Abstract
High-dose chemotherapy followed by transplantation of autologous hematopoietic progenitor cells has a proven track record of safety and efficacy in hematological malignancies and select solid tumors. The near-universal use of peripheral blood stem cells as source for autografts, routine growth factor support, and antimicrobial prophylaxis post transplantation has improved the safety of this procedure. However, the advent of highly active novel therapies in the last few years warrants reappraisal of the role of autologous transplantation in the therapeutic armamentarium of malignant disorder. This review summarizes the current role of autologous transplantation for hematological malignancies, discusses modern standards for patient selection, and highlights long-term care issues of transplant survivors from an internist's perspective. Role of tumor purging in autologous transplantation, novel transplant conditioning regimens, and post-transplant therapies to prevent disease relapse are reviewed.
Key messages
Advances in supportive care have dramatically improved the safety of autologous transplantation in the modern era, with expected rates of treatment-related mortality below 2%–3%.
Peripheral blood has replaced bone marrow as the preferred source of stem cells.
Autologous transplantation is the standard of care and potentially curative option for patients with relapsed Hodgkin and aggressive B-cell lymphomas.
Autologous transplant survivors require meticulous follow-up from primary care physicians to screen for long-term complications.
Introduction
Many decades ago researchers determined that bone marrow cells transplanted from one animal to another could restore blood production (Citation1). These preclinical investigations were taken to into the clinical arena, and hematopoietic cell transplantation (HCT) evolved as a potentially curative treatment modality for patients with selected neoplastic and, rarely, certain non-neoplastic diseases. Many tumors show a steep dose-response to cytotoxic therapy, i.e. an increase in the dose of drug or radiation markedly increases the number of cancer cells killed. Cure or effective disease control depends on delivering intensive doses of chemotherapy or chemotherapy plus radiation therapy. This intensity often exceeds the tolerance of bone marrow. Hematopoietic cell function must therefore be restored after such therapy, or these patients will experience fatal bone marrow aplasia. This is accomplished by infusing hematopoietic cells intravenously afterward; cells then ‘home’ to marrow to reconstitute lymphohematopoietic function. Thus, it is essential that blood production be restored using either autologous (‘self’) cells or cells collected from another individual, i.e. allogeneic donor cells.
In chemotherapy-sensitive hematologic malignancies, autologous HCT can provide long-term disease control, while avoiding the immunologic complications and delayed immune reconstitution inherent to allogeneic HCT. The phases of autologous HCT include: collection of autologous hematopoietic progenitor cells (HPCs) and their cryopreservation; administration of high-dose therapy (HDT; also called conditioning or preparative regimen); thawing and intravenous administration of the autologous cells; and then vigorous supportive care including antibiotics, blood component transfusions, and other measures such as electrolyte replacement therapy while awaiting recovery of hematopoiesis.
Pre-transplant patient evaluation
Recommending HCT to a potential candidate is a complex decision process intricately dependent on several variables, including underlying diagnosis, prior therapies, patient's age, performance status, degree of impairment in the vital organ function, and socio-economic support structure. Careful patient selection is crucial to successful outcomes after autologous HCT (Citation2).
Infectious disease evaluation
Careful evaluation of the patients is necessary to minimize the risk not only of new post-HCT infectious complications, but also of reactivation of past fungal and viral infections. Detailed questioning regarding sexual orientation, history of unprotected sexual intercourse, needle sharing, remote blood transfusions, travel history, etc., can help direct the infectious disease screening work-up. All HCT recipients should be tested for the presence of anti-cytomegalovirus (CMV) IgG antibodies. Testing for serum anti-herpes simplex virus IgG and anti-varicella zoster IgG is also routinely performed. All patients must be screened for HIV infection. Autologous HCT in HIV-positive patients with a CD4 count ≥ 100/μL and an undetectable or low viral load (< 10,000 copies/mL) is well tolerated and is not associated with unusually high morbidity or mortality (Citation3).
Pre-transplant dental evaluation and medically necessary oral care can eliminate potential sites of infection and trauma (Citation4).
Patients undergoing HCT should be screened for infection with hepatitis B virus (HBV) and hepatitis C virus (HCV). Infection with HBV or HCV is not an absolute contraindication for HCT. However, these patients are at increased risk of post-transplant viral reactivation, veno-occlusive disease (VOD), liver cirrhosis, and even fulminant hepatic failure (Citation5).
Organ function evaluation
Karnofsky performance status (KPS) is a useful tool for assessing a patient's functional status. In patients undergoing autologous HCT a KPS score of ≥ 70 is desirable. Some (Citation6), but not all studies have suggested an association between decreased pre-transplantation left ventricular ejection fraction (LVEF) and risk of developing post-HCT cardiotoxicity (Citation7,Citation8). Demonstration of LVEF of ≥ 45%–50% is arbitrarily considered a HCT eligibility requirement by most transplant centers.
Pulmonary function testing (PFT) performed before HCT helps to identify patients at increased risk of developing post-transplant pulmonary events. Detection of abnormal diffusion capacity for carbon monoxide (DLCO) and alveolar-arterial oxygen gradient on PFT are independent predictors of the need for mechanical ventilation and mortality post HSCT (Citation9). A serum creatinine value of ≤ 1.5 mg/dL (SI unit 132 μmol/L) and a creatinine clearance above 60 mL/min (SI unit 1 mL/s) before transplantation are desirable.
Assessment of hepatic function with the measurement of serum bilirubin concentration, transaminases, and albumin level is routinely performed during pre-transplant evaluation. Elevation of serum transaminases and alkaline phosphatase before HCT are risk factors for developing VOD following transplantation (Citation10). Determination of serum ferritin, iron profile, hepatic magnetic resonance imaging, and/or liver biopsy can help identify patients with transfusion-related iron overload. Institution of appropriate chelation therapy can lead to improvement in hepatic function.
and list common diagnostic tests and physiological criteria used by the majority of transplant centers to evaluate patient eligibility for HCT.
Table I. Recommended evaluation in a HCT candidate.
Table II. Ideal physiological criteria for selecting patients for HCT.
Counseling
Assessment of patients’ psychological health before transplantation cannot be overemphasized. It is imperative to recognize and remedy factors impairing patients’ social and psychosocial well-being, before committing them to a life-changing event such as HCT. Identifying and addressing alcohol or substance abuse issues before HCT is the key for good post-HCT patient compliance. Undergoing transplantation generally means being off work for a number of months post HCT. Support from a transplant social worker to ensure insurance coverage of transplantation, arranging for temporary disability for those in need, providing assistance with child-care, transportation, lodging, etc., are small details which produce huge impact on patients’ mental well-being and post-transplant follow-up compliance.
Advice regarding smoking cessation is often required. Smoking increases the pulmonary transplant-related mortality risk 5-fold (Citation11), underscoring the importance of smoking cessation counseling during pre-transplant evaluation (Citation12).
Hematopoietic progenitor cell collection
Autologous HPCs are collected from patients’ peripheral blood or bone marrow. These HPCs are capable of giving rise to the entire lymphohematopoietic system. These early HPCs are identified as CD34 + by immunophenotyping. Bone marrow harvesting was the traditional method of collecting HPCs for HCT. Marrow is removed from the posterior superior iliac crests with the patient in the prone position under spinal or general anesthesia. In a typical harvest, 1000 mL of marrow is aspirated (10–15 mL/kg patient weight), which requires manually entering the bone marrow space about 100 times.
Harvesting HPCs from peripheral blood is clearly an easier way to collect stem cells than traditional bone marrow harvests in the operating room. Today, nearly all autologous HCTs use peripheral blood progenitor cells as the graft source. HPCs are collected from the peripheral blood using an apheresis instrument that removes the cells by a centrifugation density gradient and returns the unwanted blood cells and plasma back to the patient. This process usually takes about ≥ 3–4 hours, and about 12–18 liters of blood are processed in the procedure. The minimum HPC dose to perform an autologous HCT successfully is generally considered to be 2 million CD34 + cells/kg recipient body weight. A dose of ≥ 4–6 million CD34 + cells/kg is considered optimal (Citation13,Citation14).
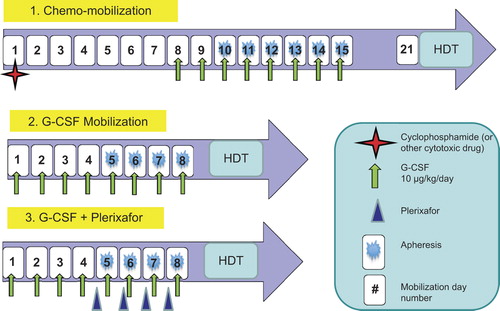
HPC mobilization is performed by using cytokines, most commonly granulocyte-colony stimulating factor (G-CSF), either alone or in combination with chemotherapy or plerixafor () (Citation14–16). Plerixafor is a small molecule that inhibits chemokine stromal cell derived factor 1-alpha from binding to CXC chemokine receptor 4, resulting in increased HPC migration into peripheral blood. The HPC mobilization method with the best risk, benefit, and cost ratio is controversial. While many lymphoma patients can successfully mobilize HPCs with ‘cytokine only’ strategies, mobilization failure rates remain a concern in patients with risk factors such as prior radiotherapy, heavily pretreated disease with more intensive chemotherapy regimens such as hyper-CVAD (cyclophosphamide, vincristine, doxorubicin and dexamethasone) or fludarabine-containing regimens, or radioimmunotherapy (RIT), advanced age, and bone marrow involvement (Citation17–20). In such cases, consideration should be given to chemotherapy- or plerixafor-based mobilization strategies (Citation13). In myeloma patients who are potential autologous HCT candidates, it is important to avoid known factors that impair HPC mobilization, e.g. melphalan-based induction regimens, long-term use of lenalidomide, and radiation therapy to a significant volume of hematopoietic marrow areas (Citation21,Citation22).
Purging contaminating tumor cells from the HPC product
Relapse after autologous HCT derives usually from proliferation of a chemotherapy-resistant clone of malignant cells surviving the HDT, or rarely from reinfusion of an autograft contaminated by tumor cells. Ex vivo purging (by monoclonal antibodies, CD34 + cell selection, etc.) (Citation23,Citation24) or ‘in vivo purging’ (e.g. rituximab therapy given to lymphoma patients during mobilization) (Citation25,Citation26) of autologous HPC grafts appear to reduce or eliminate the risk of tumor-cell reinfusion at HCT. Reduction of autograft contamination with tumor cells, however, may not lead to improved outcomes. The CUP trial, comparing salvage chemotherapy alone to chemotherapy followed by either immune-magnetically purged or unpurged HCT in relapsed follicular lymphoma (FL) (Citation27), reported no significant difference in the outcomes of purged compared to unpurged autografts. The lack of clear benefit in randomized data and a possible increase in infectious complications with ex vivo purging (Citation28,Citation29) preclude routine use of this approach.
Preparative (conditioning) regimens
In autologous HCT, the preparative regimen, which consists of high-dose chemotherapy and/or total-body irradiation (TBI), is administered first in an attempt to eliminate the malignant disease. The exact type of autologous HCT conditioning regimen depends to a certain extent on the histological diagnosis of the patient.
Several conditioning regimens have been evaluated for autologous HCT in myeloma. A randomized phase III trial compared intravenous melphalan at 200 mg/m2 (MEL200) to a combination of melphalan at 140 mg/m2 (MEL140) and 8 Gy TBI (Citation30). The MEL140-TBI regimen caused significantly more toxicity. Overall survival (OS) and progression-free survival (PFS) were similar. Another recent phase III study compared MEL200 with MEL140 + busulfan (Bu-Mel) (Citation31). In this study Bu-Mel was associated with significantly lower complete remission (CR) rates, more frequent grade 3–4 toxicities, and similar OS or PFS compared to MEL200. MEL200 is considered the standard of care for conditioning before autologous HCT in myeloma in those who can tolerate the procedure (e.g. younger patients with acceptable renal function). Advanced renal impairment is frequent (20%–30%) in myeloma (Citation32,Citation33). Autologous HCT is feasible even in myeloma patients with advanced renal impairment (serum creatinine > 3 mg/dL [SI unit > 265 μmol/L] or receiving hemodialysis). In this setting a melphalan dose of 140 mg/m2 is preferred (Citation34,Citation35). Unlike MEL200 there is no single, widely used and accepted conditioning regimen for lymphoid malignancies. Commonly used regimens include: CBV (cyclophosphamide, etoposide, carmustine), BEAM (carmustine, etoposide, cytarabine, melphalan), BEAC (carmustine, etoposide, cytarabine, cyclophosphamide), and TBI-containing regimens (Citation36), with limited retrospective data suggesting less treatment-related mortality (TRM) and more favorable outcomes following non-TBI-containing conditioning regimens (Citation37).
Radioimmunotherapy in autologous transplantation conditioning
RIT with monoclonal antibodies conjugated to a radionuclide is effective against B-cell non-Hodgkin lymphoma (NHL). Two main approaches have evolved for applying RIT as autologous HCT conditioning. One uses high-dose myeloablative RIT (with or without chemotherapy), and the other combines standard-dose RIT with HDT. A number of small phase II studies utilizing high-dose RIT with iodine-131 tositumomab (131I-T), in mostly chemosensitive relapsed B-cell NHLs, demonstrated 4-year PFS and OS of ∼ 40% and ∼ 65%, respectively, with acceptable toxicities (Citation38–40). However, since 131I-T emits γ-radiation, its administration is complicated by requirements for prolonged patient isolation, special infusion equipment, caregiver/health care worker exposure precautions, and complex dosimetry facilities. Hence, high-dose RIT by and large remains confined to centers with available expertise. To circumvent logistical challenges associated with high-dose RIT, several studies combining standard-doses of RIT with HDT to intensify auto-HCT conditioning reported encouraging outcomes (Citation41–43). However, the BMT-CTN 0401 trial which randomized chemosensitive, relapsed diffuse large B-cell lymphoma (DLBCL) to either yttrium-90 ibritumomab tiuxetan-BEAM or rituximab-BEAM conditioning did not show a benefit of RIT conditioning for disease control or survival (Citation44). The published evidence currently does not support routine addition of standard-dose RIT to auto-HCT conditioning.
Blood component transfusion, supportive care, and infection prophylaxis
After the completion of conditioning, HPCs are infused in order to rescue autologous hematopoiesis. Infused HPCs require time to regenerate hematopoiesis, and during this time the patient requires intensive supportive care to prevent complications arising from myeloablation, prolonged cytopenias, and organ damage induced by HDT. Breakdown of the normal mucosal barriers in the gastrointestinal tract leading to oral mucositis, pain, diarrhea, and increased susceptibility to infectious complications is common. Prophylaxis with fluoroquinolones (generally ciprofloxacin or levofloxacin) to prevent Gram-negative infections and an antifungal agent (e.g. fluconazole) until neutrophil recovery is recommended. Autologous HCT recipients are at risk of herpes reactivation for several months post transplantation (especially those with prior bortezomib exposure), and antiviral prophylaxis (e.g. acyclovir or valacyclovir) for 12 months post transplantation is common practice. Sulfamethoxazole/trimethoprim (or equivalent) is instituted generally after robust neutrophil recovery for Pneumocystis jirovecii prophylaxis post autologous HCT for 6–12 months. It is, however, worth mentioning that the type and duration of infectious disease prophylactic regimens display significant variability across different transplant centers and countries. Readers are referred to the Infectious Diseases Society of American website for details regarding guidelines for preventing infectious complications in HCT recipients (Citation45).
Hematopoietic recovery after autologous HCT takes approximately 2–3 weeks, and recombinant hematopoietic growth factor support early after HPC infusion (within 1–5 days) usually can reduce the duration of severe neutropenia by 2–4 days. Red blood cell and platelet transfusions are essential to prevent or treat complications and symptoms of prolonged cytopenia. Red blood cell transfusions are used to keep the hematocrit typically over 25%. Platelet transfusions are used to keep the platelet count above 10,000/μL to minimize bleeding; platelet transfusions may be required at a higher threshold in patients who demonstrate a hemorrhagic diathesis. All blood products must be irradiated to prevent inadvertent engraftment of ‘contaminating’ allogeneic lymphocytes from the transfused unit that may cause lethal transfusion-associated graft-versus-host disease (GVHD).
Acute toxicities of autologous transplantation
When the neutrophil count drops below 500/μL, there is a markedly increased susceptibility to bacterial and fungal infections. The disturbance of the mucosal barrier due to the conditioning regimen and use of intravascular access devices add to the patient's risk of infection. The longer the neutropenic period, the higher the infectious risk. Major infections encountered in autologous HCT are with Gram-negative and Gram-positive bacteria; Clostridia difficile toxin-associated diarrhea; herpes simplex virus reactivation; and fungi, particularly Candida spp. and Aspergillus spp. The use of prophylactic antibacterial, antiviral, and antifungal agents, recombinant hematopoietic growth factors, and blood HPCs has decreased the incidence of severe infections (Citation46,Citation47). Other common and reversible toxicities include nausea, vomiting, alopecia, poor appetite, rash, edema, and flushing.
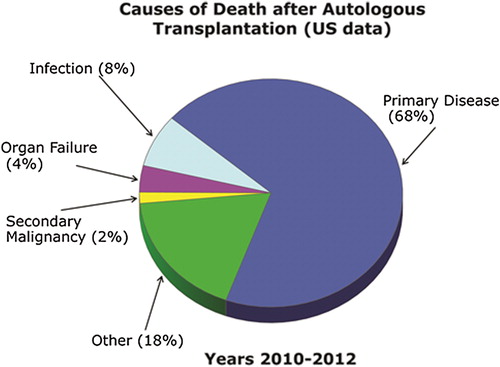
Visceral organs may also be damaged by high-dose therapy. Rare but serious problems include hepatic VOD (also known as sinusoidal obstruction syndrome), idiopathic pneumonia syndrome, and diffuse alveolar hemorrhage (Citation48,Citation49). Hepatic VOD usually presents within the first two to three weeks of the HCT as tender hepatomegaly, jaundice, and fluid retention. Lung injury can present early or later after HCT and may take months to resolve. Compared to allogeneic HCT, treatment-related mortality is low, typically < 3% during the first 100 days after transplant; relapse remains the most common cause of death (). summarizes post-autologous HCT complications and their prophylaxis (if available) and management.
Table III. Complications following autologous transplantation.
Indications for autologous transplantation
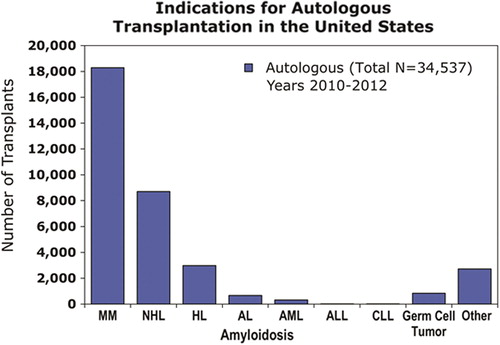
The predominant indication for autologous HCT is the treatment of cancer, although it is sometimes performed for non-neoplastic diseases (). This section focuses on the indications of autologous HCT in the setting of oncology (Citation50).
Figure 3. Common indications for autologous HCT in the United States, as reported to CIBMTR during 2010–2012. AL amyloidosis=Light chain amyloidosis, ALL=acute lymphoblastic leukemia, AML=acute myeloid leukemia, CLL=chronic lymphocytic leukemia, HL=Hodgkin lymphoma, MM=myeloma, NHL=non-Hodgkin lymphoma.
Autologous HCT for plasma cell myeloma
In the United States, myeloma and other plasma cell disorders are the most common indications for autologous HCT (). The majority of autologous HCTs for myeloma are performed as a planned procedure in newly diagnosed patients after a defined initial phase of induction therapy—‘early or upfront autologous HCT’. As myeloma is incurable in most patients, even those that do not receive an upfront autograft may undergo transplant at relapse (‘delayed autologous HCT’).
Early autologous HCT for myeloma
Randomized trials in the 1990s compared autologous HCT to conventional chemotherapy in newly diagnosed myeloma patients. The IFM (Intergroupe Francophone Myeloma) (Citation51) and the Medical Research Council (Citation52) led clinical trials demonstrating that patients randomized to receive upfront autologous HCT had significantly higher CR rates, event-free survival (EFS), and OS. Other randomized studies (), however, have not uniformly demonstrated a survival benefit (Citation53–57). In general, these studies do indicate that autologous HCT improves CR rates and EFS in comparison to conventional chemotherapy. Survival benefit has not been demonstrated in studies that permitted or planned for delayed autologous HCT at relapse, nor in studies conducted in later time periods when novel anti-myeloma agents (lenalidomide, bortezomib, carfilzomib, and pomalidomide) were available as an option after relapse.
Table IV. Selected randomized trials of conventional chemotherapy compared to single autologous HCT as upfront therapy in multiple myeloma.
Tandem autologous HCT
Barlogie and co-workers pioneered the tandem autologous transplant approach in their Total Therapy (TT) program (Citation58,Citation59). The IFM94 was the first randomized trial (Citation60) comparing single to tandem autologous HCT in previously untreated myeloma patients. The projected 7-year EFS and OS benefits were significantly better for the double autologous HCT arm. In an unplanned subgroup analysis, there was no benefit for the second autologous HCT for patients who were in a very good partial remission (VGPR) status or better after the first autograft. Another randomized study (Bologna 96) confirmed the observation that the second HCT does not benefit patients in VGPR or better after the first transplant (Citation61). More importantly this study did not show an OS benefit for the tandem procedure despite superior CR rates and EFS compared to single HCT. Most recently, in a combined Netherlands/German (HOVON65/GMMG-HD4) study, patients receiving tandem autologous HCT in the German (GMMG) component of the study were shown to have superior OS (70% versus 55% at 5 years) over otherwise similarly treated patients receiving a single autologous HCT (Citation62). Randomized studies show tandem autologous HCT produce higher CR rates and superior EFS overall, but not OS benefit (with the exception of IFM94), compared to single autologous HCT. Moreover, benefits of the second autograft appear to be limited to patients who are not in a VGPR after the first autologous HCT.
Delaying autologous HCT until relapse
The timing of autologous HCT (early versus delayed) warrants reappraisal. Mature data from randomized studies, conducted in pre-novel therapy era, suggest that survival is similar whether autologous HCT is performed early or in the delayed setting after relapse (Citation54,Citation56). Early autologous HCT was, however, associated with a longer treatment-free interval and improved quality of life (QOL) in the French MAG-95 trial (Citation54). In the novel therapy era, Palumbo and colleagues randomized patients after lenalidomide/dexamethasone induction to tandem autologous HCT or chemotherapy maintenance. Although CR rates and OS were similar, early HCT reduced the risk of progression by 50%, and median PFS (41 versus 18 months) strongly favored early autologous HCT (Citation63). Data from this trial caution us against abandoning the upfront autologous HCT strategy for myeloma.
Role of autologous transplantation in high-risk disease
Myeloma is a biologically diverse disease with clonal heterogeneity and genomic instability (Citation64,Citation65). The presence of t(4;14) or del(17p), or a high serum beta 2-microglobulin concentration predicts inferior OS (Citation66). Autologous HCT previously has yielded suboptimal results in high-risk myeloma (Citation67), and the benefit of autografting has been questioned in these patients. Recent studies of bortezomib in induction, consolidation, and maintenance phases of therapy programs incorporating tandem transplantation, as in the Arkansas TT-3 trials as well as the HOVON-65/ GMMG-HD4 study, support the role of autologous HCT in high-risk myeloma (Citation62,Citation68,Citation69).
Autologous HCT for uncommon plasma cell dyscrasias
Immunoglobulin light chain amyloidosis (AL), light chain deposition disease, heavy chain deposition disease, and POEMS syndrome are characterized by the presence of clonal plasma cells and light chain or heavy chain production leading to tissue injury. HDT with melphalan has proven effective in many of these disorders, although randomized trials are generally lacking (Citation70,Citation71). Special challenges for HCT in these disorders include higher rates of TRM, engraftment syndrome, and severe fluid retention (Citation72,Citation73). In AL, a disorder where pre-transplant organ dysfunction is common, cardiac involvement, effusions, and significant fluid retention during G-CSF administration for mobilization have emerged as predictors of higher TRM (Citation72). Careful patient selection, dose-adapted melphalan use (depending on patient age, renal function, and comorbidities), and center experience are critical. A randomized multicenter trial failed to show benefit for HCT over conventional chemotherapy in AL (Citation72,Citation74), while experience from specialized centers seems to show a significant benefit, by inducing CRs after HCT that lead to organ function recovery from AL over time (Citation71). Referring AL patients to high-volume centers for HCT should be strongly considered.
Autologous HCT for diffuse large B-cell lymphoma
HCT for high-risk DLBCL in first remission
Studies using autologous HCT as consolidation after first-line therapies in aggressive (mostly DLBCL) NHL in the rituximab era have reported contradictory findings. While two reports showed no benefit (Citation75,Citation76), the intergroup US study suggested improved PFS and OS with autologous HCT consolidation in high-risk International Prognostic Index (IPI) DLBCL (Citation77). Considering these discordant data and the high cure rates of DLBCL with modern therapies (Citation78), upfront use of autologous HCT as consolidation therapy is not recommended.
HCT for DLBCL—in relapsed disease
The role of autologous HCT in relapsed DLBCL is well-defined. The PARMA trial () (Citation79) established that DHAP (dexamethasone, high-dose ara-C, and cisplatin) salvage chemotherapy and HCT provided a significantly enhanced OS benefit in subjects with relapsed, chemotherapy-sensitive disease. Several registry-based (Citation80–83) and prospective studies in the rituximab era () (Citation84) have reproduced these results. Autologous HCT is thus standard-of-care for relapsed/chemotherapy-sensitive DLBCL.
Table V. Select studies addressing the role of autologous transplantation in lymphoid malignancies.
Autologous HCT for mantle cell lymphoma
The European Mantle Cell Lymphoma (MCL) Network trial randomized MCL patients after first-line chemotherapy to either autologous HCT consolidation (‘upfront HCT’) or interferon-alpha maintenance () (Citation85) and demonstrated a superior PFS but no OS benefit for autologous HCT. Similar randomized trials in the rituximab-era are not available. Prospective trials examining upfront autologous HCT in MCL patients following rituximab-containing induction regimens have reported encouraging 5-year PFS and OS approaching 60% and 70%, respectively () (Citation86–91). Despite the lack of randomized data, autologous HCT consolidation for MCL in first remission could be considered standard practice. A CIBMTR (Center for International Blood and Marrow Transplant Research) analysis reported a 5-year OS rate of 61% for early upfront HCT versus 44% after HCT performed later in the disease course for chemotherapy-sensitive relapse (Citation92). In relapsed MCL patients with chemotherapy-sensitive disease who are not candidates for allogeneic HCT, consolidation with autologous HCT is reasonable; however, it is not recommended for therapy-refractory MCL.
Autologous HCT for follicular lymphoma
HCT for FL—first remission
The role of autologous HCT as consolidation after initial therapy for advanced stage FL patients in first remission was examined in three trials conducted in the pre-rituximab era (Citation93–95) and one in the rituximab era () (Citation96). Autologous HCT provided a PFS benefit in three out of these four trials. OS, however, was not improved as this therapy was associated with a higher risk of second malignancies (Citation95) including therapy-related myelodysplastic syndrome/acute myeloid leukemia (tMDS/AML) (Citation93,Citation96). The use of autologous HCT consolidation for FL in first remission is not recommended.
Table VI. Post-autologous HCT vaccination schedule.
HCT for FL—relapsed disease
Use of autologous HCT in relapsed FL, too, is controversial primarily because this modality is generally not curative. In the pre-rituximab era, the CUP study compared salvage chemotherapy alone versus chemotherapy followed by either unpurged or purged HCT for relapsed FL; overall, the investigators reported a PFS and OS benefit with autologous HCT () (Citation27). In the modern era, the superiority of autologous HCT over modern salvage chemoimmunotherapies continues to be debated (Citation97). Registry data from the European Blood and Marrow Transplant Group (EBMT) (Citation98) and CIBMTR show no plateau in relapse rates of FL post autologous HCT (Citation99) and a 5%–15% risk of second malignancies () (Citation98,Citation100). Autologous HCT for relapsed FL should be considered in the context of alternative treatment choices, patient age, remission status, comorbidities, and a small but definite risk of secondary cancers. Autologous HCT is best reserved for chemotherapy-sensitive, relapsed FL in those subjects who are not candidates for curative intent allogeneic transplantation.
Autologous HCT for transformed follicular lymphoma
An EBMT report of 50 patients receiving autologous HCT for chemotherapy-sensitive FL transforming to DLBCL described 5-year PFS and OS of 30% and 51% (Citation101). A prospective Norwegian study also reported 5-year PFS and OS of 32% and 47% rates, respectively (Citation102). In the rituximab-era, a cohort analysis from the University of British Columbia suggested a survival benefit for patients undergoing autologous HCT, compared with those receiving salvage chemoimmunotherapy alone (Citation103,Citation104). Autologous HCT is reasonable for transformed FL patients with non-bulky (no nodal areas ≥ 3 cm in size), chemotherapy-sensitive disease.
Autologous HCT for T-cell lymphomas
The outcomes of relapsed T-cell NHL are dismal with conventional chemotherapy alone (Citation105), and autologous HCT has been explored as initial therapy or at the time of tumor progression. Phase II studies (with heterogeneous histological subtypes) examining upfront HCT in T-cell NHL () (Citation106–110) suggest a 3–4 year PFS ranging from 30% to 50%. Although randomized data are not available in T-cell NHL, considering the suboptimal outcomes with conventional front-line therapies it is appropriate to consider autologous HCT in most chemotherapy-sensitive patients in first remission. Since ALK-positive anaplastic large cell lymphoma has an excellent prognosis with standard chemotherapies, autologous HCT in first CR is not recommended for this specific subgroup (Citation111). Relatively encouraging outcomes for a highly select group of relapsed T-cell NHL patients with sensitive disease also have been shown with 3–5 years PFS and OS rates of approximately 15%–30% and 30%–45%, respectively (Citation112–114). Autologous HCT is also reasonable in relapsed/chemotherapy-sensitive T-cell NHL patients not deemed candidates for allogeneic HCT.
Autologous HCT for Hodgkin lymphoma
Approximately 15%–20% of Hodgkin lymphoma (HL) patients relapse and are candidates for autologous HCT. The British National Lymphoma Investigation study randomized relapsed HL subjects to chemotherapy salvage alone or to consolidation with autologous HCT (Citation115) and reported a PFS benefit in favor of the HDT arm (53% versus 10%; P = 0.02). Autologous HCT is recommended in chemotherapy-sensitive first relapse of HL because of the remarkable improvement in PFS, low TRM, and the benefit of avoiding future relapses. HL patients not achieving a CR after first-line chemotherapies (primary refractory disease) have a poor prognosis. Lazarus et al. (Citation116) showed that autologous HCT could cure about one-third of primary refractory HL patients (). Chemotherapy-unresponsive disease at autologous HCT is a poor prognostic factor, with 5-year PFS rates generally < 20% (Citation117). Autologous HCT is standard-of-care for relapsed HL, including those with primary refractory disease.
Autologous HCT for primary central nervous system (CNS) lymphoma
Upfront application of autologous HCT in primary CNS lymphomas has been investigated in a number of small phase II studies (Citation118,Citation119). While this approach is not considered standard-of-care, it is often considered in patients not achieving a CR after first-line therapies. Unlike the upfront setting, in patients with relapsed or refractory disease long-term disease control with chemotherapy or radiotherapy alone is rare. In such patients HDT is associated with CR rates of ∼50%–60%, 2–3 year OS of 40%–45%, and acceptable rates of TRM ∼ 10% (Citation120,Citation121).
Autologous transplantation in the elderly patients
Historically autologous HCT was offered only to younger patients (≤ 60–65 years). However, the advent of non-TBI-based conditioning, use of peripheral blood grafts, growth factor support, antibiotic prophylaxis, systematic revaccination, and overall improved supportive care have dramatically improved TRM of autologous HCT. As such, these improvements have translated into a consistently greater utilization of HDT in patients above 60–65 years of age. Contemporary registry data from the CIBMTR and EBMT seem to validate this practice, with seemingly no increased TRM or compromised survival outcomes in patients > 65 years of age undergoing auto-HCT (Citation122–124). Autologous HCT is feasible and safe in carefully selected elderly patients (70–75 y) with excellent organ function and performance status, and few or no comorbidities.
Role of maintenance therapies following autologous transplantation
Disease relapse following autologous HCT is unfortunately common, especially in patients with myeloma and low-grade NHL. Various consolidation and maintenance strategies have been evaluated to delay post-HCT progression/relapse in myeloma. In general, novel anti-myeloma agents have shown superior results as maintenance therapy. Lenalidomide maintenance was studied in the Cancer and Leukemia Group B (CALGB) 100104 and IFM 05–02 trials (Citation125,Citation126). Both studies demonstrated a superior PFS and EFS with lenalidomide maintenance, while CALGB 100104 also demonstrated an OS benefit in the lenalidomide arm. There was, however, a 2- to 3-fold increase in second primary malignancies in both studies associated with the lenalidomide maintenance. Pre-transplant bortezomib induction coupled with post-autologous HCT bortezomib maintenance in the HOVON-65/GMMG-HD4 trial was shown to have superior PFS and OS (Citation62). Based on these data, the risks/benefits and health care costs of novel agent maintenance should be discussed with myeloma patients post autologous HCT.
Due to the known benefits of rituximab maintenance in indolent B-cell NHL (Citation127), this strategy has been studied in the post-HCT setting (Citation28,Citation128). A randomized EBMT study assigned relapsed FL patients to either rituximab maintenance or observation alone following autologous HCT (Citation129). Maintenance therapy was well tolerated and was associated with superior 10-year PFS (54% versus 37%; P = 0.01), but no OS benefit was noted. In patients with DLBCL, the CORAL study examined post-autologous HCT rituximab maintenance and reported no PFS or OS. An increased risk of adverse events with maintenance was seen in this study (Citation130). Routine use of rituximab maintenance after HCT in B-cell NHL is not recommended.
Follow-up after autologous HCT
Patients undergoing HDT in the outpatient setting generally require daily outpatient visits for hematopoietic growth factor support, blood count monitoring, and electrolyte replacements. Following neutrophil and platelet engraftment, most transplant centers reassess response to HDT approximately 2–3 month post transplantation, with history, physical examination, laboratory evaluations, radiographic/nuclear medicine imaging, and bone marrow biopsies as appropriate. Patients with no evidence of disease and resolved acute toxicities from this point onward are generally followed long-term. Once a year follow-up is recommended in long-term survivors. In (secretory) myeloma patients, monoclonal protein assessment in serum and urine, every 3–6 months, is common practice. Many programs perform surveillance bone marrow biopsies (in myeloma and acute leukemia patients) and PET and/or CAT-scans (in lymphoma patients) to screen for relapsed disease. No data, however, exist to suggest any benefit of surveillance with marrow and radiograph evaluations in otherwise asymptomatic patients.
Post-autologous HCT immunizations
HCT results in profound immunosuppression, and a decline in protective antibody titers to vaccine-preventable diseases (e.g. measles, mumps, rubella) is observed following autologous HCT, if the recipient is not revaccinated (http://www.cdc.gov/vaccines/pubs/hemato-cell-transplts.htm). All HDT recipients should be revaccinated routinely after transplantation. Inactivated influenza vaccine should be administered beginning at least 6 months after autologous HCT and annually thereafter. Sequential administration of three doses of pneumococcal conjugate vaccine is recommended, beginning 3–6 months after the transplant, followed by a dose of pneumococcal polysaccharide vaccine. A three-dose regimen of Haemophilus influenzae Type b (Hib) vaccine is administered beginning 6 months after transplant; at least 1 month should separate the doses. Measles, mumps, and rubella (MMR) vaccine should be administered 24 months after transplant, if the HCT recipient is immunocompetent. summarizes a suggested post-autologous HCT vaccination schedule. Centers for Disease Control (CDC) recommend caution regarding live varicella vaccine (since it is a live attenuated vaccine) (Citation131) among HCT recipients. After considering the risk/benefits, if a decision is made to vaccinate with varicella vaccine, CDC recommends that the vaccine should be administered a minimum of 24 months after HCT and only if the recipient is presumed to be immunocompetent.
Late complications and survivorship issues after autologous HCT
Autologous HCT recipients require long-term follow-up and have a reduced life expectancy compared to the general population. These patients are at increased risk for late complications such as opportunistic infections, iron overload, endocrine abnormalities, osteoporosis, infertility, psychiatric disturbances, difficulty in returning to the work-force, and second malignancies including tMDS/AML (Citation49,Citation132–135). Recipients should be monitored for these potential maladies at least yearly, including administering all age-appropriate cancer screening and anti-infective vaccinations. Formal guidelines derived from prospectively observed patient cohorts to define optimal screening procedures in autologous HCT survivors are not available. American Society of Blood and Marrow Transplantation consensus guidelines (based primarily on expert opinion) recommended long-term autograft survivors to undergo yearly ocular examinations for cataracts (especially after TBI) and refractive errors, yearly assessment of cardiovascular risk factors, and education/counseling on ‘heart’-healthy lifestyle, blood pressure monitoring, and aggressive management of hypertension to prevent chronic kidney disease, screening for osteopenia/osteoporosis, yearly thyroid function evaluation (especially after TBI or RIT exposure), sexual, gonadal function, and fertility assessment in appropriate age groups, and periodic psychological evaluations (Citation135).
Conclusions and future questions
Tens of thousands of patients have derived significant benefit and many have been cured in the decades after the introduction of autologous HCT as a life-saving treatment of malignant disorders. Through painstaking clinical trials, the techniques of hematopoietic cell collection and graft manipulation, cryopreservation, and reinfusion have improved. Further, conditioning regimens and intensive supportive care have evolved considerably, dramatically advancing patient safety. Practitioners now are generally aware of the appropriate indications for referral and use of this modality as a routine tool. Sophisticated strategies include incorporating novel targeted agents into the mobilization and conditioning regimens, initiation of targeted therapy in the post-transplant period to enhance immunity and reduce relapse, and prevent late consequences.
Current directions being explored in HCT-based treatments for myeloma include newer conditioning regimens and planned post-transplant maintenance or consolidation strategies that aim to induce deeper response states. Approaches at delivering higher doses of radiation therapy to the marrow prior to autologous HCT without increasing overall toxicity are also being explored with helical tomotherapy or bone-seeking radioisotopes (Citation136,Citation137). In lymphoid malignancies results of RIT-based conditioning have been disappointing, but the future ought to look at applying novel monoclonal antibodies, targeted therapies as post-transplant maintenance and/or consolidation. Selective application of such novel maintenance and consolidation or immunotherapy strategies post HCT may help eradicate minimal residual disease (MRD) leading to durable disease control. summarizes agents with potential to advance autologous HCT outcomes in the future.
Table VII. Novel investigational agents and strategies in development.
Declaration of interest: The authors report no conflicts of interest.
References
- Santos GW. History of bone marrow transplantation. Clin Haematol. 1983;12:611–39.
- Hamadani M, Craig M, Awan FT, Devine SM. How we approach patient evaluation for hematopoietic stem cell transplantation. Bone Marrow Transplant. 2010;45:1259–68.
- Ambinder RF. The same but different: autologous hematopoietic stem cell transplantation for patients with lymphoma and HIV infection. Bone Marrow Transplant. 2009;44:1–5.
- Barker GJ. Current practices in the oral management of the patient undergoing chemotherapy or bone marrow transplantation. Support Care Cancer. 1999;7:17–20.
- Tomblyn M, Chiller T, Einsele H, Gress R, Sepkowitz K, Storek J, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15:1143–238.
- Fujimaki K, Maruta A, Yoshida M, Sakai R, Tanabe J, Koharazawa H, et al. Severe cardiac toxicity in hematological stem cell transplantation: predictive value of reduced left ventricular ejection fraction. Bone Marrow Transplant. 2001;27:307–10.
- Tang WH, Thomas S, Kalaycio M, Sobecks R, Andresen S, Jarvis J, et al. Clinical outcomes of patients with impaired left ventricular ejection fraction undergoing autologous bone marrow transplantation: can we safely transplant patients with impaired ejection fraction? Bone Marrow Transplant. 2004;34:603–7.
- Zangari M, Henzlova MJ, Ahmad S, Scigliano E, Isola L, Platnik J, et al. Predictive value of left ventricular ejection fraction in stem cell transplantation. Bone Marrow Transplant. 1999;23:917–20.
- Chien JW, Madtes DK, Clark JG. Pulmonary function testing prior to hematopoietic stem cell transplantation. Bone Marrow Transplant. 2005;35:429–35.
- McDonald GB, Hinds MS, Fisher LD, Schoch HG, Wolford JL, Banaji M, et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med. 1993;118:255–67.
- Savani BN, Montero A, Wu C, Nlonda N, Read E, Dunbar C, et al. Prediction and prevention of transplant-related mortality from pulmonary causes after total body irradiation and allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11: 223–30.
- Stagno S, Busby K, Shapiro A, Kotz M. Patients at risk: addressing addiction in patients undergoing hematopoietic SCT. Bone Marrow Transplant. 2008;42:221–6.
- Cashen AF, Lazarus HM, Devine SM. Mobilizing stem cells from normal donors: is it possible to improve upon G-CSF? Bone Marrow Transplant. 2007;39:577–88.
- Devine SM. Toward a more rational policy for autologous hematopoietic stem cell mobilization. Biol Blood Marrow Transplant. 2012; 18:1468–70.
- DiPersio JF, Micallef IN, Stiff PJ, Bolwell BJ, Maziarz RT, Jacobsen E, et al. Phase III prospective randomized double-blind placebo- controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin’s lymphoma. J Clin Oncol. 2009;27: 4767–73.
- Bensinger W, DiPersio JF, McCarty JM. Improving stem cell mobilization strategies: future directions. Bone Marrow Transplant. 2009;43: 181–95.
- Hamadani M. Reappraising the role of autologous transplantation for indolent B-cell lymphomas in the chemoimmunotherapy era: is it still relevant? Bone Marrow Transplant. 2013;48:1013–21.
- Hill BT, Rybicki L, Smith S, Dean R, Kalaycio M, Pohlman B, et al. Treatment with hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone combined with cytarabine and methotrexate results in poor mobilization of peripheral blood stem cells in patients with mantle cell lymphoma. Leuk Lymphoma. 2011;52:986–93.
- Nichols GL, Skerrett DL. Peripheral blood stem cell mobilization and harvesting after fludarabine therapy for low-grade lymphoma and chronic lymphocytic leukemia. Stem Cells Dev. 2005;14:3–5.
- Dimopoulos MA, Gertz MA, Kastritis E, Garcia-Sanz R, Kimby EK, Leblond V, et al. Update on treatment recommendations from the Fourth International Workshop on Waldenstrom’s Macroglobulinemia. J Clin Oncol. 2009;27:120–6.
- Boccadoro M, Palumbo A, Bringhen S, Merletti F, Ciccone G, Richiardi L, et al. Oral melphalan at diagnosis hampers adequate collection of peripheral blood progenitor cells in multiple myeloma. Haematologica. 2002;87:846–50.
- Kumar S, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Gastineau DA, et al. Impact of lenalidomide therapy on stem cell mobilization and engraftment post-peripheral blood stem cell transplantation in patients with newly diagnosed myeloma. Leukemia. 2007;21:2035–42.
- Freedman AS, Neuberg D, Mauch P, Soiffer RJ, Anderson KC, Fisher DC, et al. Long-term follow-up of autologous bone marrow transplantation in patients with relapsed follicular lymphoma. Blood. 1999;94:3325–33.
- Tarella C, Corradini P, Astolfi M, Bondesan P, Caracciolo D, Cherasco C, et al. Negative immunomagnetic ex vivo purging combined with high-dose chemotherapy with peripheral blood progenitor cell autograft in follicular lymphoma patients: evidence for long-term clinical and molecular remissions. Leukemia. 1999;13:1456–62.
- Tarella C, Zanni M, Magni M, Benedetti F, Patti C, Barbui T, et al. Rituximab improves the efficacy of high-dose chemotherapy with autograft for high-risk follicular and diffuse large B-cell lymphoma: a multicenter Gruppo Italiano Terapie Innnovative nei linfomi survey. J Clin Oncol. 2008;26:3166–75.
- Arcaini L, Montanari F, Alessandrino EP, Tucci A, Brusamolino E, Gargantini L, et al. Immunochemotherapy with in vivo purging and autotransplant induces long clinical and molecular remission in advanced relapsed and refractory follicular lymphoma. Ann Oncol. 2008;19:1331–5.
- Schouten HC, Qian W, Kvaloy S, Porcellini A, Hagberg H, Johnson HE, et al. High-dose therapy improves progression-free survival and survival in relapsed follicular non-Hodgkin’s lymphoma: results from the randomized European CUP trial. J Clin Oncol. 2003;21:3918–27.
- Hicks LK, Woods A, Buckstein R, Mangel J, Pennell N, Zhang L, et al. Rituximab purging and maintenance combined with auto-SCT: long-term molecular remissions and prolonged hypogammaglobulinemia in relapsed follicular lymphoma. Bone Marrow Transplant. 2009; 43:701–8.
- Crippa F, Holmberg L, Carter RA, Hooper H, Marr KA, Bensinger W, et al. Infectious complications after autologous CD34-selected peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2002;8:281–9.
- Moreau P, Facon T, Attal M, Hulin C, Michallet M, Maloisel F, et al. Comparison of 200 mg/m(2) melphalan and 8 Gy total body irradiation plus 140 mg/m(2) melphalan as conditioning regimens for peripheral blood stem cell transplantation in patients with newly diagnosed multiple myeloma: final analysis of the Intergroupe Francophone du Myelome 9502 randomized trial. Blood. 2002;99:731–5.
- Qazilbash M, Thall P, Fox P, Kebriaei P, Bashir Q, Shah N, et al. Randomized phase III trial of busulfan plus melphalan versus melphalan alone for multiple myeloma. J Clin Oncol. 2014;32(5s): abstr 8538.
- Knudsen LM, Hippe E, Hjorth M, Holmberg E, Westin J. Renal function in newly diagnosed multiple myeloma—a demographic study of 1353 patients. The Nordic Myeloma Study Group. Eur J Haematol. 1994;53:207–12.
- Torra R, Blade J, Cases A, Lopez-Pedret J, Montserrat E, Rozman C, et al. Patients with multiple myeloma requiring long-term dialysis: presenting features, response to therapy, and outcome in a series of 20 cases. Br J Haematol. 1995;91:854–9.
- Glavey SV, Gertz MA, Dispenzieri A, Kumar S, Buadi F, Lacy M, et al. Long-term outcome of patients with mutiple myeloma-related advanced renal failure following auto-SCT. Bone Marrow Transplant. 2013;48:1543–7.
- Badros A, Barlogie B, Siegel E, Roberts J, Langmaid C, Zangari M, et al. Results of autologous stem cell transplant in multiple myeloma patients with renal failure. Br J Haematol. 2001;114:822–9.
- Kharfan-Dabaja MA, Nishihori T, Otrock ZK, Haidar N, Mohty M, Hamadani M. Monoclonal antibodies in conditioning regimens for hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2013;19:1288–300.
- Salar A, Sierra J, Gandarillas M, Caballero MD, Marin J, Lahuerta JJ, et al. Autologous stem cell transplantation for clinically aggressive non-Hodgkin’s lymphoma: the role of preparative regimens. Bone Marrow Transplant. 2001;27:405–12.
- Press OW, Eary JF, Appelbaum FR, Martin PJ, Nelp WB, Glenn S, et al. Phase II trial of 131I-B1 (anti-CD20) antibody therapy with autologous stem cell transplantation for relapsed B cell lymphomas. Lancet. 1995;346:336–40.
- Kaminski MS, Zasadny KR, Francis IR, Fenner MC, Ross CW, Milik AW, et al. Iodine-131-anti-B1 radioimmunotherapy for B-cell lymphoma. J Clin Oncol. 1996;14:1974–81.
- Liu SY, Eary JF, Petersdorf SH, Martin PJ, Maloney DG, Appelbaum FR, et al. Follow-up of relapsed B-cell lymphoma patients treated with iodine-131-labeled anti-CD20 antibody and autologous stem-cell rescue. J Clin Oncol. 1998;16:3270–8.
- Vose JM, Bierman PJ, Enke C, Hankins J, Bociek G, Lynch JC, et al. Phase I trial of iodine-131 tositumomab with high-dose chemotherapy and autologous stem-cell transplantation for relapsed non-Hodgkin’s lymphoma. J Clin Oncol. 2005;23:461–7.
- Shimoni A, Zwas ST, Oksman Y, Hardan I, Shem-Tov N, Yerushalmi R, et al. Yttrium-90-ibritumomab tiuxetan (Zevalin) combined with high-dose BEAM chemotherapy and autologous stem cell transplantation for chemo-refractory aggressive non-Hodgkin’s lymphoma. Exp Hematol. 2007;35:534–40.
- Krishnan A, Nademanee A, Fung HC, Raubitschek AA, Molina A, Yamauchi D, et al. Phase II trial of a transplantation regimen of yttrium-90 ibritumomab tiuxetan and high-dose chemotherapy in patients with non-Hodgkin’s lymphoma. J Clin Oncol. 2008; 26:90–5.
- Vose JM, Carter S, Burns LJ, Ayala E, Press OW, Moskowitz CH, et al. Phase III randomized study of rituximab/carmustine, etoposide, cytarabine, and melphalan (BEAM) compared with iodine-131 tositumomab/BEAM with autologous hematopoietic cell transplantation for relapsed diffuse large B-cell lymphoma: results from the BMT CTN 0401 trial. J Clin Oncol. 2013 31:1662–8.
- Available at: http://www.idsociety.org/uploadedFiles/IDSA/Guidelines-Patient_Care/PDF_Library/OI.pdf (accessed 11 June 2014).
- Hiemenz JW. Management of infections complicating allogeneic hematopoietic stem cell transplantation. Semin Hematol. 2009;46: 289–312.
- Bobak D, Arfons LM, Creger RJ, Lazarus HM. Clostridium difficile-associated disease in human stem cell transplant recipients: coming epidemic or false alarm? Bone Marrow Transplant. 2008;42:705–13.
- Ho VT, Revta C, Richardson PG. Hepatic veno-occlusive disease after hematopoietic stem cell transplantation: update on defibrotide and other current investigational therapies. Bone Marrow Transplant. 2008;41:229–37.
- Tichelli A, Rovo A, Gratwohl A. Late pulmonary, cardiovascular, and renal complications after hematopoietic stem cell transplantation and recommended screening practices. Hematology Am Soc Hematol Educ Program. 2008:125–33.
- Ljungman P, Bregni M, Brune M, Cornelissen J, de Witte T, Dini G, et al. Allogeneic and autologous transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe 2009. Bone Marrow Transplant. 2010;45:219–34.
- Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996;335:91–7.
- Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–83.
- Blade J, Rosinol L, Sureda A, Ribera JM, Diaz-Mediavilla J, Garcia-Larana J, et al. High-dose therapy intensification compared with continued standard chemotherapy in multiple myeloma patients responding to the initial chemotherapy: long-term results from a prospective randomized trial from the Spanish cooperative group PETHEMA. Blood. 2005;106:3755–9.
- Fermand JP, Ravaud P, Chevret S, Divine M, Leblond V, Belanger C, et al. High-dose therapy and autologous peripheral blood stem cell transplantation in multiple myeloma: up-front or rescue treatment? Results of a multicenter sequential randomized clinical trial. Blood. 1998;92:3131–6.
- Fermand JP, Katsahian S, Divine M, Leblond V, Dreyfus F, Macro M, et al. High-dose therapy and autologous blood stem-cell transplantation compared with conventional treatment in myeloma patients aged 55 to 65 years: long-term results of a randomized control trial from the Group Myelome-Autogreffe. J Clin Oncol. 2005;23:9227–33.
- Barlogie B, Kyle RA, Anderson KC, Greipp PR, Lazarus HM, Hurd DD, et al. Standard chemotherapy compared with high-dose chemoradiotherapy for multiple myeloma: final results of phase III US Intergroup Trial S9321. J Clin Oncol. 2006;24:929–36.
- Segeren CM, Sonneveld P, van der Holt B, Vellenga E, Croockewit AJ, Verhoef GE, et al. Overall and event-free survival are not improved by the use of myeloablative therapy following intensified chemotherapy in previously untreated patients with multiple myeloma: a prospective randomized phase 3 study. Blood. 2003;101:2144–51.
- Barlogie B, Jagannath S, Vesole DH, Naucke S, Cheson B, Mattox S, et al. Superiority of tandem autologous transplantation over standard therapy for previously untreated multiple myeloma. Blood. 1997;89: 789–93.
- Barlogie B, Jagannath S, Desikan KR, Mattox S, Vesole D, Siegel D, et al. Total therapy with tandem transplants for newly diagnosed multiple myeloma. Blood. 1999;93:55–65.
- Attal M, Harousseau JL, Facon T, Guilhot F, Doyen C, Fuzibet JG, et al. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med. 2003;349:2495–502.
- Cavo M, Tosi P, Zamagni E, Cellini C, Tacchetti P, Patriarca F, et al. Prospective, randomized study of single compared with double autologous stem-cell transplantation for multiple myeloma: Bologna 96 clinical study. J Clin Oncol. 2007;25:2434–41.
- Sonneveld P, Schmidt-Wolf IG, van der Holt B, El Jarari L, Bertsch U, Salwender H, et al. Bortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: results of the randomized phase III HOVON-65/ GMMG-HD4 trial. J Clin Oncol. 2012;30:2946–55.
- Boccadoro M, Cavallo F, Maria Gay F, di Raimondo F. Melphalan/prednisone/lenalidomide (MPR) versus high-dose melphalan and autologous transplantation (MEL200) plus lenalidomide maintenance or no maintenance in newly diagnosed multiple myeloma (MM) patients. J Clin Oncol. 2013;31(suppl): abstr 8509.
- Egan JB, Shi CX, Tembe W, Christoforides A, Kurdoglu A, Sinari S, et al. Whole-genome sequencing of multiple myeloma from diagnosis to plasma cell leukemia reveals genomic initiating events, evolution, and clonal tides. Blood. 2012;120:1060–6.
- Keats JJ, Chesi M, Egan JB, Garbitt VM, Palmer SE, Braggio E, et al. Clonal competition with alternating dominance in multiple myeloma. Blood. 2012;120:1067–76.
- Avet-Loiseau H, Attal M, Moreau P, Charbonnel C, Garban F, Hulin C, et al. Genetic abnormalities and survival in multiple myeloma: the experience of the Intergroupe Francophone du Myelome. Blood. 2007;109:3489–95.
- Gertz M, Lacy M, Dispenzieri A, Greipp P. Clinical implications of t(11;14)(q13;q32), t(4;14)(p16.3;q32) and -17p13 (p53) deletions in myeloma patients treated with high dose therapy. Blood. 2005;106: 2837–40.
- Neben K, Lokhorst HM, Jauch A, Bertsch U, Hielscher T, van der Holt B, et al. Administration of bortezomib before and after autologous stem cell transplantation improves outcome in multiple myeloma patients with deletion 17p. Blood. 2012;119:940–8.
- Pineda-Roman M, Zangari M, Haessler J, Anaissie E, Tricot G, van Rhee F, et al. Sustained complete remissions in multiple myeloma linked to bortezomib in total therapy 3: comparison with total therapy 2. Br J Haematol. 2008;140:625–34.
- D’Souza A, Lacy M, Gertz M, Kumar S, Buadi F, Hayman S, et al. Long-term outcomes after autologous stem cell transplantation for patients with POEMS syndrome (osteosclerotic myeloma): a single-center experience. Blood. 2012;120:56–62.
- Cibeira MT, Sanchorawala V, Seldin DC, Quillen K, Berk JL, Dember LM, et al. Outcome of AL amyloidosis after high-dose melphalan and autologous stem cell transplantation: long-term results in a series of 421 patients. Blood. 2011;118:4346–52.
- Comenzo RL, Gertz MA. Autologous stem cell transplantation for primary systemic amyloidosis. Blood. 2002;99:4276–82.
- Dispenzieri A, Lacy MQ, Hayman SR, Kumar SK, Buadi F, Dingli D, et al. Peripheral blood stem cell transplant for POEMS syndrome is associated with high rates of engraftment syndrome. Eur J Haematol. 2008;80:397–406.
- Jaccard A, Moreau P, Leblond V, Leleu X, Benboubker L, Hermine O, et al. High-dose melphalan versus melphalan plus dexamethasone for AL amyloidosis. N Engl J Med. 2007;357:1083–93.
- Schmitz N, Nickelsen M, Ziepert M, Haenel M, Borchmann P, Schmidt C, et al. Conventional chemotherapy (CHOEP-14) with rituximab or high-dose chemotherapy (MegaCHOEP) with rituximab for young, high-risk patients with aggressive B-cell lymphoma: an open-label, randomised, phase 3 trial (DSHNHL 2002-1). Lancet Oncol. 2012;13:1250–9.
- Le Gouill S, Milpied NJ, Lamy T, Delwail V, Gressin R, Guyotat D, et al. First-line rituximab (R) high-dose therapy (R-HDT) versus R-CHOP14 for young adults with diffuse large B-cell lymphoma: preliminary results of the GOELAMS 075 prospective multicenter randomized trial. ASCO Meeting Abstracts. 2011;29(15_suppl):8003.
- Stiff PJ, Unger JM, Cook JR, Constine LS, Couban S, Stewart DA, et al. Autologous transplantation as consolidation for aggressive non- Hodgkin’s lymphoma. N Engl J Med. 2013;369:1681–90.
- Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–42.
- Philip T, Guglielmi C, Hagenbeek A, Somers R, Van der Lelie H, Bron D, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med. 1995;333:1540–5.
- Vose JM, Zhang MJ, Rowlings PA, Lazarus HM, Bolwell BJ, Freytes CO, et al. Autologous transplantation for diffuse aggressive non-Hodgkin’s lymphoma in patients never achieving remission: a report from the Autologous Blood and Marrow Transplant Registry. J Clin Oncol. 2001;19:406–13.
- Mounier N, Canals C, Gisselbrecht C, Cornelissen J, Foa R, Conde E, et al. High-dose therapy and autologous stem cell transplantation in first relapse for diffuse large B cell lymphoma in the rituximab era: an analysis based on data from the European Blood and Marrow Transplantation Registry. Biol Blood Marrow Transplant. 2012;18:788–93.
- Lazarus HM, Zhang MJ, Carreras J, Hayes-Lattin BM, Ataergin AS, Bitran JD, et al. A comparison of HLA-identical sibling allogeneic versus autologous transplantation for diffuse large B cell lymphoma: a report from the CIBMTR. Biol Blood Marrow Transplant. 2010;16: 35–45.
- Fenske TS, Hari PN, Carreras J, Zhang MJ, Kamble RT, Bolwell BJ, et al. Impact of pre-transplant rituximab on survival after autologous hematopoietic stem cell transplantation for diffuse large B cell lymphoma. Biol Blood Marrow Transplant. 2009;15:1455–64.
- Gisselbrecht C, Glass B, Mounier N, Singh Gill D, Linch DC, Trneny M, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28:4184–90.
- Dreyling M, Lenz G, Hoster E, Van Hoof A, Gisselbrecht C, Schmits R, et al. Early consolidation by myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission significantly prolongs progression-free survival in mantle-cell lymphoma: results of a prospective randomized trial of the European MCL Network. Blood. 2005;105:2677–84.
- Damon LE, Johnson JL, Niedzwiecki D, Cheson BD, Hurd DD, Bartlett NL, et al. Immunochemotherapy and autologous stem-cell transplantation for untreated patients with mantle-cell lymphoma: CALGB 59909. J Clin Oncol. 2009;27:6101–8.
- Geisler CH, Kolstad A, Laurell A, Andersen NS, Pedersen LB, Jerkeman M, et al. Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo-purged stem cell rescue: a nonrandomized phase 2 multicenter study by the Nordic Lymphoma Group. Blood. 2008;112:2687–93.
- Geisler CH, Kolstad A, Laurell A, Jerkeman M, Raty R, Andersen NS, et al. Nordic MCL2 trial update: six-year follow-up after intensive immunochemotherapy for untreated mantle cell lymphoma followed by BEAM or BEAC + autologous stem-cell support: still very long survival but late relapses do occur. Br J Haematol. 2012;158:355–62.
- Tam CS, Bassett R, Ledesma C, Korbling M, Alousi A, Hosing C, et al. Mature results of the M. D. Anderson Cancer Center risk-adapted transplantation strategy in mantle cell lymphoma. Blood. 2009;113: 4144–52.
- Hermine O, Hoster E, Walewski J, Ribrag V, Brousse N, Thieblemont C, et al. Alternating courses of 3x CHOP and 3x DHAP plus rituximab followed by a high dose ARA-C containing myeloablative regimen and autologous stem cell transplantation (ASCT) increases overall survival when compared to 6 courses of CHOP plus rituximab followed by myeloablative radiochemotherapy and ASCT in mantle cell lymphoma: final analysis of the MCL younger trial of the European Mantle Cell Lymphoma Network (MCL net). Blood ASH Annual Meeting Abstracts. 2012;120:151.
- Arne K, Laurell A, Jerkeman M, Gronbaek K, Elonen E, Raty R, et al. Nordic MCL3 study: zevalin combined with high-dose chemotherapy followed by autologous stem cell support as late intensification for mantle cell lymphoma (MCL) patients < 66 years not in CR after induction chemoimmunotherapy: no benefit of zevalin. Blood ASH Annual Meeting Abstracts. 2012;120:747.
- Fenske TS, Carreras J, Zhang M, Laport G, Montoto S, Maloney D, et al. Outcome of patients with mantle cell lymphoma undergoing autologous versus reduced-intensity allogeneic transplantation. Ann Oncol. 2011;22(Suppl 4):iv87.
- Lenz G, Dreyling M, Schiegnitz E, Forstpointner R, Wandt H, Freund M, et al. Myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission prolongs progression-free survival in follicular lymphoma: results of a prospective, randomized trial of the German Low-Grade Lymphoma Study Group. Blood. 2004; 104:2667–74.
- Sebban C, Mounier N, Brousse N, Belanger C, Brice P, Haioun C, et al. Standard chemotherapy with interferon compared with CHOP followed by high-dose therapy with autologous stem cell transplantation in untreated patients with advanced follicular lymphoma: the GELF-94 randomized study from the Groupe d’Etude des Lymphomes de l’Adulte (GELA). Blood. 2006;108:2540–4.
- Gyan E, Foussard C, Bertrand P, Michenet P, Le Gouill S, Berthou C, et al. High-dose therapy followed by autologous purged stem cell transplantation and doxorubicin-based chemotherapy in patients with advanced follicular lymphoma: a randomized multicenter study by the GOELAMS with final results after a median follow-up of 9 years. Blood. 2009;113:995–1001.
- Ladetto M, De Marco F, Benedetti F, Vitolo U, Patti C, Rambaldi A, et al. Prospective, multicenter randomized GITMO/IIL trial comparing intensive (R-HDS) versus conventional (CHOP-R) chemoimmunotherapy in high-risk follicular lymphoma at diagnosis: the superior disease control of R-HDS does not translate into an overall survival advantage. Blood. 2008;111:4004–13.
- Sebban C, Brice P, Delarue R, Haioun C, Souleau B, Mounier N, et al. Impact of rituximab and/or high-dose therapy with autotransplant at time of relapse in patients with follicular lymphoma: a GELA study. J Clin Oncol. 2008;26:3614–20.
- Montoto S, Canals C, Rohatiner AZ, Taghipour G, Sureda A, Schmitz N, et al. Long-term follow-up of high-dose treatment with autologous haematopoietic progenitor cell support in 693 patients with follicular lymphoma: an EBMT registry study. Leukemia. 2007; 21:2324–31.
- van Besien K, Loberiza FR Jr, Bajorunaite R, Armitage JO, Bashey A, Burns LJ, et al. Comparison of autologous and allogeneic hematopoietic stem cell transplantation for follicular lymphoma. Blood. 2003;102: 3521–9.
- Rohatiner AZ, Nadler L, Davies AJ, Apostolidis J, Neuberg D, Matthews J, et al. Myeloablative therapy with autologous bone marrow transplantation for follicular lymphoma at the time of second or subsequent remission: long-term follow-up. J Clin Oncol. 2007;25: 2554–9.
- Williams CD, Harrison CN, Lister TA, Norton AJ, Blystad AK, Coiffier B, et al. High-dose therapy and autologous stem-cell support for chemosensitive transformed low-grade follicular non-Hodgkin’s lymphoma: a case-matched study from the European Bone Marrow Transplant Registry. J Clin Oncol. 2001;19:727–35.
- Eide MB, Lauritzsen GF, Kvalheim G, Kolstad A, Fagerli UM, Maisenholder M, et al. High dose chemotherapy with autologous stem cell support for patients with histologically transformed B-cell non-Hodgkin lymphomas. A Norwegian multi centre phase II study. Br J Haematol. 2011;152:600–10.
- Al-Tourah AJ, Gill KK, Chhanabhai M, Hoskins PJ, Klasa RJ, Savage KJ, et al. Population-based analysis of incidence and outcome of transformed non-Hodgkin’s lymphoma. J Clin Oncol. 2008;26: 5165–9.
- Villa D, Crump M, Panzarella T, Savage KJ, Toze CL, Stewart DA, et al. Autologous and allogeneic stem-cell transplantation for transformed follicular lymphoma: a report of the Canadian blood and marrow transplant group. J Clin Oncol. 2013;31:1164–71.
- Mak V, Hamm J, Chhanabhai M, Shenkier T, Klasa R, Sehn LH, et al. Survival of patients with peripheral t-cell lymphoma after first relapse or progression: spectrum of disease and rare long-term survivors. J Clin Oncol. 2013;31:1970–6.
- Corradini P, Tarella C, Zallio F, Dodero A, Zanni M, Valagussa P, et al. Long-term follow-up of patients with peripheral T-cell lymphomas treated up-front with high-dose chemotherapy followed by autologous stem cell transplantation. Leukemia. 2006;20:1533–8.
- Rodriguez J, Conde E, Gutierrez A, Lahuerta JJ, Arranz R, Sureda A, et al. The adjusted International Prognostic Index and beta- 2-microglobulin predict the outcome after autologous stem cell transplantation in relapsing/refractory peripheral T-cell lymphoma. Haematologica. 2007;92:1067–74.
- Mercadal S, Briones J, Xicoy B, Pedro C, Escoda L, Estany C, et al. Intensive chemotherapy (high-dose CHOP/ESHAP regimen) followed by autologous stem-cell transplantation in previously untreated patients with peripheral T-cell lymphoma. Ann Oncol. 2008;19: 958–63.
- Reimer P, Rudiger T, Geissinger E, Weissinger F, Nerl C, Schmitz N, et al. Autologous stem-cell transplantation as first-line therapy in peripheral T-cell lymphomas: results of a prospective multicenter study. J Clin Oncol. 2009;27:106–13.
- d’Amore F, Relander T, Lauritzsen GF, Jantunen E, Hagberg H, Anderson H, et al. Up-front autologous stem-cell transplantation in peripheral T-cell lymphoma: NLG-T-01. J Clin Oncol. 2012;30: 3093–9.
- Savage KJ. Therapies for peripheral T-cell lymphomas. Hematology Am Soc Hematol Educ Program. 2011;2011:515–24.
- Chen AI, McMillan A, Negrin RS, Horning SJ, Laport GG. Long-term results of autologous hematopoietic cell transplantation for peripheral T cell lymphoma: the Stanford experience. Biol Blood Marrow Transplant. 2008;14:741–7.
- Nademanee A, Palmer JM, Popplewell L, Tsai NC, Delioukina M, Gaal K, et al. High-dose therapy and autologous hematopoietic cell transplantation in peripheral T cell lymphoma (PTCL): analysis of prognostic factors. Biol Blood Marrow Transplant. 2011;17:1481–9.
- Yang DH, Kim WS, Kim SJ, Bae SH, Kim SH, Kim IH, et al. Prognostic factors and clinical outcomes of high-dose chemotherapy followed by autologous stem cell transplantation in patients with peripheral T cell lymphoma, unspecified: complete remission at transplantation and the prognostic index of peripheral T cell lymphoma are the major factors predictive of outcome. Biol Blood Marrow Transplant. 2009;15: 118–25.
- Linch DC, Winfield D, Goldstone AH, Moir D, Hancock B, McMillan A, et al. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin’s disease: results of a BNLI randomised trial. Lancet. 1993;341:1051–4.
- Lazarus HM, Rowlings PA, Zhang MJ, Vose JM, Armitage JO, Bierman PJ, et al. Autotransplants for Hodgkin’s disease in patients never achieving remission: a report from the Autologous Blood and Marrow Transplant Registry. J Clin Oncol. 1999;17:534–45.
- Gopal AK, Metcalfe TL, Gooley TA, Pagel JM, Petersdorf SH, Bensinger WI, et al. High-dose therapy and autologous stem cell transplantation for chemoresistant Hodgkin lymphoma: the Seattle experience. Cancer. 2008;113:1344–50.
- Montemurro M, Kiefer T, Schuler F, Al-Ali HK, Wolf HH, Herbst R, et al. Primary central nervous system lymphoma treated with high-dose methotrexate, high-dose busulfan/thiotepa, autologous stem-cell transplantation and response-adapted whole-brain radiotherapy: results of the multicenter Ostdeutsche Studiengruppe Hamato- Onkologie OSHO-53 phase II study. Ann Oncol. 2007;18: 665–71.
- Illerhaus G, Marks R, Ihorst G, Guttenberger R, Ostertag C, Derigs G, et al. High-dose chemotherapy with autologous stem-cell transplantation and hyperfractionated radiotherapy as first-line treatment of primary CNS lymphoma. J Clin Oncol. 2006;24:3865–70.
- Soussain C, Hoang-Xuan K, Taillandier L, Fourme E, Choquet S, Witz F, et al. Intensive chemotherapy followed by hematopoietic stem-cell rescue for refractory and recurrent primary CNS and intraocular lymphoma: Societe Francaise de Greffe de Moelle Osseuse-Therapie Cellulaire. J Clin Oncol. 2008;26:2512–18.
- Soussain C, Suzan F, Hoang-Xuan K, Cassoux N, Levy V, Azar N, et al. Results of intensive chemotherapy followed by hematopoietic stem-cell rescue in 22 patients with refractory or recurrent primary CNS lymphoma or intraocular lymphoma. J Clin Oncol. 2001; 19:742–9.
- McCarthy PL Jr, Hahn T, Hassebroek A, Bredeson C, Gajewski J, Hale G, et al. Trends in use of and survival after autologous hematopoietic cell transplantation in North America, 1995-2005: significant improvement in survival for lymphoma and myeloma during a period of increasing recipient age. Biol Blood Marrow Transplant. 2013; 19:1116–23.
- Jantunen E, Canals C, Attal M, Thomson K, Milpied N, Buzyn A, et al. Autologous stem-cell transplantation in patients with mantle cell lymphoma beyond 65 years of age: a study from the European Group for Blood and Marrow Transplantation (EBMT). Ann Oncol. 2012; 23:166–71.
- Bashir Q, Shah N, Parmar S, Wei W, Rondon G, Weber DM, et al. Feasibility of autologous hematopoietic stem cell transplant in patients aged >/ = 70 years with multiple myeloma. Leuk Lymphoma. 2012;53:118–22.
- Attal M, Lauwers-Cances V, Marit G, Caillot D, Moreau P, Facon T, et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366:1782–91.
- McCarthy PL, Owzar K, Hofmeister CC, Hurd DD, Hassoun H, Richardson PG, et al. Lenalidomide after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366:1770–81.
- Salles G, Seymour JF, Offner F, Lopez-Guillermo A, Belada D, Xerri L, et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet. 2011;377:42–51.
- Neumann F, Harmsen S, Martin S, Kronenwett R, Kondakci M, Aivado M, et al. Rituximab long-term maintenance therapy after autologous stem cell transplantation in patients with B-cell non-Hodgkin’s lymphoma. Ann Hematol. 2006;85:530–4.
- Pettengell R, Schmitz N, Gisselbrecht C, Smith G, Patton WN, Metzner B, et al. Rituximab purging and/or maintenance in patients undergoing autologous transplantation for relapsed follicular lymphoma: a prospective randomized trial from the lymphoma working party of the European group for blood and marrow transplantation. J Clin Oncol. 2013;31:1624–30.
- Gisselbrecht C, Schmitz N, Mounier N, Singh Gill D, Linch DC, Trneny M, et al. Rituximab maintenance therapy after autologous stem-cell transplantation in patients with relapsed CD20(+) diffuse large B-cell lymphoma: final analysis of the collaborative trial in relapsed aggressive lymphoma. J Clin Oncol. 2012;30:4462–9.
- Forlenza CJ, Small TN. Live (vaccines) from New York. Bone Marrow Transplant. 2013;48:749–54.
- Majhail NS, Lazarus HM, Burns LJ. Iron overload in hematopoietic cell transplantation. Bone Marrow Transplant. 2008;41:997–1003.
- Rizzo JD, Wingard JR, Tichelli A, Lee SJ, Van Lint MT, Burns LJ, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation: joint recommendations of the European Group for Blood and Marrow Transplantation, the Center for International Blood and Marrow Transplant Research, and the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2006;12:138–51.
- Burns LJ. Late effects after autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2009;15(1 Suppl):21–24.
- Majhail NS, Rizzo JD, Lee SJ, Aljurf M, Atsuta Y, Bonfim C, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18:348–71.
- Christoforidou AV, Saliba RM, Williams P, Qazilbash M, Roden L, Aleman A, et al. Results of a retrospective single institution analysis of targeted skeletal radiotherapy with (166)Holmium-DOTMP as conditioning regimen for autologous stem cell transplant for patients with multiple myeloma. Impact on transplant outcomes. Biol Blood Marrow Transplant. 2007;13:543–9.
- Wong JY, Rosenthal J, Liu A, Schultheiss T, Forman S, Somlo G. Image-guided total-marrow irradiation using helical tomotherapy in patients with multiple myeloma and acute leukemia undergoing hematopoietic cell transplantation. Int J Radiat Oncol Biol Phys. 2009;73:273–9.
- Lazarus HM, Loberiza FR Jr, Zhang MJ, Armitage JO, Ballen KK, Bashey A, et al. Autotransplants for Hodgkin’s disease in first relapse or second remission: a report from the autologous blood and marrow transplant registry (ABMTR). Bone Marrow Transplant. 2001;27: 387–96.
- Palumbo A, Bringhen S, Petrucci MT, Musto P, Rossini F, Nunzi M, et al. Intermediate-dose melphalan improves survival of myeloma patients aged 50 to 70: results of a randomized controlled trial. Blood. 2004;104:3052–7.