membrane integrity (Citation20) caused by the anchoring of the lipophilic side chain in the bilayer (Citation21). Importantly also, this novel mode of action confers to oritavancin a rapid and intense bactericidal character, as well as a maintained activity on slow-growing bacteria or on biofilms (Citation22). Among the other remarkable features of this molecule, one should mention its prolonged half-life (terminal half-life > 360 h), which can be attributed to both a high protein binding (85%–90%) (Citation4) and an exceptional capacity to accumulate within eukaryotic cells (Citation23), reaching concentrations as high as 560 mg/L in alveolar macrophages of healthy adults having received a cumulative dose of 4 g (Citation24) (). Coupled to the bactericidal character of the drug, this high accumulation confers to oritavancin a high efficacy against intracellular bacteria, including small colony variants of S. aureus, which are generally particularly recalcitrant to antibiotic action (Citation25,Citation26).
Figure 1. Chemical structure of oritavancin as compared to vancomycin. Major changes are highlighted together with their main consequences for activity or pharmacokinetics.
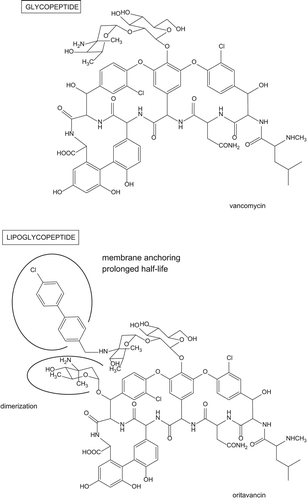
Table II. Susceptibility of relevant pathogens to antibiotics in development and their comparators.
Table III. Main pharmacokinetic properties of antibiotics.
Oritavancin demonstrated its therapeutic interest in early phase II–III clinical trials, where it proved as effective as comparators for the treatment of complicated skin and soft tissue infections caused by Gram-positive bacteria including MRSA (oritavancin 1.5 or 3 mg/kg once daily for 3–7 days versus 15 mg/kg twice daily for 3–7 days followed by oral cephalexin for up to 10–14 days), and for the treatment of S. aureus-associated bacteremia (5–10 mg/kg oritavancin once daily for 10–14 days versus vancomycin 15 mg/kg twice daily or a beta-lactam for 10–14 days) with no evidence of increased incidence of side effects (see (Citation27) for review). Yet its development was slowed down by successive changes in companies (from Eli Lilly to Intermune Inc., San Francisco, CA (2002), to Targanta Therapeutics Corporation, Montreal, Quebec (2006), to The Medicines Companies, Parsippany, NJ (2009) (Citation28)). A first application to the Food and Drug Administration (FDA) in 2009 was unsuccessful, with a request for an additional phase III trial with more MRSA-infected patients as well as further evaluation of oritavancin effects on macrophage functions, in relation to the huge cellular accumulation of the drug. In vitro data documented that the drug could indeed cause a mixed storage disorder in lysosomes (Citation29) as well as inhibition of latex bead phagocytosis (Citation30), but at concentrations far higher than those observed in alveolar macrophages from treated volunteers. Moreover, no changes in bacterial phagocytosis, killing capacities, or reactive oxygen species production were observed in conditions mimicking human exposure (Citation30,Citation31), ruling out a major risk of toxicity associated to the cellular tropism of the drug. New phase III trials were designed (), in which the therapeutic scheme was revisited based on recently acquired pharmacodynamic data favoring the administration of a single dose of 1200 mg. The main arguments supporting this unique administration are the concentration-dependent bactericidal character of the drug and its prolonged half-life (Citation32). The corresponding pharmacokinetic data are illustrated in , highlighting a high free Cmax and prolonged terminal half-life (Citation33). A pilot phase II trial supported this concept (Citation34). It demonstrated that the clinical response was better in patients with acute bacterial skin and skin structure infections treated by a single 1200 mg dose or 800 mg on day 1 followed by 400 mg on day 5 than in those receiving a conventional daily administration of 200 mg for 3–7 days, with no sign of adverse events. Interestingly enough, this therapeutic scheme would be compatible with outpatient parenteral antimicrobial therapy (Citation35,Citation36), which is associated with many benefits (improved quality of life, reduced costs and risks of nosocomial infections). Preliminary reports from these recent phase III studies were released and show equivalent efficacy for a single 1200 mg dose of oritavancin as for a 7–10-day treatment with vancomycin (15 mg/kg BID) (Citation37,Citation38), with no sign of side effects. The US FDA has accepted a new drug application for oritavancin with priority review, with action date scheduled for 6 August 2014. On its side, the European Medicines Agency (EMA) has accepted for review a marketing authorization application in February 2014.
Table IV. Recent clinical trials posted on the clinicaltrials.gov repository for antibiotics under development.
Ketolides: focus on solithromycin
Ketolides are a subclass of macrolide antibiotics characterized by the absence of a 3-O-cladinose sugar (replaced by a keto group), a 11,12- or 6,11-cyclic moiety, and a heteroaryl-alkyl side chain attached to the macrocyclic ring through a suitable linker (Citation39). They show an improved activity against strains resistant to conventional macrolides. Macrolides inhibit protein synthesis by binding to the domain V of the 50S ribosomal subunit and preventing elongation of the peptidic chain. The main mechanism of resistance consists in the methylation of the ribosomal subunit at the position A2058, which considerably reduces the affinity of the antibiotic for its target by creating steric hindrance at the antibiotic binding site. Another mechanism of resistance consists in the expression of efflux systems, which reduce the intrabacterial concentration of the drug. The latter mechanism is responsible for the intrinsic resistance of most Gram-negative bacteria to macrolides and is also associated to acquired, inducible resistance in Gram-positive bacteria, mainly in streptococci. Macrolide resistance is widely spread over the world, reaching 25% in the US (Citation40), 70% in Asia with still higher figures in specific counties (Citation41), and ranging from 50% (Malta) to less than 5% (The Netherlands, Norway, Latvia) in Europe, with intermediate values in most countries (5%–10% in seven countries, 10%–25% in eight countries, and 25%–40% in nine countries) (Citation42). Worryingly also, resistance is emerging and spreading in bacteria responsible for sexually transmitted diseases for which macrolides often represent a first choice (Citation43,Citation44).
By their additional side chain, ketolides bind to both domains V and II of the ribosomal subunit, so that they keep sufficient affinity for methylated ribosomes to inhibit protein synthesis effectively in macrolide-resistant strains. Moreover, the absence of cladinose makes them unable to induce methylase production (Citation45). Ketolides are also less subject to active efflux by Streptococcus pneumoniae while remaining extruded by S. pyogenes (Citation39,Citation46). Taken together, these properties insured a renewed interest for these antibiotics in the treatment of respiratory tract infections (Citation39). Telithromycin is the only ketolide on the market since 2001 in Europe and 2004 in the US. Yet, its use has been drastically reduced since 2007 because rare but severe side effects were reported, leading to the addition of warnings to the summary of product characteristics (Citation47,Citation48), advising of a risk of acute hepatic failure and severe liver injury, as well as of visual disturbance, transient loss of consciousness, and life-threatening respiratory failure in patients with myasthenia gravis. It has been suggested that these adverse effects could result from a blockade of nicotinic acetylcholine receptors present at the vagus nerve innervating the liver, the ciliary ganglion of the eye, and at the neuromuscular junction, thanks to the pyridine-imidazole group present on the side chain of telithromycin (Citation49).
A series of other molecules are currently in clinical development (), which belong to three subfamilies (Citation50), namely 11-N ketolides, including fluoroketolides like solithromycin, 6-O ketolides, such as cethromycin, and bridged bicyclic ketolides like modithromycin (EDP-420) or EDP-322 (developed as its EDP-788 prodrug).
Figure 2. Chemical structure of ketolides in development as compared to telithromycin. Major changes are highlighted together with their main consequences for activity or pharmacokinetics. The structure of EDP-322 having not yet been released, modithromycin is presented as an exemplative typical bridged bicyclic ketolide.
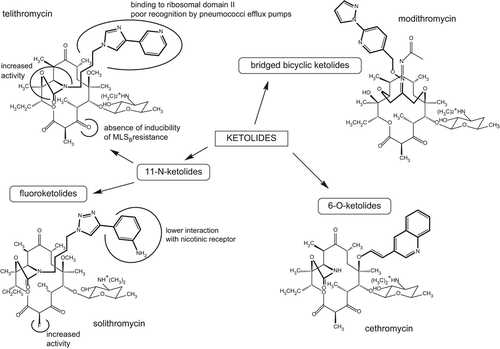
Cethromycin (Abbott compound ABT-773, further developed by Advanced Life Sciences, Inc., Woodridge, IL, USA) completed two phase III trials where it was compared at a dose of 300 mg QD to clarithromycin (250 mg BID) for the treatment of community-acquired pneumonia (Citation51). However, it was denied by the FDA in 2009, being considered as safe but not effective enough to justify its marketing, as it essentially showed equivalence to clarithromycin (Citation52).
The Enanta Pharmaceuticals (Watertown, MA, USA) compound modithromycin entered phase II clinical trials in 2006 but has now been supplanted by EDP-322, which is at the present time in phase I. While modithromycin was essentially studied in vitro and in vivo as an antipneumococcal drug (Citation53–55), EDP-322 is rather positioned as a potent anti-MRSA agent.
The fluoroketolide solithromycin (CEM-101; Cempra Inc., Chapel Hill, NC, USA) is currently recruiting patients for two phase III clinical trials where it is compared with oral moxifloxacin for the treatment of community-acquired pneumonia. As compared to telithromycin, solithromycin may offer an improved safety profile. Since its side chain does not possess the pyridine-imidazole group of telithromycin, it is a 30-times less potent inhibitor of nicotinic receptors than telithromycin (Citation49). Accordingly, none of the rare adverse effects of telithromycin had been observed in current phase I/II trials with solithromycin (Citation56). With respect to its mode of action, solithromycin, as other ketolides, remains capable of binding to ribosomes that are mono- or even dimethylated, thanks to three structural features. First, the absence of cladinose gives more movement freedom to the desosamine sugar and allows repositioning of the antibiotic in the ribosomal binding site. This avoids the steric clash between the antibiotic and the dimethylated A2058 that explains poor binding of conventional macrolides to dimethylated ribosomes and subsequent resistance. Second, the additional aryl-alkyl side chain can interact with a base pair formed by A752 and U2609 in the 23S RNA from both native and methylated ribosomes, further contributing to strengthen the antibiotic binding to ribosomes from susceptible and resistant strains. Third, the 2-fluorine substituent, which is not present in telithromycin, may possibly account for the higher intrinsic activity of solithromycin as compared to telithromycin (Citation57). As for other macrolides and ketolides, solithromycin's pharmacokinetic profile is characterized by a broad tissue distribution and high cellular accumulation (Citation58,Citation59), reaching elevated concentrations in alveolar macrophages (24-h AUC: 1500 mg.h/L; ratio to serum concentration: 180) and epithelial lining fluid (24-h AUC: 80 mg.h/L; ratio to serum concentration: 10). Its half-life of 6.65 h related to a high protein binding (85%) (Citation60) allows for a once-a-day administration, with a proposed therapeutic scheme by oral route consisting in a loading dose of 800 mg followed by a 4-day treatment with a daily dose of 400 mg. In these conditions, serum levels reached a Cmax and an AUC of approx. 0.8 mg/L and 7 mg.h/L (for a 400 mg dose) () and of approximately 1.3 mg/L and 14 mg.h/L (for an 800 mg dose) (Citation60). This scheme allows reaching the pharmacodynamic target demonstrated as predictive of efficacy for this drug, namely an AUC/MIC > 1.3 h in epithelial lining fluid (ELF) (Citation61) with a probability of 99.9% for MICs as high as 1 mg/L (Citation62). However, a loading dose does not seem necessary when administering the drug by intravenous route (Citation63). MIC90 against contemporary isolates of respiratory pathogens were 0.25 mg/L for S. pneumoniae (0.5 mg/L for multiresistant strains), 0.015 mg/L for Legionella pneumophila, and 0.5 mg/L for Moraxella catarrhalis. As other macrolides, solithromycin is less active on Haemophilus influenzae (MIC90 2 mg/L). Nevertheless, all MIC90 values remain lower than those recorded for telithromycin (Citation64). Solithromycin also shows useful activity against staphylococci and enterococci (Citation65), or pathogens causing sexually transmissible diseases (Chlamydia trachomatis (Citation66), Neisseria gonorrhoeae (Citation67–69), Mycoplasma spp. (Citation70,Citation71)). Interestingly, solithromycin MICs remain low against strains resistant to conventional macrolides or to other antibiotic classes (Citation72,Citation73), suggesting it may represent a useful alternative to currently recommended drugs in areas with high resistance rates. Lastly, solithromycin demonstrates activity against biofilms formed by S. pneumoniae (Citation74), which may be an advantage when dealing with chronic infections where biofilms are thought to play a major role in recurrence of the infection. Taken together, these properties are advantageous for the treatment of respiratory or genital infections. They also rationalize activity against intracellular pathogens like S. aureus, Listeria monocytogenes, L. pneumophila, and N. gonorrhoeae (Citation58,Citation67), against which solithromycin proves at least as effective but more potent than other macrolides, based essentially on its lower MICs and not on its higher accumulation level. In the clinics, solithromycin has, at this stage, already proven as effective as levofloxacin with a more favorable safety profile in a phase II trial for the treatment of community-acquired bacterial pneumonia, where patients were randomized to receive either 800 mg solithromycin orally on day 1, followed by 400 mg daily on days 2 to 5, or 750 mg levofloxacin during 5 days (Citation75). Notably, no influence on the QTc interval was observed so far (Citation56). A phase II clinical trial is examining the efficacy of a single dose for the treatment of uncomplicated gonorrhea. The drug has received the Qualified Infectious Disease Product (QIDP) status and Fast Track designation for community-acquired pneumonia from the US FDA in September 2013.
Quinolones
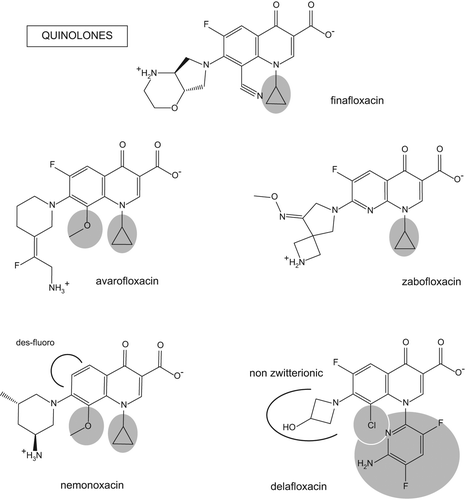
Quinolone antibiotics represent one of the largest antibiotic classes when considering that already in 2005 about 10,000 compounds had been patented and 800 million patients had been treated (Citation76,Citation77). Fluoroquinolones inhibit bacterial replication by forming a ternary complex with DNA and class II topoisomerases (DNA gyrase and topoisomerase IV), two enzymes responsible for DNA supercoiling. Quinolones have been categorized in successive generations (Citation77–79) based on the nature of their substituents, which governs their interaction with their pharmacological target and their spectrum of activity (Citation77,Citation79). The substituents that best increase potency are a cyclopropyl or, alternatively, a difluorophenyl in position 1, a fluorine in position 6, and a halogen, methoxy, or fused third ring in position 8 (). Molecules harboring a piperazine-based substituent in position 7 are in general more active on Gram-negative bacteria and preferentially target DNA gyrase, while those presenting a pyrrolidine-based substituent rather show activity on Gram-positive bacteria and target topoisomerase IV. Dual targeting molecules present a broad spectrum of activity. Nalidixic acid (by-product of antimalarial research) is representative of the first generation, with only a narrow spectrum, low serum levels, and toxicity issues. The second generation is characterized by the addition of a fluorine substituent at position 6 (hence the name of fluoroquinolones often given to the whole class), which markedly increases activity. Ciprofloxacin is the most widely used molecule in this generation and remains one of the most active fluoroquinolones on Gram-negative bacteria. Levofloxacin, the active isomer of ofloxacin, another second-generation molecule, is considered by certain authors as constituting an independent generation (Citation78). Moxifloxacin is the leading molecule in the next generation, which is characterized by a spectrum of activity rather oriented towards Gram-positive bacteria including anaerobes (activity on Gram-negative anaerobes like Bacteroides is too weak to envision its use for the treatment of intra-abdominal infections (Citation80)). Among more recent generations, one can find molecules like gemifloxacin (marketed in the US and in Korea), which also include Gram-positive anaerobes in their spectrum, or garenoxacin (marketed in Japan), which lacks the fluorine in position 6, giving rise to the desfluoroquinolones subclass (Citation77). Despite the tremendous number of molecules produced, only a few of them were brought on the market, among which some were withdrawn or have seen their use restricted because of toxicity issues (Citation81). More frequent reasons for withdrawal include tendinitis (pefloxacin), rash (sparfloxacin, clinafloxacin), QTc prolongation (grepafloxacin), dysglycemia (gatifloxacin, clinafloxacin), hemolysis (temafloxacin), and hepatotoxicity (trovafloxacin) (Citation77,Citation79,Citation81). Resistance to fluoroquinolones is primarily caused by target mutations, which can accumulate and lead to high-level resistance. First-step mutations occur in general in the primary target enzyme (thus more often in GyrA subunit of DNA gyrase in Gram-negative bacteria; ParC subunit of topoisomerase IV in Gram-positive bacteria) (Citation77). Yet, this may vary depending also on the bacterial species, the reverse being notably observed in S. pneumoniae (Citation77,Citation82). Active efflux is contributing to decreased susceptibility as well. Gram-positive bacteria do express narrow-spectrum pumps extruding only fluoroquinolones. NorA was historically described as the fluoroquinolone transporter in S. aureus (Citation83), but more recent studies suggest a potential role of other efflux pumps in clinical isolates, like NorB, NorC, MdeA (Citation84) or the plasmid-encoded QacA and QacB (Citation85). In S. pneumoniae, PmrA was the first described transporter (Citation86), but the heterodimer PatA/PatB is now considered as the main efflux system playing a role in resistance of clinical isolates (Citation87). Of note, there are huge differences in the recognition of different fluoroquinolones by these pumps, norfloxacin and ciprofloxacin being more affected than moxifloxacin, for example (Citation88,Citation89), due to their more hydrophilic character and to the absence of a bulky substituent in position 7. In Gram-negative bacteria, fluoroquinolones are virtually universal substrates of many broad-spectrum transporters (Citation90). By reducing intrabacterial concentration of antibiotics, efflux can contribute to select target mutations and therefore participate in increasing levels of resistance (Citation91). More anecdotal resistance mechanisms include the plasmid-mediated production of the protein Qnr that impairs the binding of fluoroquinolones to DNA (Citation92), or the N-acetylation of fluoroquinolones harboring a piperidine substituent in position 7 (norfloxacin and ciprofloxacin) by an AAC(6’)-Ib-cr enzyme originally inactivating aminoglycosides (Citation93). As recently reviewed in extenso (Citation94,Citation95), epidemiological surveys performed over the globe demonstrate increasing rates of fluoroquinolone resistance, but huge discrepancies among countries, with the highest figures being observed in the Asia–Pacific region and lower ones in Europe and North America. Considering resistance to ciprofloxacin in Gram-negative species, it can reach more than 20% in uropathogens or bacteria causing intra-abdominal infections. Worryingly enough, much higher values (> 70%) are reported in enterobacteriaceae displaying other mechanisms of resistance (including production of extended-spectrum beta-lactamases (Citation96)) or associated with complicated infections. Among enteropathogens, Campylobacter species show the highest resistance rate (80% in some reports), but Salmonella and Shigella often harbor plasmid-mediated resistance and started to spread in the Middle East. More than half of Escherichia coli causing traveler's diarrhea are fluoroquinolone-resistant in Asia or Africa. In anaerobes, resistance rates are elevated in some countries (∼50%); selection may have occurred after using early-generation fluoroquinolones displaying poor antianaerobic activity. Among respiratory tract pathogens, Haemophilus influenzae and Moraxella catarrhalis remain susceptible while resistance rates in Streptococcus pneumoniae are so far usually low (< 4%). Again, at-risk situations include elderly patients, nursing homes, or hospitals. In spite of fluoroquinolone contra-indication in pediatrics, resistance has been detected in pneumococci isolated from children, possibly due to transmission from adults. In Gram-negative pathogens causing health care-associated pneumonia, resistance rates vary enormously among countries and regions, making local surveillance data indispensable. In bacteria causing skin infections, fluoroquinolone resistance is so common among MRSA that current molecules can no more be considered as valuable therapeutic options. MSSA (methicillin-susceptible S. aureus) remain more susceptible, as well as Streptococcus pyogenes (with, however, some local spots of higher resistance, like in Belgium (Citation97)). Among pathogens causing sexually transmitted diseases, high variations in resistance rates are observed. The more alarming reports concern Neisseria gonorrhoeae, with values ranging from 10% in the US to 60% in Europe and more than 90% in Asia.
Figure 3. Chemical structure of new fluoroquinolones in clinical development. The substituents in position 1 and 8 known to confer high intrinsic activity are on a gray background. Other specific features are highlighted by black arcs.
In this context, research of new molecules over the last years has focused on the identification of molecules displaying high intrinsic activity including against strains resistant to current fluoroquinolones. This goal was achieved by in-depth structure–activity relationship studies in order to identify the substituents that allow a dual targeting of both topoisomerase IV and DNA-gyrase together with a high binding affinity to these enzymes.
Among the numerous quinolones under study at the present time, five new molecules are in clinical development (). They all show increased intrinsic activity, including against strains resistant to current fluoroquinolones, due to an improved interaction with their target enzymes.
Finafloxacin (BAY35-3377; Bayer HealthCare, Wuppertal, Germany; now developed by MerLion Pharmaceuticals GmbH; Singapore and Berlin, Germany) is an 8-cyano-fluoroquinolone. It has the particularity of showing increased bacterial uptake, and therefore enhanced activity, at acidic pH (Citation98–100), a condition which usually reduces the potency of this class of drugs. This constitutes an advantage for the treatment of infections localized in an acidic environment like the urinary and genital tracts, the gastric mucosa, the airways of patients suffering from chronic inflammatory diseases, or abscesses (Citation99) as well as against intracellular infections with phagolysosomal bacteria like S. aureus (Citation98). Finafloxacin MICs against ciprofloxacin-susceptible or resistant Gram-negative bacteria are similar to those of ciprofloxacin at neutral pH but 3–5 dilutions lower at pH 5.2 (Citation99–102). It is, however, less active than ciprofloxacin on Pseudomonas aeruginosa or other ESKAPE pathogens like Klebsiella or Enterobacter spp. (Citation101). In contrast to ciprofloxacin, finafloxacin is not a substrate for the fluoroquinolone efflux system by QepA1 of Escherichia coli (possibly related to its lower hydrophilicity) and is not affected the AAC(6’)-Ib-cr acetylase, as it does not display the piperazine ring substrate for this enzyme in position 7 (Citation100). In phase I studies by oral route, finafloxacin Cmax was close to 11 mg/L for an 800 mg dose and an AUC of 28 mg.h/L (). Cmax increased linearly with the dose, but AUC normalized to the dose increased of about 50% for doses ≥ 400 mg, because elimination was slowed down (t1/2 of approximately 5 h for doses lower than 400 mg and 10 h for higher doses). This deviation from linearity is, however, thought to result from inaccurate detection of low concentrations (Citation103). Considering as pharmacodynamic criterion of efficacy an AUC/MIC > 125 h−1 for infections caused by Gram-negative bacteria (Citation104), a pharmacodynamic breakpoint of 0.25 mg/L could be proposed for this dose. Yet, the drug can reach much higher concentrations in the urine than in serum (Citation103), which may insure its efficacy even for less susceptible bacteria, especially if taking into account the acidic pH of this fluid. The accumulation of the drug within eukaryotic cells (about 5-fold) explains activity on intracellular organisms like S. aureus, L. pneumophila, or L. monocytogenes, with a relative potency and a maximal efficacy similar to those of ciprofloxacin (Citation98). Intracellular potency is, however, improved when cells are incubated at acidic pH, because this condition increases the accumulation of the drug within eukaryotic cells as well. The US Food and Drug Administration has designated finafloxacin for oral and intravenous use as a Qualified Infectious Disease Product and for Fast Track development for the treatment of complicated urinary tract infections (cUTI) including pyelonephritis, complicated intra-abdominal infections (cIAI), and acute bacterial skin and skin structure infections (ABSSSI). It is currently in phase II of clinical development (). Moreover, the drug has also completed two phase III trials for the treatment of ear infections using topical application, in partnership with Alcon Pharmaceuticals Ltd (Forth Worth, TX, USA).
Avarofloxacin (JNJ-Q2; Janssen Pharmaceutica, subdivision of Johnson & Johnson, licensed to Furiex Pharmaceuticals, Morrisville, NC, USA, in 2011) harbors a cyclopropyl in position 1, a methoxy in position 8, and a bulky, 6-membered aminated substituent in position 7, itself substituted by a fluorine. Avarofloxacin proves more active than moxifloxacin against Gram-positive pathogens, including MRSA or S. pneumoniae resistant to fluoroquinolones (). It is also slightly (1–2 dilutions) more active than moxifloxacin against other respiratory pathogens like H. influenzae or M. catarrhalis (Citation105) and than ciprofloxacin against N. gonorrhoeae, against which it also keeps activity on ciprofloxacin-resistant strains (Citation106). In vitro, animal and human data assessing cardiovascular safety disclosed a profile comparable to that of moxifloxacin (Citation107). Considering that a free AUC/MIC = 14 h−1 generates a static effect in animal models of skin infections by S. aureus, PK/PD simulations showed a target attainment rate of 0.966 for MIC ≤ 0.5 mg/L if using the drug at an oral dose of 250 mg or an intravenous dose of 150 mg twice daily (Citation108).
This dosage was therefore selected in clinical trials. Pharmacokinetic data from phase I () reported a Cmax of approx. 2 mg/L, and AUC of 28 mg.h/L and a half-life of approximately 14 h for an oral dose of 250 mg, with ELF/plasma and alveolar macrophages ratios ranging, respectively, between 17 and 64, and 74 and 157 (Citation109). A first published report of a phase II study showed comparable cure rates for avarofloxacin (150 mg intravenously twice daily followed by 250 mg orally twice daily) and moxifloxacin (400 mg once daily, intravenously or orally) for the treatment of community-acquired infection (Citation110). Yet, the number of patients was too small (16 in each arm) to perform in-depth statistical analyses. Avarofloxacin received a Qualification as Infectious Disease Product and Fast Track designation from the US FDA in February 2013 and is ready to start phase III trials for the treatment of acute bacterial skin and skin-structure infections and community-acquired pneumonia.
Zabofloxacin (DW-224a; Dong Wha Pharmaceuticals Industry, Ltd; Anyang City, Korea) is constructed on a naphthyridone nucleus. It presents a cyclopropyl substituent in position 1 and a bulky, pyrrolidine-based substituent in position 7, which directs its spectrum towards Gram-positive bacteria. It targets both DNA gyrase and topoisomerase IV in S. pneumoniae, which reduces the risk of selection of resistance (Citation111). It is in general 2–16 times more potent than moxifloxacin on Gram-positive bacteria but 2–4 times less potent than ciprofloxacin against Gram-negative bacteria (Citation112). It is also more potent than ciprofloxacin against N. gonorrhoeae or C. trachomatis, suggesting it could be an appealing alternative to ciprofloxacin against macrolide-resistant strains (Citation113). A phase I trial evidenced a Cmax of approximately 2 mg/L and a 24-h AUC of approximately 11 mg.h/L after a single oral dose of 400 mg () (Citation114). A phase II trial comparing zabofloxacin (400 mg QD for 3 or 5 days) to moxifloxacin (400 mg QD for 7 days) for the treatment of mild to moderate community-acquired pneumonia concluded the clinical and microbiological cure rates were similar, with no sign of side effects (Citation115). A second phase II trial has compared it to levofloxacin in the same indication (). Zabofloxacin is claimed to be in late stage of phase III development for respiratory tract infections by Gram-positive resistant bacteria, but no additional information has been made available.
Nemonoxacin (TG-873870; TaiGen Biotechnology Co., Ltd., Taiwan) is a desfluoroquinolone harboring a methoxy group in position 8 and a 6-membered aminated substituent in position 6. It is globally 2–8-fold more potent than moxifloxacin against most Gram-positive cocci but 4-fold less potent than ciprofloxacin against Gram-negative bacilli (Citation116). Additionally, it also shows useful activity on Chlamydia spp. (Citation117) or Clostridium difficile (Citation118).
In preclinical studies, nemonoxacin was minimally metabolized (less than 5% metabolites recovered) and did not influence human hepatic CYP3A4 activity; it had minimal effect on cardiac conduction as measured by ECG QTc interval prolongation and a low phototoxic potential (119 and references cited therein). Human pharmacokinetic data for the oral route () showed a Cmax around 3.5–5 mg/L and an AUC of approximately 32 mg.h/L for a dose of 500 mg, a low protein binding (16%) but a half-life of 10–15 h (longer upon administration of higher doses). This allows for a once-a-day mode of administration while maintaining free serum levels about the MIC of target pathogens (Citation119,Citation120). AUC was decreased of about 17% by food intake. The most common adverse effect observed in phase I was headache (Citation120). In vitro pharmacodynamic studies suggest that a 3-log kill could be achieved against S. pneumoniae for free AUC/MIC ratio > 47.5 h−1 (Citation121). This is consistent with the globally accepted concept that the probability for therapeutic success with quinolones is free AUC/MIC ratio > 25–40 h−1 for Gram-positive bacteria (Citation122). A pharmacodynamic breakpoint of ∼0.5 mg/L could be proposed on these bases, which is well above the MIC distribution of pneumococci () and can partly cover MRSA. The drug completed two phase II clinical trials with the oral formulation for community-acquired pneumonia and diabetic foot infection, and one phase II clinical trial with the intravenous formulation for community-acquired pneumonia, as well as one phase III trial for the treatment of community-acquired pneumonia by oral route. The results of one of these trials were published (Citation123) and showed that nemonoxacin (750 mg or 500 mg) orally for 7 days was as effective as levofloxacin (500 mg), with 1) clinical success rates close to 90%, 2) bacterial eradication of 90% in the 750 mg dose and the levofloxacin groups versus 85% in the 500 mg dose group, and 3) good tolerability. The US FDA granted nemonoxacin Qualified Infectious Disease Product and Fast Track designations for community-acquired bacterial pneumonia (CAP) and acute bacterial skin and skin structure infections (ABSSSI) in December 2013.
Delafloxacin (WQ-3034, discovered by Wakunaga Pharmaceutical Co., Ltd., Osaka & Hiroshima, Japan; further developed as ABT-492, Abbott Park, IL, USA, and then as RX-3341 by Rib-X Pharmaceuticals Inc., New Haven, CT, USA; now Melinta Therapeutics, New Haven, CT) has the unique property of being an anionic fluoroquinolone, as it lacks a positively charged substituent in position 7. This chemical feature rationalizes why it accumulates much more in both bacteria and eukaryotic cells at acidic pH (Citation124). Delafloxacin shows very low MICs against Gram-positive pathogens, with values typically 4 dilutions lower than those of moxifloxacin, even against strains showing elevated MIC to the reference drug (Citation124). At acidic pH, the difference in potency between the two antibiotics can reach 7 dilutions (Citation124). The high potency of delafloxacin is thought to result from the specific shape, size, and polarity of the molecule as compared to conventional fluoroquinolones (Citation125). Although designed as an anti-Gram-positive drug, it is also at least as potent as ciprofloxacin against Gram-negative bacteria, including P. aeruginosa (). Selection of resistance in S. aureus is infrequent (10−9 to 10−11), and concentrations preventing the selection of mutations (MPC (Citation126)) range from 1 to 4 times the initial MIC, with values 8- to 32-fold lower than for other quinolones (Citation127). Delafloxacin also proved active in in vitro models of biofilm or intracellular infection by S. aureus (Citation124,Citation128), despite the fact it mainly localizes in the cytoplasmic compartment of cells (Citation124). This may be due to the high diffusibility of fluoroquinolones, which may help them freely to cross biological membranes within the cells to gain access to the infected compartment. Efficacy was further documented in animal models of granuloma pouch by Gram-negative bacteria and thigh infection or renal abscess by S. aureus (Citation129–131).
In phase I trials (), delafloxacin showed a Cmax of 10 and 16 mg/L and an AUC of 24 and 40 mg.h/L after IV administration of 300 and 450 mg, respectively, with a free fraction of 84% and a half-life of 8–12 h (Citation132,Citation133).
Similar values (slightly lower Cmax (7 mg/L)) were observed in phase II patients treated for acute bacterial skin and skin structure infections by a daily dose of 300 mg intravenous and BID (Citation134). Elimination is mainly renal, but metabolites have been detected, among which a glucuronoconjugate (Citation135). The dose should be reduced from 300 to 200 mg IV in case of renal insufficiency (Citation136). Based on a pharmacodynamic target of fAUC/MIC = 25–40 h−1 for Gram-positive infections (Citation122), a pharmacodynamic breakpoint of 0.5 mg/L could be proposed, which covers most of the strains, including fluoroquinolone-resistant ones. This is in accordance with PK/PD animal data (Citation137) and Monte Carlo simulations, which concluded to a > 90% target attainment rate for MICs ≤ 0.5 mg/L upon administration of 300–450 mg BID (Citation133), and rationalizes the doses used in phase III trials (). Safety data available so far did not reveal any specific adverse events (Citation132,Citation138), including on the cardiac function (no prolongation of QTc interval (Citation139)). Published data from phase II clinical trials demonstrated equal efficacy in the treatment of acute bacterial infection of skin and skin structure infection (ABSSSI) for delafloxacin 300 mg IV BID as compared to linezolid or vancomycin but better efficacy when considering as end-point a reduction > 20% or > 30% in lesion size after 48–72 hours (Citation138,Citation140). The US FDA has granted delafloxacin the status of a Qualified Infectious Disease Product for the indications of acute bacterial skin and skin structure infections (ABSSSI) and community-acquired bacterial pneumonia (CABP) in October 2012. It is currently in phase III of clinical development for acute bacterial infection of skin and skin structure infection and uncomplicated gonorrhea based on its excellent in vitro activity against N. gonorrhoeae, including ciprofloxacin-resistant strains (Citation141).
Conclusion
At the end of this survey, one can see that the molecules in the last stages of development mainly address the question of resistance in Gram-positive pathogens, among which MRSA and VRE belong to the so-called ESKAPE pathogens. Oritavancin consistently shows low MIC on VRE, multiresistant pneumococci, MRSA, but also VRSA and, to some extent, VISA. Solithromycin and new quinolones (except finafloxacin) bring in general an impressive response to resistance to earlier-generation molecules in staphylococci and pneumococci. Some variations do, however, exist among fluoroquinolones, with avarofloxacin and delafloxacin being the more potent on MRSA. Delafloxacin and finafloxacin display a clear advantage for infections located in acidic territories caused, respectively, by Gram-positive and Gram-negative bacteria. While being the only molecule rather directed towards Gram-negative bacteria, finafloxacin does not offer any advantage over ciprofloxacin on ESKAPE pathogens.
Oritavancin can be considered as a member of a totally new antibiotic class, since its mode of action is clearly different from that of conventional glycopeptides. It could usefully complement the only marketed molecule in this class, telavancin, by a somewhat higher activity on VRSA and VRE and, most conspicuously, by its original mode of administration, which clearly offers a series of advantages in terms of ease of use and reduction of hospitalization duration.
Ketolides and new quinolones essentially offer improved intrinsic activity as compared to earlier molecules, which was reached by optimizing their binding to the pharmacological targets. Hopefully enough, the dose of all of them has being rationally established based on PK/PD concepts, which should help limiting the risk of selection of resistance if used appropriately. Yet, we clearly need much more data related to their clinical efficacy on multidrug-resistant pathogens and their safety profile. For registration, health authorities demand at this stage the demonstration of an equivalence to standard treatment, which, by definition, prevents the investigators from enrolling patients infected by bacteria resistant to the comparator. These resistant strains are indeed actually the main targets for the new drugs. The scientific community is therefore pushing for the inclusion of non-comparative trials directed to the evaluation of new antibiotics against these specific populations or of superiority trials in the development plan of new antibiotics (Citation142). With respect to safety issues, experience with previously marketed molecules has shown that severe adverse reactions were too rare to be detected during clinical development. Post-marketing surveillance is therefore essential. Moreover, one can argue that reasonable use, meaning limited to infections sufficiently severe really to require antibiotic treatment, should contribute to contain this risk. This would go through the establishment of rational and regularly updated guidelines for the treatment of bacterial infections in order to preserve the interest of these new molecules we are looking forward to seeing on the market.
Notice of Correction
Following acceptance of this manuscript and publication online ahead of print on 24 July 2014, FDA approval has been obtained in August 2014 for the use of oritavancin for the treatment of acute bacterial skin and skin structure infections.
Acknowledgements
F. Van Bambeke is Maître de recherches of the Belgian Fonds de la Recherche Scientifique (FSR-FNRS).
Declaration of interest: F. Van Bambeke has received research grants from The Medicine Company, Cempra Pharmaceuticals, MerLion Pharmaceuticals, and Melinta Therapeutics.
References
- Boucher HW, Talbot GH, Benjamin DK Jr, Bradley J, Guidos RJ, Jones RN, et al. 10 x ‘20 Progress—development of new drugs active against gram-negative bacilli: an update from the Infectious Diseases Society of America. Clin Infect Dis. 2013;56:1685–94.
- Gould IM, Bal AM. New antibiotic agents in the pipeline and how they can help overcome microbial resistance. Virulence. 2013;4:185–91.
- d’Urso de Souza Mendes C, de Souza Antunes AM. Pipeline of known chemical classes of antibiotics. Antibiotics. 2013;2:500–34.
- Van Bambeke F, Van Laethem Y, Courvalin P, Tulkens PM. Glycopeptide antibiotics: from conventional molecules to new derivatives. Drugs. 2004;64:913–36.
- Edelsberg J, Weycker D, Barron R, Li X, Wu H, Oster G, et al. Prevalence of antibiotic resistance in US hospitals. Diagn Microbiol Infect Dis. 2014;78:255–62.
- Jones RN, Flonta M, Gurler N, Cepparulo M, Mendes RE, Castanheira M. Resistance surveillance program report for selected European nations (2011). Diagn Microbiol Infect Dis. 2014;78: 429–36.
- Jones RN, Guzman-Blanco M, Gales AC, Gallegos B, Castro AL, Martino MD, et al. Susceptibility rates in Latin American nations: report from a regional resistance surveillance program (2011). Braz J Infect Dis. 2013;17:672–81.
- Zhanel GG, Adam HJ, Low DE, Blondeau J, Decorby M, Karlowsky JA, et al. Antimicrobial susceptibility of 15,644 pathogens from Canadian hospitals: results of the CANWARD 2007–2009 study. Diagn Microbiol Infect Dis. 2011;69:291–306.
- Molton JS, Tambyah PA, Ang BS, Ling ML, Fisher DA. The global spread of healthcare-associated multidrug-resistant bacteria: a perspective from Asia. Clin Infect Dis. 2013;56:1310–18.
- Limbago BM, Kallen AJ, Zhu W, Eggers P, McDougal LK, Albrecht VS. Report of the 13th vancomycin-resistant Staphylococcus aureus isolate from the United States. J Clin Microbiol. 2014;52:998–1002.
- Foucault ML, Courvalin P, Grillot-Courvalin C. Fitness cost of VanA-type vancomycin resistance in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2009;53:2354–9.
- Kelley PG, Gao W, Ward PB, Howden BP. Daptomycin non- susceptibility in vancomycin-intermediate Staphylococcus aureus (VISA) and heterogeneous-VISA (hVISA): implications for therapy after vancomycin treatment failure. J Antimicrob Chemother. 2011; 66:1057–60.
- van Hal SJ, Lodise TP, Paterson DL. The clinical significance of vancomycin minimum inhibitory concentration in Staphylococcus aureus infections: a systematic review and meta-analysis. Clin Infect Dis. 2012;54:755–71.
- Beauregard DA, Williams DH, Gwynn MN, Knowles DJ. Dimerization and membrane anchors in extracellular targeting of vancomycin group antibiotics. Antimicrob Agents Chemother. 1995;39:781–5.
- Nicas TI, Cole CT, Preston DA, Schabel AA, Nagarajan R. Activity of glycopeptides against vancomycin-resistant gram-positive bacteria. Antimicrob Agents Chemother. 1989;33:1477–81.
- Cooper RD, Snyder NJ, Zweifel MJ, Staszak MA, Wilkie SC, Nicas TI, et al. Reductive alkylation of glycopeptide antibiotics: synthesis and antibacterial activity. J Antibiot (Tokyo). 1996;49:575–81.
- Morrissey I, Seifert H, Canton R, Nordmann P, Stefani S, Macgowan A, et al. Activity of oritavancin against methicillin-resistant staphylococci, vancomycin-resistant enterococci and beta-haemolytic streptococci collected from western European countries in 2011. J Antimicrob Chemother. 2013;68:164–7.
- Mendes RE, Sader HS, Flamm RK, Farrell DJ, Jones RN. Oritavancin activity against Staphylococcus aureus causing invasive infections in U.S. and European hospitals: a 5-year international surveillance program. Antimicrob Agents Chemother. 2014;58:2921–4.
- Zhanel GG, Schweizer F, Karlowsky JA. Oritavancin: mechanism of action. Clin Infect Dis. 2012;54(Suppl 3):S214–19.
- Belley A, McKay GA, Arhin FF, Sarmiento I, Beaulieu S, Fadhil I, et al. Oritavancin disrupts membrane integrity of Staphylococcus aureus and vancomycin-resistant enterococci to effect rapid bacterial killing. Antimicrob Agents Chemother. 2010;54:5369–71.
- Domenech O, Dufrene YF, Van Bambeke F, Tukens PM, Mingeot-Leclercq MP. Interactions of oritavancin, a new semi-synthetic lipoglycopeptide, with lipids extracted from Staphylococcus aureus. Biochim Biophys Acta. 2010;1798:1876–85.
- Belley A, Neesham-Grenon E, McKay G, Arhin FF, Harris R, Beveridge T, et al. Oritavancin kills stationary-phase and biofilm Staphylococcus aureus cells in vitro. Antimicrob Agents Chemother. 2009;53:918–25.
- Van Bambeke F, Carryn S, Seral C, Chanteux H, Tyteca D, Mingeot-Leclercq MP, et al. Cellular pharmacokinetics and pharmacodynamics of the glycopeptide antibiotic oritavancin (LY333328) in a model of J774 mouse macrophages. Antimicrob Agents Chemother. 2004;48:2853–60.
- Rodvold KA. Plasma and intrapulmonary concentrations of oritavancin and vancomycin in normal healthy adults. 14th European Congress of Clinical Microbiology and Infectious Diseases, Prague, Czech Republic 1–4 May 2004; Oral presentation 254.
- Garcia LG, Lemaire S, Kahl BC, Becker K, Proctor RA, Denis O, et al. Pharmacodynamic evaluation of the activity of antibiotics against hemin- and menadione-dependent small-colony variants of Staphylococcus aureus in models of extracellular (broth) and intracellular (THP-1 monocytes) infections. Antimicrob Agents Chemother. 2012;56:3700–11.
- Nguyen HA, Denis O, Vergison A, Theunis A, Tulkens PM, Struelens MJ, et al. Intracellular activity of antibiotics in a model of human THP-1 macrophages infected by a Staphylococcus aureus small-colony variant strain isolated from a cystic fibrosis patient: pharmacodynamic evaluation and comparison with isogenic normal-phenotype and revertant strains. Antimicrob Agents Chemother. 2009;53:1434–42.
- Van Bambeke F. Glycopeptides in clinical development: pharmacological profile and clinical perspectives. Curr Opin Pharmacol. 2004;4:471–8.
- Allen NE. From vancomycin to oritavancin: the discovery and development of a novel lipoglycopeptide antibiotic. Anti-Infective Agents in Medicinal Chemistry. 2010;9:23–47.
- Van Bambeke F, Saffran J, Mingeot-Leclercq MP, Tulkens PM. Mixed-lipid storage disorder induced in macrophages and fibroblasts by oritavancin (LY333328), a new glycopeptide antibiotic with exceptional cellular accumulation. Antimicrob Agents Chemother. 2005;49:1695–700.
- Lemaire S, Mingeot-Leclercq MP, Tulkens PM, Van Bambeke F. Study of macrophage functions in murine J774 cells and human activated THP-1 cells exposed to oritavancin, a lipoglycopeptide with high cellular accumulation. Antimicrob Agents Chemother. 2014; 58:2059–66.
- Baquir B, Lemaire S, Van Bambeke F, Tulkens PM, Lin L, Spellberg B. Macrophage killing of bacterial and fungal pathogens is not inhibited by intense intracellular accumulation of the lipoglycopeptide antibiotic oritavancin. Clin Infect Dis. 2012;54(Suppl 3): S229–32.
- Ambrose PG, Drusano GL, Craig WA. In vivo activity of oritavancin in animal infection models and rationale for a new dosing regimen in humans. Clin Infect Dis. 2012;54(Suppl 3):S220–8.
- Belley A, Arhin FF, Sarmiento I, Deng H, Rose W, Moeck G. Pharmacodynamics of a simulated single 1,200-milligram dose of oritavancin in an in vitro pharmacokinetic/pharmacodynamic model of methicillin-resistant staphylococcus aureus infection. Antimicrob Agents Chemother. 2013;57:205–11.
- Dunbar LM, Milata J, McClure T, Wasilewski MM. Comparison of the efficacy and safety of oritavancin front-loaded dosing regimens to daily dosing: an analysis of the SIMPLIFI trial. Antimicrob Agents Chemother. 2011;55:3476–84.
- Tice A. Oritavancin: a new opportunity for outpatient therapy of serious infections. Clin Infect Dis. 2012;54(Suppl 3):S239–43.
- LaPensee K, Fan W, Good S, Jiang H, Lodise TP. A single-dose of oritavancin compared to 7–10 days of vancomycin: clinical response and safety in the MRSA subgroup treated in the ambulatory setting (solo trials). 24th European Congress of Clinical Microbiology and Infectious Diseases, Barcelona, Spain 10–13 May 2014; e-Poster 415.
- Corey R, Perez A, Moeck G, Jiang H, Good S. Single-dose oritavancin compared to 7–10 days of vancomycin in the treatment of Gram- positive acute bacterial skin and skin structure infections; the SOLO I study. 53d Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), Denver, CO 10–13 September 2013; Poster L204.
- Corey R, Perez A, Moeck G, Jiang H, Good S. Single-dose oritavancin compared to 7–10 days of vancomycin in the treatment of Gram-positive acute bacterial skin and skin structure infections; the SOLO II study. 53d Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), Denver, CO 10–13 September 2013; Poster L206c.
- Van Bambeke F, Harms JM, Van Laethem Y, Tulkens PM. Ketolides: pharmacological profile and rational positioning in the treatment of respiratory tract infections. Expert Opin Pharmacother. 2008;9: 267–83.
- Link-Gelles R, Thomas A, Lynfield R, Petit S, Schaffner W, Harrison L, et al. Geographic and temporal trends in antimicrobial nonsusceptibility in Streptococcus pneumoniae in the post-vaccine era in the United States. J Infect Dis. 2013;208:1266–73.
- Kim SH, Song JH, Chung DR, Thamlikitkul V, Yang Y, Wang H, et al. Changing trends in antimicrobial resistance and serotypes of Streptococcus pneumoniae isolates in Asian countries: an Asian Network for Surveillance of Resistant Pathogens (ANSORP) study. Antimicrob Agents Chemother. 2012;56:1418–26.
- European Center for Disease Prevention and Control. Antimicrobial resistance surveillance in Europe - 2012 report. Stockholm, Sweden: European Centre for Disease Prevention and Control; 2013. p. 1–218.
- Chisholm SA, Neal TJ, Alawattegama AB, Birley HD, Howe RA, Ison CA. Emergence of high-level azithromycin resistance in Neisseria gonorrhoeae in England and Wales. J Antimicrob Chemother. 2009;64:353–8.
- Pond MJ, Nori AV, Witney AA, Lopeman RC, Butcher PD, Sadiq ST. High prevalence of antibiotic-resistant Mycoplasma genitalium in nongonococcal urethritis: the need for routine testing and the inadequacy of current treatment options. Clin Infect Dis. 2014;58:631–7.
- Douthwaite S, Champney WS. Structures of ketolides and macrolides determine their mode of interaction with the ribosomal target site. J Antimicrob Chemother. 2001;48(Suppl T1):1–8.
- Zhanel GG, Hisanaga T, Nichol K, Wierzbowski A, Hoban DJ. Ketolides: an emerging treatment for macrolide-resistant respiratory infections, focusing on S. pneumoniae. Expert Opin Emerg Drugs. 2003;8: 297–321.
- European Medicines Agency. EMEA statement on the safety of Ketek (telithromycin). 27 January 2006. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2009/11/WC500015092.pdf (accessed 13 April 2014).
- Food and Drug Administration. Telithromycin (marketed as Ketek) information. 2007. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm107824.htm (accessed 13 April 2014).
- Bertrand D, Bertrand S, Neveu E, Fernandes P. Molecular characterization of off-target activities of telithromycin: a potential role for nicotinic acetylcholine receptors. Antimicrob Agents Chemother. 2010;54:5399–402.
- Van Bambeke F. Macrolides and ketolides. In: Vinks A, Derendorf H, Mouton JW, editors. Fundamentals of antimicrobial pharmacokinetics and pharmacodynamics. New York, NY: Springer; 2014. p. 257–78.
- English ML, Fredericks CE, Milanesio NA, Rohowsky N, Xu ZQ, Jenta TR, et al. Cethromycin versus clarithromycin for community-acquired pneumonia: comparative efficacy and safety outcomes from two double-blinded, randomized, parallel-group, multicenter, multinational noninferiority studies. Antimicrob Agents Chemother. 2012; 56:2037–47.
- Food and Drug Administration advisory committee. Cethromycin safe but not proven effective. 4 June 2009. Available at: http://www.healio.com/infectious-disease/practice-management/news/online/%7B3b1f728c-9639-40a2-b1fb-3eb288e17322%7D/fda-advisory-committee-cethromycin-safe-but-not-proven-effective (accesssed 13 April 2014).
- Sato T, Tateda K, Kimura S, Iwata M, Ishii Y, Yamaguchi K. In vitro antibacterial activity of modithromycin, a novel 6,11-bridged bicyclolide, against respiratory pathogens, including macrolide- resistant Gram-positive cocci. Antimicrob Agents Chemother. 2011; 55:1588–93.
- Sato T, Kawai Y, Matsuda H, Tateda K, Kimura S, Ishii Y, et al. In vitro and in vivo antibacterial activity of modithromycin against streptococci and Haemophilus influenzae. J Antimicrob Chemother. 2011;66:1547–1554.
- Maglio D, Sun HK, Patel T, Banevicius MA, Nightingale CH, Arya A, et al. Pharmacodynamic profiling of modithromycin: Assessment in a pneumococcal murine pneumonia model. Int J Antimicrob Agents. 2014;43:540–6.
- Oldach D, Jamieson BD, Clark K, Keedy K, Fernandes P. Systemic safety profile of solithromycin in Phase 1 & 2 clinical trials. 52d Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), San Francisco, CA, 9–12 September 2012; Poster A1286.
- Llano-Sotelo B, Dunkle J, Klepacki D, Zhang W, Fernandes P, Cate JH, et al. Binding and action of CEM-101, a new fluoroketolide antibiotic that inhibits protein synthesis. Antimicrob Agents Chemother. 2010;54:4961–70.
- Lemaire S, Van Bambeke F, Tulkens PM. Cellular accumulation and pharmacodynamic evaluation of the intracellular activity of CEM-101, a novel fluoroketolide, against Staphylococcus aureus, Listeria monocytogenes, and Legionella pneumophila in human THP-1 macrophages. Antimicrob Agents Chemother. 2009;53:3734–43.
- Rodvold KA, Gotfried MH, Still JG, Clark K, Fernandes P. Comparison of plasma, epithelial lining fluid, and alveolar macrophage concentrations of solithromycin (CEM-101) in healthy adult subjects. Antimicrob Agents Chemother. 2012;56:5076–81.
- Still JG, Schranz J, Degenhardt TP, Scott D, Fernandes P, Gutierrez MJ, et al. Pharmacokinetics of solithromycin (CEM-101) after single or multiple oral doses and effects of food on single-dose bioavailability in healthy adult subjects. Antimicrob Agents Chemother. 2011;55: 1997–2003.
- Andes DR, Okusanya OO, Forrest A, Bhavnani SM, Fernandes P, Ambrose P. Pharmacokinetic-pharmacodynamic (PK-PD) analysis of solithromycin against Streptococcus pneumoniae using data from a murine-lung infection model. 50th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), Boston, MA, 12–15 September 2010; PosterA1–688.
- Okusanya OO, Bhavnani SM, Forrest A, Fernandes P, Ambrose P. Pharmacokinetic-pharmacodynamic target attainment analysis supporting solithromycin (CEM-101) phase 2 dose selection. 50th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), Boston, MA, 12–15 September 2010; PosterA1–692.
- Okusanya OO, Bhavnani SM, Forrest A, Bulik CC, Oldach D, Fernandes P, et al. Population pharmacokinetic (PPK) and pharmacokinetic-pharmacodynamic (PK-PD) target attainment (TA) analyses for solithromycin (SOL, CEM-101) to support intravenous (IV). 52d Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), San Francisco, CA, 9–12 September 2012; Poster A1269.
- Farrell DJ, Sader HS, Castanheira M, Biedenbach DJ, Rhomberg PR, Jones RN. Antimicrobial characterisation of CEM-101 activity against respiratory tract pathogens, including multidrug-resistant pneumococcal serogroup 19A isolates. Int J Antimicrob Agents. 2010;35:537–43.
- Putnam SD, Sader HS, Farrell DJ, Biedenbach DJ, Castanheira M. Antimicrobial characterisation of solithromycin (CEM-101), a novel fluoroketolide: activity against staphylococci and enterococci. Int J Antimicrob Agents. 2011;37:39–45.
- Roblin PM, Kohlhoff SA, Parker C, Hammerschlag MR. In vitro activity of CEM-101, a new fluoroketolide antibiotic, against Chlamydia trachomatis and Chlamydia (Chlamydophila) pneumoniae. Antimicrob Agents Chemother. 2010;54:1358–9.
- Mallegol J, Fernandes P, Seah C, Guyard C, Melano RG. Determination of in vitro activity of solithromycin at different pHs and its intracellular activity tested against clinical isolates of Neisseria gonorrhoeae from a laboratory collection. Antimicrob Agents Chemother. 2013 Jun 24. [Epub ahead of print]
- Olsen B, Pham TL, Golparian D, Johansson E, Tran HK, Unemo M. Antimicrobial susceptibility and genetic characteristics of Neisseria gonorrhoeae isolates from Vietnam, 2011. BMC Infect Dis. 2013;13:40.
- Golparian D, Fernandes P, Ohnishi M, Jensen JS, Unemo M. In vitro activity of the new fluoroketolide solithromycin (CEM-101) against a large collection of clinical Neisseria gonorrhoeae isolates and international reference strains, including those with high-level antimicrobial resistance: potential treatment option for gonorrhea? Antimicrob Agents Chemother. 2012;56:2739–42.
- Jensen JS, Fernandes P, Unemo M. In vitro activity of the new fluoroketolide solithromycin (CEM-101) against macrolide resistant and susceptible Mycoplasma genitalium strains. Antimicrob Agents Chemother. 2014;58:3151–6.
- Waites KB, Crabb DM, Duffy LB. Comparative in vitro susceptibilities of human mycoplasmas and ureaplasmas to a new investigational ketolide, CEM-101. Antimicrob Agents Chemother. 2009;53:2139–41.
- Lemaire S, van der Linden M, Tulkens PM. Activity of solithromycin against clinical isolates of S. pneumoniae with variable susceptibilities to conventional antibiotics. 28th International Congress on Chemotherapy and Infection, Yokoama, Japan, 5–8 June 2013; Poster 105.
- Biedenbach DJ, Sader HS, Jones RN, Farrell DJ. Antimicrobial activity of solithromycin (CEM-101), a novel fluoroketolide, tested against isolates collected in Europe during 2010 surveillance. 21th European Congress of Clinical Microbiology and Infectious Diseases & 27th International Congress of Chemotherapy, Milan, Italy, 7–10 May 2011; Poster 1136.
- Vandevelde NM, Tulkens PM, Van Bambeke F. Antibiotic activity against naive and induced Streptococcus pneumoniae biofilms in an in vitro pharmacodynamic model. Antimicrob Agents Chemother. 2014;58:1348–58.
- Oldach D, Clark K, Schranz J, Das A, Craft JC, Scott D, et al. Randomized, double-blind, multicenter phase 2 study comparing the efficacy and safety of oral solithromycin (CEM-101) to those of oral levofloxacin in the treatment of patients with community-acquired bacterial pneumonia. Antimicrob Agents Chemother. 2013;57: 2526–34.
- Emami S, Shafiee A, Forounmadi A. Quinolones: recent structural and clinical developments. Ir J Pharm Res. 2005;4:123–36.
- Van Bambeke F, Michot JM, Van Eldere J, Tulkens PM. Quinolones in 2005: an update. Clin Microbiol Infect. 2005;11:256–80.
- Walsh CT, Wencewicz TA. Prospects for new antibiotics: a molecule-centered perspective. J Antibiot (Tokyo). 2014;67:7–22.
- Sousa J, Alves G, Fortuna A, Falcao A. Third- and fourth-generation fluoroquinolone antibacterials: a systematic review of safety and toxicity profiles. Curr Drug Saf. 2014 Jan 6. [Epub ahead of print]
- Stein GE, Goldstein EJ. Fluoroquinolones and anaerobes. Clin Infect Dis. 2006;42:1598–607.
- Van Bambeke F, Tulkens PM. Safety profile of the respiratory fluoroquinolone moxifloxacin: comparison with other fluoroquinolones and other antibacterial classes. Drug Saf. 2009;32:359–78.
- Drlica K, Zhao X. DNA gyrase, topoisomerase IV, and the 4-quinolones. Microbiol Mol Biol Rev. 1997;61:377–92.
- Munoz-Bellido JL, Alonzo MM, Martinez Andres JA, Guttierrez Zufiaurre MN, Ortiz G, Segovia HM, et al. Efflux pump-mediated quinolone resistance in Staphylococcus aureus strains wild type for gyrA, gyrB, grlA, and norA. Antimicrob Agents Chemother. 1999;43:354–6.
- Costa SS, Falcao C, Viveiros M, Machado D, Martins M, Melo-Cristino J, et al. Exploring the contribution of efflux on the resistance to fluoroquinolones in clinical isolates of Staphylococcus aureus. BMC Microbiol. 2011;11:241.
- Alam MM, Kobayashi N, Uehara N, Watanabe N. Analysis on distribution and genomic diversity of high-level antiseptic resistance genes qacA and qacB in human clinical isolates of Staphylococcus aureus. Microb Drug Resist. 2003;9:109–21.
- Gill MJ, Brenwald NP, Wise R. Identification of an efflux pump gene, pmrA, associated with fluoroquinolone resistance in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1999;43:187–9.
- Garvey MI, Baylay AJ, Wong RL, Piddock LJ. Overexpression of patA and patB, which encode ABC transporters, is associated with fluoroquinolone resistance in clinical isolates of Streptococcus pneumoniae. Antimicrob Agents Chemother. 2011;55:190–6.
- Lismond A, Carbonnelle S, Tulkens PM, Van Bambeke F. Efflux of novel quinolones in contemporary Streptococcus pneumoniae isolates from community-acquired pneumonia. J Antimicrob Chemother. 2011;66:948–51.
- Schmitz FJ, Fluit AC, Luckefahr M, Engler B, Hofmann B, Verhoef J, et al. The effect of reserpine, an inhibitor of multidrug efflux pumps, on the in-vitro activities of ciprofloxacin, sparfloxacin and moxifloxacin against clinical isolates of Staphylococcus aureus. J Antimicrob Chemother. 1998;42:807–10.
- Van Bambeke F, Glupczynski Y, Plesiat P, Pechere JC, Tulkens PM. Antibiotic efflux pumps in prokaryotic cells: occurrence, impact on resistance and strategies for the future of antimicrobial therapy. J Antimicrob Chemother. 2003;51:1055–65.
- Lomovskaya O, Lee A, Hoshino K, Ishida H, Mistry A, Warren MS, et al. Use of a genetic approach to evaluate the consequences of inhibition of efflux pumps in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1999;43:1340–6.
- Tran JH, Jacoby GA. Mechanism of plasmid-mediated quinolone resistance. Proc Natl Acad Sci U S A. 2002;99:5638–42.
- Robicsek A, Strahilevitz J, Jacoby GA, Macielag M, Abbanat D, Park CH, et al. Fluoroquinolone-modifying enzyme: a new adaptation of a common aminoglycoside acetyltransferase. Nat Med. 2006;12:83–8.
- Dalhoff A. Resistance surveillance studies: a multifaceted problem—the fluoroquinolone example. Infection. 2012;40:239–62.
- Dalhoff A. Global fluoroquinolone resistance epidemiology and implictions for clinical use. Interdiscip Perspect Infect Dis. 2012;2012:976273.
- Titelman E, Iversen A, Kahlmeter G, Giske CG. Antimicrobial susceptibility to parenteral and oral agents in a largely polyclonal collection of CTX-M-14 and CTX-M-15-producing Escherichia coli and Klebsiella pneumoniae. APMIS. 2011;119:853–63.
- Van Heirstraeten L, Leten G, Lammens C, Goossens H, Malhotra-Kumar S. Increase in fluoroquinolone non-susceptibility among clinical Streptococcus pyogenes in Belgium during 2007-10. J Antimicrob Chemother. 2012;67:2602–5.
- Lemaire S, Van Bambeke F, Tulkens PM. Activity of finafloxacin, a novel fluoroquinolone with increased activity at acid pH, towards extracellular and intracellular Staphylococcus aureus, Listeria monocytogenes and Legionella pneumophila. Int J Antimicrob Agents. 2011;38:52–9.
- Higgins PG, Stubbings W, Wisplinghoff H, Seifert H. Activity of the investigational fluoroquinolone finafloxacin against ciprofloxacin-sensitive and -resistant Acinetobacter baumannii isolates. Antimicrob Agents Chemother. 2010;54:1613–15.
- Emrich NC, Heisig A, Stubbings W, Labischinski H, Heisig P. Antibacterial activity of finafloxacin under different pH conditions against isogenic strains of Escherichia coli expressing combinations of defined mechanisms of fluoroquinolone resistance. J Antimicrob Chemother. 2010;65:2530–3.
- Stubbings W, Leow P, Yong GC, Goh F, Korber-Irrgang B, Kresken M, et al. In vitro spectrum of activity of finafloxacin, a novel, pH-activated fluoroquinolone, under standard and acidic conditions. Antimicrob Agents Chemother. 2011;55:4394–7.
- Dalhoff A, Stubbings W, Schubert S. Comparative in vitro activities of the novel antibacterial finafloxacin against selected Gram-positive and Gram-negative bacteria tested in Mueller-Hinton broth and synthetic urine. Antimicrob Agents Chemother. 2011;55:1814–18.
- Patel H, Andresen A, Vente A, Heilmann HD, Stubbings W, Seiberling M, et al. Human pharmacokinetics and safety profile of finafloxacin, a new fluoroquinolone antibiotic, in healthy volunteers. Antimicrob Agents Chemother. 2011;55:4386–93.
- Jacobs MR. Optimisation of antimicrobial therapy using pharmacokinetic and pharmacodynamic parameters. Clin Microbiol Infect. 2001;7:589–96.
- Biedenbach DJ, Farrell DJ, Flamm RK, Liverman LC, McIntyre G, Jones RN. Activity of JNJ-Q2, a new fluoroquinolone, tested against contemporary pathogens isolated from patients with community-acquired bacterial pneumonia. Int J Antimicrob Agents. 2012;39:321–5.
- Biedenbach DJ, Turner LL, Jones RN, Farrell DJ. Activity of JNJ-Q2, a novel fluoroquinolone, tested against Neisseria gonorrhoeae, including ciprofloxacin-resistant strains. Diagn Microbiol Infect Dis. 2012;74:204–6.
- Eichenbaum G, Pugsley MK, Gallacher DJ, Towart R, McIntyre G, Shukla U, et al. Role of mixed ion channel effects in the cardiovascular safety assessment of the novel anti-MRSA fluoroquinolone JNJ-Q2. Br J Pharmacol. 2012;166:1694–707.
- Van Wart SA, Melhem M, Davenport JM, Ambrose P, Bhavnani SM, Andes DR, et al. Population pharmacokinetic (PPK) and pharmacokinetic-pharmacodynamic (PK-PD) target attainment (TA) analyses to support JNJ-Q2 dose selection for patients with acute bacterial skin and skin structure infections (ABSSSI). 52d Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), San Francisco, CA, 9–12 September 2012; Poster A-1961.
- Davenport JM, Covington PS, Gotfried MH, Medlock M, Watanalumlerd P, McIntyre G, et al. Summary of pharmacokinetics and tissue distribution of a broad-spectrum fluoroquinolone, JNJ-Q2. Clin Pharmacol Drug Dev. 2012;1:121–30.
- Covington PS, Davenport JM, Andrae DA, Stryjewski ME, Turner LL, McIntyre G, et al. A phase 2 study of the novel fluoroquinolone JNJ-Q2 in community-acquired bacterial pneumonia. J Antimicrob Chemother. 2013;68:2691–3.
- Park HS, Jung SJ, Kwak JH, Choi DR, Choi EC. DNA gyrase and topoisomerase IV are dual targets of zabofloxacin in Streptococcus pneumoniae. Int J Antimicrob Agents. 2010;36:97–8.
- Park HS, Kim HJ, Seol MJ, Choi DR, Choi EC, Kwak JH. In vitro and in vivo antibacterial activities of DW-224a, a new fluoronaphthyridone. Antimicrob Agents Chemother. 2006;50:2261–4.
- Jones RN, Biedenbach DJ, Ambrose PG, Wikler MA. Zabofloxacin (DW-224a) activity against Neisseria gonorrhoeae including quinolone-resistant strains. Diagn Microbiol Infect Dis. 2008;62:110–12.
- Han H, Kim SE, Shin KH, Lim C, Lim KS, Yu KS, et al. Comparison of pharmacokinetics between new quinolone antibiotics: the zabofloxacin hydrochloride capsule and the zabofloxacin aspartate tablet. Curr Med Res Opin. 2013;29:1349–55.
- Kim YS, Kim MJ, Back KR, Lim CH, Rock JA, Seong SK, et al. A phase 2, multi-dose, double-blind, randomized, multicenter, safety and efficacy study of zabofloxacin vs moxifloxacin in the treatment of mild to moderate community-acquired pneumonia. 52d Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), San Francisco, CA, 9–12 September 2012; Poster L1–294.
- Adam HJ, Laing NM, King CR, Lulashnyk B, Hoban DJ, Zhanel GG. In vitro activity of nemonoxacin, a novel nonfluorinated quinolone, against 2,440 clinical isolates. Antimicrob Agents Chemother. 2009;53:4915–20.
- Chotikanatis K, Kohlhoff SA, Hammerschlag MR. In vitro activity of nemonoxacin, a novel nonfluorinated quinolone antibiotic, against Chlamydia trachomatis and Chlamydia pneumoniae. Antimicrob Agents Chemother. 2014;58:1800–1.
- Liao CH, Ko WC, Lu JJ, Hsueh PR. Characterizations of clinical isolates of clostridium difficile by toxin genotypes and by susceptibility to 12 antimicrobial agents, including fidaxomicin (OPT-80) and rifaximin: a multicenter study in Taiwan. Antimicrob Agents Chemother. 2012;56:3943–9.
- Lin L, Chang LW, Tsai CY, Hsu CH, Chung DT, Aronstein WS, et al. Dose escalation study of the safety, tolerability, and pharmacokinetics of nemonoxacin (TG-873870), a novel potent broad-spectrum nonfluorinated quinolone, in healthy volunteers. Antimicrob Agents Chemother. 2010;54:405–10.
- Chung DT, Tsai CY, Chen SJ, Chang LW, King CH, Hsu CH, et al. Multiple-dose safety, tolerability, and pharmacokinetics of oral nemonoxacin (TG-873870) in healthy volunteers. Antimicrob Agents Chemother. 2010;54:411–17.
- Liang W, Chen YC, Cao YR, Liu XF, Huang J, Hu JL, et al. Pharmacokinetics and pharmacodynamics of nemonoxacin against Streptococcus pneumoniae in an in vitro infection model. Antimicrob Agents Chemother. 2013;57:2942–7.
- Wright DH, Brown GH, Peterson ML, Rotschafer JC. Application of fluoroquinolone pharmacodynamics. J Antimicrob Chemother. 2000;46:669–83.
- van Rensburg DJ, Perng RP, Mitha IH, Bester AJ, Kasumba J, Wu RG, et al. Efficacy and safety of nemonoxacin versus levofloxacin for community-acquired pneumonia. Antimicrob Agents Chemother. 2010;54:4098–106.
- Lemaire S, Tulkens PM, Van Bambeke F. Contrasting effects of acidic pH on the extracellular and intracellular activities of the anti-gram-positive fluoroquinolones moxifloxacin and delafloxacin against Staphylococcus aureus. Antimicrob Agents Chemother. 2011; 55:649–58.
- Duffy E, Devito JA, Remy J, Burak E. Delafloxacin chemical properties lead to increased potency against Gram-positive pathogens, including quinolone-resistant pathogens II. 50th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), Boston, MA, 12–15 September 2010; Poster E183.
- Drlica K, Zhao X. Mutant selection window hypothesis updated. Clin Infect Dis. 2007;44:681–8.
- Remy JM, Tow-Keogh CA, McConnell TS, Dalton JM, Devito JA. Activity of delafloxacin against methicillin-resistant Staphylococcus aureus: resistance selection and characterization. J Antimicrob Chemother. 2012;67:2814–20.
- Bauer J, Siala W, Tulkens PM, Van Bambeke F. A combined pharmacodynamic quantitative and qualitative model reveals the potent activity of daptomycin and delafloxacin against Staphylococcus aureus biofilms. Antimicrob Agents Chemother. 2013;57:2726–37.
- Burak E, Hopkins S, Pillar C, Lawrence L. In vitro activity of delaoxacin against methicillin-resistant Staphylococcus aureus from the United States, Europe and Asia. 19th European Congress of Clinical Microbiology and Infectious Diseases, Helsinki, Finland, 16–19 May 2009; Poster1080.
- Ding Y, Villet MA, Lee JC, Hooper DC. Treatment of renal abscesses caused by Staphylococcus aureus MW2, using delafloxacin and moxifloxacin. 21th European Congress of Clinical Microbiology and Infectious Diseases & 27th International Congress of Chemotherapy, Milan, Italy, 7–10 May 2011; Poster 1506.
- Marra A, Bortolon E, Molstad D, Wu Y, Jing H, Burak E. Evaluation of delafloxacin in rat granuloma pouch infections caused by Gram- negative pathogens. 50th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), Boston, MA, 12–15 September 2010; Poster A1–680.
- Lawrence L, Benedict M, Hart J, Hawkins A, Danping L, Medlock M, et al. Pharmacokinetics (PK) and safety of single doses of delafloxacin administered intravenously in healthy human subjects. 51th Interescience Conference on Antimicrobial Agents and Chemotherpay, Chicago, IL, 17–20 September 2011; Poster A2-045a.
- Rubino CM, Bhavnani SM, Burak E, Ambrose PG. Pharmacokinetic-pharmacodynamic target attainment analyses supporting delafloxacin phase 3 dose regimen decisions. 50th Interscience Conference on Antimicrobial Agents and Chemotherapy, Boston, MA, 12–15 September 2010; Poster A1-681.
- Hoover R, Lawrence L, Longcor J, Greenfield J. Pharmacokinetics (PK) of delafloxacin (DLX), vancomycin (VAN), and linezolid (LNZ) in a phase 2 exploratory study in subjects with acute bacterial skin and skin structure infections (ABSSSI). 52d Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), San Francisco, CA, 9–12 September 2012; Poster A1957.
- Lawrence L, Longcor J, Li D, Reeve M, Hoover R, McEwen AB, et al. Metabolism and mass balance of [14C]-Delafloxacin in healthy human volunteers following intravenous administration. 52d Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), San Francisco, CA, 9–12 September 2012; Poster A1956.
- Hoover R, Lawrence L, Smith C, Longcor J. Pharmacokinetics (PK) of delafloxacin (DLX) in patients with varying degrees of renal impairment. 53d Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), Denver, CO, 10–13 September 2013; Poster A-017E.
- Burak E, Bortolon E, Molstad D, Wu Y, Jing H, Girard D. Pharmacokinetics and pharmacodynamics of delafloxacin in S. aureus murine thigh infection models. 49th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA, 12–15 September 2009; Poster A1–1941.
- Longcor J, Hopkins S, Lawrence L, Green S, Mahra P, Manos P, et al. Results of a phase 2 study of delafloxacin (DLX) compared to vancomycin (VAN) and linezolid (LNZ) in acute bacterial skin and skin structure infections (ABSSSI). 52d Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), San Francisco, CA, 9–12 September 2012; Poster L1–1663.
- Lawrence L, Benedict M, Litwin J, Thorn M, Medlock M, Hopkins S, et al. A thorough phase 1 QTc study of delafloxacin (DLX) compared with placebo and moxifloxacin (MXF). 52d Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), San Francisco, CA, 9–12 September 2012; Poster A1958.
- Longcor J, Lawrence L, Duffy E, Hopkins S. Objective measures of clinical efficacy in a phase 2b exploratory study of delafloxacin compared to vancomycin and linezolid in adults with acute bacterial skin and skin structure infections (ABSSSI). 52d Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), San Francisco, CA, 9–12 September 2012; Poster L1–1667c.
- Roberts MC, Remy J, Longcor J, Marra A, Sun E, Duffy E. In vitro activity of delafloxacin against Neisseria gonorrhoeae clinical isolates. STI & AIDS World Congress 2013, Vienna, Austria, 14–17 June 2013; Poster.
- White paper: recommendations on the conduct of superiority and organism-specific clinical trials of antibacterial agents for the treatment of infections caused by drug-resistant bacterial pathogens. Clin Infect Dis. 2012;55:1031–46.
- Saravolatz LD, Pawlak J, Johnson LB. In vitro activity of oritavancin against community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA), vancomycin-intermediate S. aureus (VISA), vancomycin-resistant S. aureus (VRSA) and daptomycin-non-susceptible S. aureus (DNSSA). Int J Antimicrob Agents. 2010;36:69–72.
- Arhin FF, Draghi DC, Pillar CM, Parr TR Jr, Moeck G, Sahm DF. Comparative in vitro activity profile of oritavancin against recent gram-positive clinical isolates. Antimicrob Agents Chemother. 2009;53:4762–71.
- Farrell DJ, Castanheira M, Sader HS, Jones RN. The in vitro evaluation of solithromycin (CEM-101) against pathogens isolated in the United States and Europe (2009). J Infect. 2010;61:476–83.
- Andrews JM, Weller TM, Ashby JP, Walker RM, Wise R. The in vitro activity of ABT773, a new ketolide antimicrobial agent. J Antimicrob Chemother. 2000;46:1017–22.
- Luna VA, Xu ZQ, Eiznhamer DA, Cannons AC, Cattani J. Susceptibility of 170 isolates of the USA300 clone of MRSA to macrolides, clindamycin and the novel ketolide cethromycin. J Antimicrob Chemother. 2008;62:639–40.
- Arhin FF, Sarmiento I, Parr TR Jr, Moeck G. Comparative in vitro activity of oritavancin against Staphylococcus aureus strains that are resistant, intermediate or heteroresistant to vancomycin. J Antimicrob Chemother. 2009;64:868–70.
- Chen YH, Liu CY, Lu JJ, King CH, Hsueh PR. In vitro activity of nemonoxacin (TG-873870), a novel non-fluorinated quinolone, against clinical isolates of Staphylococcus aureus, enterococci and Streptococcus pneumoniae with various resistance phenotypes in Taiwan. J Antimicrob Chemother. 2009;64:1226–9.
- Singh KV, Malathum K, Murray BE. In vitro activities of a new ketolide, ABT-773, against multidrug-resistant gram-positive cocci. Antimicrob Agents Chemother. 2001;45:3640–3.
- Lauderdale TL, Shiau YR, Lai JF, Chen HC, King CH. Comparative in vitro activities of nemonoxacin (TG-873870), a novel nonfluorinated quinolone, and other quinolones against clinical isolates. Antimicrob Agents Chemother. 2010;54:1338–42.
- Farrell DJ, Liverman LC, Biedenbach DJ, Jones RN. JNJ-Q2, a new fluoroquinolone with potent in vitro activity against Staphylococcus aureus, including methicillin- and fluoroquinolone-resistant strains. Antimicrob Agents Chemother. 2011;55: 3631–4.
- Kwon AR, Min YH, Ryu JM, Choi DR, Shim MJ, Choi EC. In vitro and in vivo activities of DW-224a, a novel fluoroquinolone antibiotic agent. J Antimicrob Chemother. 2006;58:684–8.
- Burak E, Devito JA, Remy J, Duffy E. Delafloxacin chemical properties lead to increased potency against Gram-positive pathogens, including quinolone-resistant pathogens I. 50th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), Boston, MA, 12–15 September 2010; Poster E182.
- Morrow BJ, He W, Amsler KM, Foleno BD, Macielag MJ, Lynch AS, et al. In vitro antibacterial activities of JNJ-Q2, a new broad-spectrum fluoroquinolone. Antimicrob Agents Chemother. 2010;54:1955–64.
- Mendes RE, Woosley LN, Farrell DJ, Sader HS, Jones RN. Oritavancin activity against vancomycin-susceptible and vancomycin-resistant Enterococci with molecularly characterized glycopeptide resistance genes recovered from bacteremic patients, 2009-2010. Antimicrob Agents Chemother. 2012;56:1639–42.
- Kosowska-Shick K, Credito K, Pankuch GA, Lin G, Bozdogan B, McGhee P, et al. Antipneumococcal activity of DW-224a, a new quinolone, compared to those of eight other agents. Antimicrob Agents Chemother. 2006;50:2064–71.
- McGhee P, Clark C, Kosowska-Shick KM, Nagai K, Dewasse B, Beachel L, et al. In vitro activity of CEM-101 against Streptococcus pneumoniae and Streptococcus pyogenes with defined macrolide resistance mechanisms. Antimicrob Agents Chemother. 2010;54:230–8.

