Abstract
Objective. Treatment with tumour necrosis factor antagonists (anti-TNF) has been recognized as a risk factor for tuberculosis (TB) reactivation. Our aim was to evaluate risk of TB reactivation in rheumatologic and non-rheumatologic diseases treated with the same anti-TNF agents with and without concomitant therapies.
Methods. We searched for randomized controlled trials (RCTs) evaluating infliximab, adalimumab, and certolizumab in both rheumatologic and non-rheumatologic diseases until 2012. Results were calculated as pooled rates and/or pooled odd ratios (OR).
Results. Overall, 40 RCTs with a total of 14,683 patients (anti-TNF: 10,010; placebo: 4673) were included. TB reactivation was 0.26% (26/10,010) in the anti-TNF group and 0% (0/4673) in the control group, corresponding to an OR of 24.8 (95% CI 2.4–133). TB risk was higher when anti-TNF agents were combined with methotrexate or azathioprine as compared with either controls (24/4241 versus 0/4673; OR 54; 95% CI 5.3–88) or anti-TNF monotherapy (24/4241 versus 2/5769; OR 13.3; 95% CI 3.7–100). When anti-TNF was used as monotherapy, TB risk tended to be higher than placebo (2/5769 versus 0/4673; OR 4; 95% CI 0.2–15.7).
Conclusions. TB risk with anti-TNF agents appeared to be increased when these agents were used in combination with methotrexate or azathioprine as compared with monotherapy regimen. TB risk seemed to be higher than placebo, even when monotherapy is prescribed.
Key messages
Treatment with tumour necrosis factor antagonists (anti-TNF) has been recognized as a risk factor for tuberculosis (TB) reactivation.
We found that TB risk was higher when anti-TNF agents were combined with immunosuppressive therapy as compared with either controls (OR 54) or anti-TNF monotherapy (OR 13.3).
Introduction
Tumour necrosis factor (TNF) has been shown to play a critical role in the host response against tuberculosis (TB), including granuloma integrity and containment of disease (Citation1–3). A key aspect of host defence against TB is represented by the antigen-specific adaptive T cell response that is impaired by anti-TNF therapy. Recently, it was hypothesized that the depletion of a granulysin-expressing subset of effector memory CD8+ T cells (CD8+ TEMRA cells) induced by infliximab (IFX) could result in suboptimal control of mycobacterial growth, leading to the potential spreading of TB infection (Citation4). Antibodies against TNF are known to cause a reactivation of latent TB in animal models of the disease (Citation5), and TB cases have been consistently reported in patients with rheumatoid arthritis (RA) and Crohn's disease (CD) (Citation6), especially in populations at high prevalence of TB infection (Citation7,Citation8). When considering the increasing use of anti-TNF therapies in these diseases, the burden of TB reactivation may be clinically relevant. TB reactivation tends to occur early following initiation of the drug, with extra-pulmonary and disseminated manifestations occurring relatively more frequently than in immunocompetent patients (Citation6). Consequently, TB screening prior to anti-TNF treatment is currently recommended (Citation9–12).
Because of its low incidence, anti-TNF-related TB risk has been mainly estimated in large uncontrolled populations, such as long-term open-label extension trials, registries, and post-marketing surveillance databases (Citation8,Citation13–18). However, these estimates were likely inaccurate due to several biases from lack of adequate control groups and clinical under-reporting. On the other hand, estimates from individual randomized controlled trials (RCTs) are more likely to be reliable, but are limited by small sample size and/or short study duration. To overcome these limitations, the anti-TNF-related risk of TB reactivation has been estimated in systematic reviews, where pooling data from individual RCTs substantially increases the confidence in the final estimate (Citation19–21). Although this methodology has been mainly applied to assess the treatment-related TB risk in individual diseases (Citation19–21), a recent meta-analysis estimated the adverse effects of different biologic drugs—including anti-TNF—in several diseases (i.e. all but HIV infection) (Citation22). A 4.7-fold increase of TB reactivation risk during anti-TNF therapy as compared to placebo has been shown in an overall study population of 163 RCTs (5010 participants) and 46 extension studies (11,954 participants) (Citation23).
However, the role of other factors potentially associated with TB reactivation during anti-TNF therapy has not been fully ascertained. Therefore, we performed a systematic review of RCTs of only three anti-TNF agents used in both rheumatologic and non-rheumatologic diseases—IFX, adalimumab (ADA), and certolizumab pegol (CERT)—in order to compare the risk of TB between the treatment and placebo groups, and to identify potential predictive factors associated with TB reactivation such as the type of disease or the concomitant use of immunosuppressive drugs.
Methods
Methods of the analysis and inclusion criteria were based on PRISMA recommendations (Citation24). The present review is a selective update of a previously published systematic review by Singh et al. which included articles published up to January 2010 (Citation22).
Eligibility criteria
We considered all RCTs in which the selected anti-TNF agents were compared with at least one arm of placebo, and the risk of TB reactivation was assessed. For this analysis, we decided to limit the present review to RCTs including anti-TNF used in more than one disease, namely IFX, ADA, and CERT that are applied in RA, ankylosing spondylitis (AS), inflammatory bowel diseases (IBD), psoriasis, and psoriatic arthritis (PA). We decided to exclude the very few trials in which anti-TNF agents were sporadically used in other diseases, such as viral hepatitis, sarcoidosis, etc. We also excluded RCTs with < 30 patients or with a follow-up duration < 12 weeks. These criteria were applied both when extracting studies already included in the previous review (Citation22), and when considering new studies from the literature search. If there was any suspicion of cohort overlap between studies, only the most recent study was considered for inclusion. RCTs with an open-label phase with anti-TNF before randomization were also excluded.
Information sources
A literature search was performed for the period January 2010 to March 2012. Relevant publications were identified by MEDLINE and Cochrane databases. We identified eligible studies with the MeSH terms Tuberculosis, Rheumatic diseases, Inflammatory bowel diseases, Psoriasis, Infliximab, Adalimumab, Certolizumab pegol, and Randomized controlled trial. Only RCTs published in English language were considered. We used bibliographies of all relevant studies identified to do a recursive search. When the selected randomized study provided insufficient information regarding the TB risk, authors were contacted.
Study selection
Titles and abstracts of papers were screened by four reviewers (R.L., A.Z., V.B., C.H.). Studies were independently evaluated for relevance by the reviewers (using the full text of the study). Any disagreements were resolved through discussion. Data were extracted from the included studies by one of the four reviewers and checked by a second reviewer, and the data were extracted into tables. Any disagreements were resolved through discussion with a third reviewer.
Data collection
From each paper, reviewers independently abstracted the following information: country of origin, type of disease, type and dose of anti-TNF, number of patients included in the RCT, study duration, concomitant therapies systematically included in the active arms or in the control arm according to study protocol, performance of TB screening prior to therapy, and number of TB cases in each arm. In addition, clinical information about TB cases was collected when available.
Risk of bias in individual studies
Information on the methodological quality of each study was recorded, and quality assessment was performed using the CONSORT checklist (Citation24). However, since our study focused on a secondary end-point of these studies (i.e. TB reactivation), we simplified the interpretation of the quality of the primary end-point (i.e. therapeutic response). Thus, studies with random sequence generation, adequate concealment of allocation, blinding of participants and personnel, blinding of outcome assessment, and adequate outcome were considered at low risk of bias. Regarding our study end-point (i.e. TB incidence), we evaluated whether a systematic reporting of adverse events was provided in each individual study.
Summary measures
The primary end-point of this systematic review was to address the risk of TB in the anti-TNF arms as compared with those allocated in the control group, and whether there was an association of such risk with other variables, such as the type of disease or concomitant medications.
Planned methods of analysis
For each of the two arms (anti-TNF and controls), we aggregated the absolute number of cases with TB. Comparison among groups was performed by chi-square test. Pooled estimates and 95% confidence intervals (CI) were calculated. The Number Cruncher Statistical System software (NCSS, Kaysville, UT, USA) was used for the computations. When considering the rare incidence of TB in any of the two arms, we preferred not to adopt a more complex meta-analytical approach, because of the well-known bias of this methodology with rare events (Citation25).
Results
Study selection
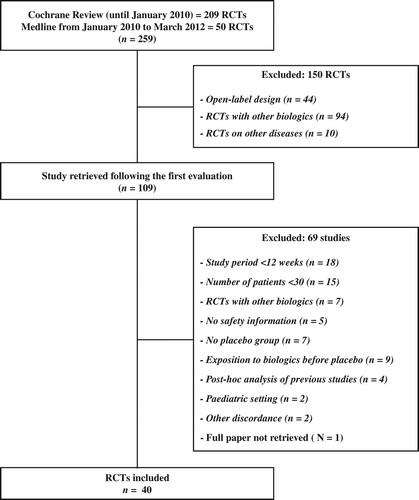
A flow diagram of this systematic review, with the number of papers retrieved, included, and excluded, as well as the reasons for exclusion, is shown in . In summary, 259 studies were identified by the included databases for the selected period, including 59 extracted from the previous meta-analysis (Citation22). After removing non-pertinent papers, 109 were regarded as relevant. Out of these, 69 were excluded for the reasons reported in , while 40 trials were selected for inclusion in the systematic review (Citation26–65).
Characteristics of the included studies
Main characteristics of the included studies are provided in . The studies were performed in either USA and Europe (n = 18), only USA (n = 11), only Europe (n = 6), or non-USA/European countries (n = 5). RA was the underlying disease in 14 studies, AS in 5, psoriasis in 9, PA in 4, and IBD in 8 cases (7 Crohn's disease; 1 ulcerative colitis). The anti-TNF agent used was IFX in 20 studies, ADA in 14, and CERT in 6. In 13 studies, the anti-TNF agent was used with a concomitant immunosuppressive agent according to the study protocol (11 studies with methotrexate and 2 with azathioprine), and in 28 studies anti-TNF agent was used as monotherapy (in one study the active group was divided in IFX as monotherapy and IFX+ AZA) (Citation32).
Table I. Characteristics of included trials.
Participants
A total of 14,683 subjects were randomized in the selected studies (). Of these, 10,010 were allocated in the active arm, and the remaining 4673 in the control arm. In detail, 2941 controls received only placebo, while the remaining 1732 were allocated to placebo plus methotrexate (MTX) or azathioprine (AZA). The number of patients enrolled for study ranged from 47 to 1212, with a median of 275. The median duration of follow-up was 24 weeks, ranging from 12 to 54 weeks. The doses ranged from 5 to 20 mg/kg for IFX, from 100 to 400 mg for CERT, from 20 to 80 mg for ADA, from 2 to 2.5 mg/kg for AZA, and from 12.5 to 25 mg for MTX. TB screening was clearly stated to be performed in 27 RCTs, no screening was carried out in 3 series, while this information was unavailable in other 10 trials (). In detail, TB screening was performed with only chest X-ray in 5 studies, only purified protein derivative (PPD) test in 2, and with PPD plus chest X-ray in the remaining 20 series. Only a few studies provided information on how TB screening-positive cases were evaluated. As shown in , positive cases were excluded from the RCT in 18 studies, while preventive TB therapy was administered in 8 studies. Finally, 4 studies excluded those with a clinical history of TB.
Table II. Screening for tuberculosis (TB) infection and incidence of TB in the included studies.
Quality of the studies
All the included trials appeared to be at low risk of bias when evaluated by criteria defining the quality of the primary end-point. All of the included studies systematically reported adverse events.
TB risk
Overall, 26 (0.26%) out of 10,010 patients developed TB in the active treatment group, corresponding to an incidence rate of 481 cases per 100,000 patient-years. No cases of TB were observed in the 4673 patients receiving placebo or placebo plus immunosuppressive therapy (). This difference was statistically significant (OR 24.8; 95% CI 2.4–133; P < 0.01). When stratifying for concomitant therapy, patients treated with anti-TNF in monotherapy (2/5769 versus 0/4673; OR 4; 95% CI 0.2–15.7; P = 0.5), as well as those treated with anti-TNF agents combined with MTX or AZA (24/4241 versus 0/4673; OR 54; 95% CI 5.3–288; P < 0.001), appeared to be at higher TB risk, although a statistically significant difference was showed only in the latter group. When differentiating between the two types of controls, TB risk with combination therapy was significantly higher than both placebo alone (24/4241 versus 0/2941; OR 34; 95% CI 3.3–181; P < 0.001) and placebo plus immunosuppressive agents (24/4241 versus 0/1732; OR 20; 95% CI 2–107; P < 0.001). TB risk was also significantly higher when comparing combined therapy with monotherapy (24/4241 versus 2/5769; OR 13.3; 95% CI 3.7–100; P < 0.001), observing in the former group an incidence rate of 832 cases per 100,000 patient-years and of 91 in latter group. When stratifying for disease, patients treated with anti-TNF agents for rheumatologic diseases were not at higher TB risk as compared with non-rheumatologic diseases (24/8367 versus 2/1643; OR 1.9; 95% CI 0.5–14; P = 0.29).
Table III. TB risk according to different treatments.
TB cases
Data on TB cases are provided in . There were 9 pulmonary localizations, 9 extra-pulmonary infections (including 2 disseminated), and information about site of infection was missing for the remaining 7 patients. Overall, 10 (40%) TB cases were observed in Russia, Estonia, Ukraine, and Bulgaria, whilst only 1 case occurred in USA. Screening for TB at entry was performed in 21 (84%) of these patients, being negative and positive in 11 and 6 cases, respectively (in 4 cases not reported). Of note, TB infection occurred in 2 patients who had positive screening despite the fact that a preventive anti-TB therapy was performed. Nineteen patients recovered with anti-TB therapy, and 1 died; outcome information was missing for the remaining 5.
Table IV. Data of patients who developed tuberculosis (TB) in different trials.
Discussion
According to our systematic review, the combination therapy of anti-TNF agents with MTX or AZA results in a 13-fold increase in the risk of TB reactivation, as compared with anti-TNF agents used in monotherapy. Combination therapy is usually performed in rheumatic diseases and, more recently, also in IBD patients (Citation30,Citation32,Citation66). Our findings are relevant for several reasons. First, our estimates are based on pooling of 40 RCTs with an overall population of over 14,683 patients. The high quality of the underlying studies is likely to exclude allocation bias between active and placebo arms, as well as an under-reporting of TB cases between combined and monotherapy. Due to the large number of patients pooled, it is unlikely that new individual RCTs will affect the main result of our analysis. Second, we showed for the first time that TB risk is affected by concomitant medications. This is in agreement with the results of a recent meta-analysis of RCTs of ADA and IFX—where > 70% of patients were treated with a combination therapy—that showed a 2-fold increased risk of serious infections compared with placebo (Citation19). Thus, a more intensive screening and surveillance approach seems to be advisable when combined therapy is used. Of note, our results are also in agreement with a recent analysis from the FDA adverse event-reporting system (Citation67), showing a 4-fold increased risk of mycobacterial infections in those with combined therapy versus monotherapy. Third, we confirmed a higher risk of TB reactivation also in patients with anti-TNF monotherapy. The fact that the difference as compared with placebo did not reach statistical significance in our analysis may be simply referred to the small number of events, since only two TB cases occurred in the monotherapy arm. Of note, in previous systematic reviews (Citation19−22), TB cases were not separated between monotherapy and combined therapy, so that it was more likely to reach the statistically significant difference. Thus, we confirm the importance of TB screening even in patients with anti-TNF monotherapy. Our data would indicate that the additional TB risk is related with a synergistic effect between anti-TNF agents and MTX or AZA rather than to an intrinsic risk of each immunosuppressive drug. Indeed, the simple addition of MTX or AZA to placebo did not increase the risk of TB as compared with placebo alone. This finding seems to be in contrast with the results of the CORRONA registry in which the combination therapy with MTX and TNF antagonists was not associated with a synergistic risk of overall and opportunistic infections compared with either agents prescribed alone (Citation68). However, this result could be explained by the unexpectedly increased risk of opportunistic infections, including TB, found in the cohort of patients treated with MTX alone. Such a finding is not supported by the majority of previous studies which failed to show any increase of infection risk with MTX (Citation69,Citation70), so that no screening for TB is actually recommended in patients treated with MTX as well as AZA (Citation71). Fourth, although TB development tended to be higher in patients with rheumatological diseases as compared to those with IBD or psoriasis (0.29% versus 0.12%), the difference was not statistically significant. Indeed, there is no plausible explanation in the pathogenesis and/or natural history of rheumatologic and non-rheumatologic diseases to support an intrinsic role of the underlying disease in affecting the TB risk. Indeed, although it has been reported that RA patients are at 2-fold increased risk of developing infections when compared with the general population (Citation72), no increased TB risk was demonstrated.
There are limitations in the present analysis. Our results are not immediately generalizable to anti-TNF agents, such as etanercept, that are mainly used in rheumatologic conditions. Secondly, we did not use a formal meta-analytic approach for the well-known limitations when dealing with rare events (Citation25). However, the very low frequency of TB events in any study marginalizes the relevance of inter-study heterogeneity. Third, there was some heterogeneity in the TB screening and intervention in positive cases among studies. However, the sensitivity analysis minimized the role of such heterogeneity. Fourth, the median duration of the studies included in our systematic review was only 24 weeks (range 12–54). However, we feel this interval to be adequate in order to assess TB risk, since > 70% of anti-TNF-related TB cases have been shown to occur within 90 days from the beginning of anti-TNF therapy (Citation6). Of note, the absolute estimate of TB risk—ranging between 91 and 832 cases per 100,000 patient-years—is in agreement with previous incidence rates reported in the literature (Citation8,Citation13). Moreover, the overall risk of infections seems to be no longer increased after the first year of follow-up (Citation73). Fifth, glucocorticoid use has been associated with an almost 8-fold increased risk of TB reactivation, especially in those receiving high doses (≥ 15 mg of prednisone) for prolonged periods (≥ 1 month), as well as with a 2–3-fold increase in the risk of hospitalized infections (Citation74,Citation75). Therefore, since the ‘on demand’ use of glucocorticoids was allowed in the RCTs analysed in our systematic review, this could have influenced the TB risk. However, glucocorticoid use appeared to be similar between active treatment and control arms, minimizing the chances of a selection bias.
In conclusion, our systematic review showed a 13-fold increase risk of TB reactivation when using anti-TNF therapy in combination with MTX or AZA as compared with monotherapy. This warrants the adoption of more intensive screening strategies for these patients. This should not obviously reduce the awareness of TB risk in patients with anti-TNF monotherapy, as already supported by the international guidelines.
Declaration of interest: The authors declare no conflicts of interest.
References
- Mohan VP, Scanga CA, Yu K, Scott HM, Tanaka KE, Tsang E, et al. Effects of tumor necrosis factor alpha on host immune response in chronic persistent tuberculosis: possible role for limiting pathology. Infect Immun. 2001;69:1847–55.
- Lin PL, Plessner HL, Voitenok NN, Flynn JL. Tumor necrosis factor and tuberculosis. J Investig Dermatol Symp Proc. 2007;12:22–5.
- Engele M, Stössel E, Castiglione KJ, Schwerdtner N, Wagner M, Bölcskei P, et al. Induction of TNF in human alveolar macrophages as a potential evasion mechanism of virulent Mycobacterium tuberculosis. J Immunol. 2002;168:1328–37.
- Bruns H, Meinken C, Schauenberg P, Härter G, Kern P, Modlin RL, et al. Anti-TNF immunotherapy reduces CD8 + T cell-mediated antimicrobial activity against Mycobacterium tuberculosis in humans. J Clin Invest. 2009;119:1167–77.
- Flynn JL, Goldstein MM, Chan J, Triebold KJ, Pfeffer K, Lowenstein CJ, et al. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–72.
- Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001; 345:1098–104.
- Carmona L, Hernandez-Garcia C, Vadillo C, Pato E, Balsa A, Gonzalez-Alvaro I, et al. Increased risk of tuberculosis in patients with rheumatoid arthritis. J Rheumatol. 2003;30:1436–9.
- Gomez-Reino JJ, Carmona L, Valverde VR, Mola EM, Montero MD. Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk: a multicenter active-surveillance report. Arthritis Rheum. 2003;48:2122–7.
- British Thoracic Society Standards of Care Committee. BTS recommendations for assessing risk and for managing Mycobacterium tuberculosis infection and disease in patients due to start anti-TNF-alpha treatment. Thorax. 2005;60:800–5.
- Solovic I, Sester M, Gomez-Reino JJ, Rieder HL, Ehlers S, Milburn HJ, et al. The risk of tuberculosis related to tumour necrosis factor antagonist therapies: a TBNET consensus statement. Eur Respir J. 2010; 36:1185–206.
- Dignass A, Van Assche G, Lindsay JO, Lemann M, Soderholm J, Colombel JF, et al. The second European evidence-based consensus on the diagnosis and management of Crohn's disease: current management. J Crohns Colitis. 2010;4:28–62.
- Orlando A, Armuzzi A, Papi C, Annese V, Ardizzone S, Biancone L, et al. The Italian Society of Gastroenterology (SIGE) and the Italian Group for the study of Inflammatory Bowel Disease (IG-IBD) Clinical Practice Guidelines: The use of tumor necrosis factor-alpha antagonist therapy in inflammatory bowel disease. Dig Liver Dis. 2011;43:1–20.
- Tubach F, Salmon D, Ravaud P, Allanore Y, Goupille P, Breban M, et al. Risk of tuberculosis is higher with anti-tumor necrosis factor monoclonal antibody therapy than with soluble tumor necrosis factor receptor therapy: the three-year prospective French Research Axed on Tolerance of Biotherapies registry. Arthritis Rheum. 2009;60:1884–94.
- Burmester GR, Panaccione R, Gordon KB, McIlraith MJ, Lacerda AP. Adalimumab: long-term safety in 23,458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn's disease. Ann Rheum Dis. 2013;72:517–24.
- Colombel JF, Loftus EV Jr, Tremaine WJ, Egan LJ, Harmsen WS, Schleck CD, et al. The safety profile of infliximab in patients with Crohn's disease: the Mayo clinic experience in 500 patients. Gastroenterology. 2004;126:19–31.
- Fidder H, Schnitzler F, Ferrante M, Noman M, Katsanos K, Segaert S, et al. Long-term safety of infliximab for the treatment of inflammatory bowel disease: a single-centre cohort study. Gut. 2009;58:501–8.
- Lichtenstein GR, Feagan BG, Cohen RD, Salzberg BA, Diamond RH, Price S, et al. Serious infection and mortality in patients with Crohn's disease: more than 5 years of follow-up in the TREAT registry. Am J Gastroenterol. 2012;107:1409–22.
- Dixon WG, Hyrich KL, Watson KD, Lunt M, Galloway J, Ustianowski A, et al. Drug-specific risk of tuberculosis in patients with rheumatoid arthritis treated with anti-TNF therapy: results from the British Society for Rheumatology Biologics Register (BSRBR). Ann Rheum Dis. 2010; 69:522–8.
- Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295:2275–85.
- Leombruno JP, Einarson TR, Keystone EC. The safety of anti-tumour necrosis factor treatments in rheumatoid arthritis: meta and exposure-adjusted pooled analyses of serious adverse events. Ann Rheum Dis. 2009;68:1136–45.
- Dommasch ED, Abuabara K, Shin DB, Nguyen J, Troxel AB, Gelfand JM. The risk of infection and malignancy with tumor necrosis factor antagonists in adults with psoriatic disease: a systematic review and meta-analysis of randomized controlled trials. J Am Acad Dermatol. 2011;64:1035–50.
- Singh JA, Wells GA, Christensen R, Tanjong Ghogomu E, Maxwell L, Macdonald JK, et al. Adverse effects of biologics: a network meta- analysis and Cochrane overview. Cochrane Database Syst Rev. 2011;(2):CD008794.
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:65–94.
- Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P, Group C. Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med. 2008;148:295–309.
- Walker E, Hernandez AV, Kattan MW. Meta-analysis: its strengths and limitations. Cleve Clin J Med. 2008;75:431–9.
- Targan SR, Hanauer SB, van Deventer SJ, Mayer L, Present DH, Braakman T, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. Crohn's Disease cA2 Study Group. N Engl J Med. 1997;337:1029–35.
- Present DH, Rutgeerts P, Targan S, Hanauer SB, Mayer L, van Hogezand RA, et al. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med. 1999;340:1398–405.
- Winter TA, Wright J, Ghosh S, Jahnsen J, Innes A, Round P. Intravenous CDP870, a PEGylated Fab’ fragment of a humanized antitumour necrosis factor antibody, in patients with moderate-to-severe Crohn's disease: an exploratory study. Aliment Pharmacol Ther. 2004;20:1337–46.
- Schreiber S, Rutgeerts P, Fedorak RN, Khaliq-Kareemi M, Kamm MA, Boivin M, et al. A randomized, placebo-controlled trial of certolizumab pegol (CDP870) for treatment of Crohn's disease. Gastroenterology. 2005;129:807–18.
- Lemann M, Mary JY, Duclos B, Veyrac M, Dupas JL, Delchier JC, et al. Infliximab plus azathioprine for steroid-dependent Crohn's disease patients: a randomized placebo-controlled trial. Gastroenterology. 2006;130:1054–61.
- Sandborn WJ, Feagan BG, Stoinov S, Honiball PJ, Rutgeerts P, Mason D, et al. Certolizumab pegol for the treatment of Crohn's disease. N Engl J Med. 2007;357:228–38.
- Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010;362:1383–95.
- Sandborn WJ, Rutgeerts P, Feagan BG, Reinisch W, Olson A, Johanns J, et al. Colectomy rate comparison after treatment of ulcerative colitis with placebo or infliximab. Gastroenterology. 2009;137:1250–60.
- Maini R, St Clair EW, Breedveld F, Furst D, Kalden J, Weisman M, et al. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet. 1999;354:1932–9.
- Weinblatt ME, Keystone EC, Furst DE, Moreland LW, Weisman MH, Birbara CA, et al. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 2003;48:35–45.
- Furst DE, Schiff MH, Fleischmann RM, Strand V, Birbara CA, Compagnone D, et al. Adalimumab, a fully human anti tumor necrosis factor-alpha monoclonal antibody, and concomitant standard antirheumatic therapy for the treatment of rheumatoid arthritis: results of STAR (Safety Trial of Adalimumab in Rheumatoid Arthritis). J Rheumatol. 2003;30:2563–71.
- van de Putte LB, Atkins C, Malaise M, Sany J, Russell AS, van Riel PL, et al. Efficacy and safety of adalimumab as monotherapy in patients with rheumatoid arthritis for whom previous disease modifying antirheumatic drug treatment has failed. Ann Rheum Dis. 2004;63:508–16.
- Keystone EC, Kavanaugh AF, Sharp JT, Tannenbaum H, Hua Y, Teoh LS, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum. 2004;50:1400–11.
- St Clair EW, van der Heijde DM, Smolen JS, Maini RN, Bathon JM, Emery P, et al. Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: a randomized, controlled trial. Arthritis Rheum. 2004;50:3432–43.
- Abe T, Takeuchi T, Miyasaka N, Hashimoto H, Kondo H, Ichikawa Y, et al. A multicenter, double-blind, randomized, placebo controlled trial of infliximab combined with low dose methotrexate in Japanese patients with rheumatoid arthritis. J Rheumatol. 2006;33:37–44.
- Westhovens R, Yocum D, Han J, Berman A, Strusberg I, Geusens P, et al. The safety of infliximab, combined with background treatments, among patients with rheumatoid arthritis and various comorbidities: a large, randomized, placebo-controlled trial. Arthritis Rheum. 2006;54: 1075–86.
- Schiff M, Keiserman M, Codding C, Songcharoen S, Berman A, Nayiager S, et al. Efficacy and safety of abatacept or infliximab vs placebo in ATTEST: a phase III, multi-centre, randomised, double-blind, placebo-controlled study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Ann Rheum Dis. 2008;67:1096–103.
- Miyasaka N. Clinical investigation in highly disease-affected rheumatoid arthritis patients in Japan with adalimumab applying standard and general evaluation: the CHANGE study. Mod Rheumatol. 2008;18:252–62.
- Keystone E, Heijde D, Mason D Jr, Landewe R, Vollenhoven RV, Combe B, et al. Certolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis: findings of a fifty-two-week, phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum. 2008;58:3319–29.
- Fleischmann R, Vencovsky J, van Vollenhoven RF, Borenstein D, Box J, Coteur G, et al. Efficacy and safety of certolizumab pegol monotherapy every 4 weeks in patients with rheumatoid arthritis failing previous disease-modifying antirheumatic therapy: the FAST4WARD study. Ann Rheum Dis. 2009;68:805–11.
- Smolen J, Landewe RB, Mease P, Brzezicki J, Mason D, Luijtens K, et al. Efficacy and safety of certolizumab pegol plus methotrexate in active rheumatoid arthritis: the RAPID 2 study. A randomised controlled trial. Ann Rheum Dis. 2009;68:797–804.
- Chen DY, Chou SJ, Hsieh TY, Chen YH, Chen HH, Hsieh CW, et al. Randomized, double-blind, placebo-controlled, comparative study of human anti-TNF antibody adalimumab in combination with methotrexate and methotrexate alone in Taiwanese patients with active rheumatoid arthritis. J Formos Med Assoc. 2009;108:310–19.
- Braun J, Brandt J, Listing J, Zink A, Alten R, Golder W, et al. Treatment of active ankylosing spondylitis with infliximab: a randomised controlled multicentre trial. Lancet. 2002;359:1187–93.
- van der Heijde D, Dijkmans B, Geusens P, Sieper J, DeWoody K, Williamson P, et al. Efficacy and safety of infliximab in patients with ankylosing spondylitis: results of a randomized, placebo-controlled trial (ASSERT). Arthritis Rheum. 2005;52:582–91.
- van der Heijde D, Kivitz A, Schiff MH, Sieper J, Dijkmans BA, Braun J, et al. Efficacy and safety of adalimumab in patients with ankylosing spondylitis: results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2006;54:2136–46.
- Lambert RG, Salonen D, Rahman P, Inman RD, Wong RL, Einstein SG, et al. Adalimumab significantly reduces both spinal and sacroiliac joint inflammation in patients with ankylosing spondylitis: a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2007;56:4005–14.
- Inman RD, Maksymowych WP. A double-blind, placebo-controlled trial of low dose infliximab in ankylosing spondylitis. J Rheumatol. 2010;37:1203–10.
- Gottlieb AB, Evans R, Li S, Dooley LT, Guzzo CA, Baker D, et al. Infliximab induction therapy for patients with severe plaque-type psoriasis: a randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol. 2004;51:534–42.
- Reich K, Nestle FO, Papp K, Ortonne JP, Evans R, Guzzo C, et al. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet. 2005;366:1367–74.
- Gordon KB, Langley RG, Leonardi C, Toth D, Menter MA, Kang S, et al. Clinical response to adalimumab treatment in patients with moderate to severe psoriasis: double-blind, randomized controlled trial and open-label extension study. J Am Acad Dermatol. 2006;55:598–606.
- Menter A, Feldman SR, Weinstein GD, Papp K, Evans R, Guzzo C, et al. A randomized comparison of continuous vs. intermittent infliximab maintenance regimens over 1 year in the treatment of moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2007;56:31.e31–15.
- Saurat JH, Stingl G, Dubertret L, Papp K, Langley RG, Ortonne JP, et al. Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION). Br J Dermatol. 2008;158:558–66.
- Menter A, Tyring SK, Gordon K, Kimball AB, Leonardi CL, Langley RG, et al. Adalimumab therapy for moderate to severe psoriasis: a randomized, controlled phase III trial. J Am Acad Dermatol. 2008; 58:106–15.
- Asahina A, Nakagawa H, Etoh T, Ohtsuki M. Adalimumab in Japanese patients with moderate to severe chronic plaque psoriasis: efficacy and safety results from a phase II/III randomized controlled study. J Dermatol. 2010;37:299–310.
- Torii H, Nakagawa H. Infliximab monotherapy in Japanese patients with moderate-to-severe plaque psoriasis and psoriatic arthritis. A randomized, double-blind, placebo-controlled multicenter trial. J Dermatol Sci. 2010;59:40–9.
- Leonardi C, Langley RG, Papp K, Tyring SK, Wasel N, Vender R, et al. Adalimumab for treatment of moderate to severe chronic plaque psoriasis of the hands and feet: efficacy and safety results from REACH, a randomized, placebo-controlled, double-blind trial. Arch Dermatol. 2011;147:429–36.
- Antoni CE, Kavanaugh A, Kirkham B, Tutuncu Z, Burmester GR, Schneider U, et al. Sustained benefits of infliximab therapy for dermatologic and articular manifestations of psoriatic arthritis: results from the infliximab multinational psoriatic arthritis controlled trial (IMPACT). Arthritis Rheum. 2005;52:1227–36.
- Antoni C, Krueger GG, de Vlam K, Birbara C, Beutler A, Guzzo C, et al. Infliximab improves signs and symptoms of psoriatic arthritis: results of the IMPACT 2 trial. Ann Rheum Dis. 2005;64:1150–7.
- Mease PJ, Gladman DD, Ritchlin CT, Ruderman EM, Steinfeld SD, Choy EH, et al. Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 2005;52:3279–89.
- Genovese MC, Mease PJ, Thomson GT, Kivitz AJ, Perdok RJ, Weinberg MA, et al. Safety and efficacy of adalimumab in treatment of patients with psoriatic arthritis who had failed disease modifying antirheumatic drug therapy. J Rheumatol. 2007;34:1040–50.
- Panaccione R, Ghosh S, Middleton S, Márquez JR, Scott BB, Flint L, et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology. 2014;146:392–400.
- Deepak P, Stobaugh DJ, Ehrenpreis ED. Infectious complications of TNF-alpha inhibitor monotherapy versus combination therapy with immunomodulators in inflammatory bowel diseases: analysis of Food and Drug Administration adverse event reporting system. J Gastrointestin Liver Dis. 2013;22:269–76.
- Greenberg JD, Reed G, Kremer JM, Tindall E, Kavanaugh A, Zheng C, et al. Association of methotrexate and tumour necrosis factor antagonists with risk of infectious outcomes including opportunistic infections in the CORRONA registry. Ann Rheum Dis. 2010;69:380–6.
- Wolfe F, Caplan L, Michaud K. Treatment for rheumatoid arthritis and the risk of hospitalization for pneumonia: associations with prednisone, disease-modifying antirheumatic drugs, and anti-tumor necrosis factor therapy. Arthritis Rheum. 2006;54:628–34.
- Doran MF, Crowson CS, Pond GR, O’Fallon WM, Gabriel SE. Predictors of infection in rheumatoid arthritis. Arthritis Rheum. 2002; 46:2294–2300.
- Bogas M, Machado P, Mourao AF, Costa L, Santos MJ, Fonseca JE, et al. Methotrexate treatment in rheumatoid arthritis: management in clinical remission, common infection and tuberculosis. Results from a systematic literature review. Clin Rheumatol. 2010;29:629–35.
- Doran MF, Crowson CS, Pond GR, O’Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46:2287–93.
- Askling J, Fored CM, Brandt L, Baecklund E, Bertilsson L, Feltelius N, et al. Time-dependent increase in risk of hospitalisation with infection among Swedish RA patients treated with TNF antagonists. Ann Rheum Dis. 2007;66:1339–44.
- Smitten AL, Choi HK, Hochberg MC, Suissa S, Simon TA, Testa MA, et al. The risk of hospitalized infection in patients with rheumatoid arthritis. J Rheumatol. 2008;35:387–93.
- Jick SS, Lieberman ES, Rahman MU, Choi HK. Glucocorticoid use, other associated factors, and the risk of tuberculosis. Arthritis Rheum. 2006;55:19–26.