Abstract
Introduction. Maternal obesity has long-term consequences for the offspring's later health, including an increased risk of type 2 diabetes and cardiovascular disease. The underlying mechanisms explaining these associations are, however, not fully understood.
Methods. A total of 2003 individuals from the Helsinki Birth Cohort Study born 1934–44, underwent measurements of body size, body composition, and clinical characteristics at a mean age of 62 years. Data on maternal anthropometry were available from hospital records.
Results. Maternal BMI was positively associated with BMI in the offspring. Higher maternal BMI was associated with less favorable body composition in the offspring. There was a significant interaction between birth weight and maternal BMI on offspring body fat percentage (P for interaction 0.003). In mothers with low BMI, a higher offspring birth weight was associated with lower fat percentage, while among those with maternal BMI in the highest fourth, higher offspring birth weight predicted higher body fat percentage.
Discussion. Our findings suggest that a disadvantageous body composition is programmed in early life. This may in part underlie the association between maternal obesity and later cardio-metabolic health of the offspring. These findings support the importance of prevention of overweight in women of child-bearing age.
High maternal BMI is positively associated with adverse health outcomes among adult offspring.
Higher maternal BMI was associated with a less favorable body composition among the offspring in adult life.
In mothers with low BMI, a higher offspring birth weight was associated with lower body fat percentage in adulthood, while among those with maternal BMI in the highest fourth, higher offspring birth weight predicted higher body fat percentage, in adult life.
Introduction
Overweight and obesity are associated with adverse health consequences including an increased risk of type 2 diabetes and cardiovascular disease (Citation1,Citation2). There is evidence showing that maternal overweight and obesity have long-term health consequences on offspring's later health. Several studies have shown associations between maternal obesity and obesity and obesity-related outcomes in the offspring (Citation3–6). These associations are stronger for maternal than for paternal obesity, suggesting that they are not solely explained by a common genetic background (Citation7).
A Scottish study showed that maternal obesity in pregnancy was associated with an increased risk of cardiovascular events and premature death in adult offspring (Citation8). We have previously shown increased death rates from coronary heart disease in men whose mothers had high body mass index (BMI) during pregnancy (Citation9). In the Helsinki Birth Cohort Study (HBCS), a cohort of 13,345 men and women born during 1934–44, higher maternal BMI during pregnancy was associated with overall mortality, cancer and cardiovascular disease mortality and morbidity, and type 2 diabetes, among the offspring (Citation10). There were gender differences; for example the association between maternal BMI and type 2 diabetes was stronger in women.
These described long-term adverse health effects can be mediated through various mechanisms including environmental, genetic, and epigenetic factors. One plausible underlying mechanism is in utero programming (Citation5,Citation11–16). Traditionally, early life programming has primarily been studied in relation to a small body size of birth. However, not only a small body size at birth is associated with adverse health outcomes; a large body size at birth is associated with an increased risk of overweight and obesity (Citation17–21).
In the present study we focus upon clinical characteristics and cardio-metabolic risk factors in relation to maternal body mass index during pregnancy in individuals from the HBCS. The objective of this study was to identify pathways and risk factors of diseases as well as underlying causes explaining the epidemiological associations between maternal BMI and offspring morbidity and mortality.
Materials and methods
The epidemiological cohort of the HBCS includes 13,345 men and women who were born between 1934 and 1944 in one of the two major maternity hospitals in Helsinki and who visited child welfare clinics in the city and lived in Finland in 1971 when a unique ID number was allocated to each resident of the country. Details of the birth records and child welfare clinic records have been described previously (Citation22,Citation23). Briefly the birth records included the mother's height and weight measured at the maternity hospital prior to delivery. Body mass index (BMI) was calculated as weight (kg) divided by height2 (m2). The weight and length of the baby were recorded at the maternity hospital, and we calculated the ponderal index (birth weight/length3).
For the clinical study, we used random-number tables to select a subset of people in the initial epidemiological study group (n = 8,760) who were still alive and living in Finland. In order to achieve a sample size in excess of 2,000 people for this study we selected 2,691 subjects for the study, and 2,003 of them visited the clinic. The procedures used at the clinic have been described previously (Citation24). The subjects attended the clinic after an overnight fast. The clinical examination included a 2-hour 75-g oral glucose tolerance test and measurements of height, weight, waist circumference, and blood pressure. Body composition was assessed by bio-impedance, using an eight-polar tactile electrode system (InBody 3.0). Height and weight were measured in light indoor clothing and without shoes. Height was measured to the nearest 0.1 cm and weight to the nearest 0.1 kg. Height was measured with a Kawi stadiometer. Weight was measured on Seca Alpha 770 scales.
Blood pressure was measured from the right arm while the subject was in the sitting position, and it was recorded as the mean of two successive readings from a mercury sphygmomanometer. Blood was drawn for measurements of lipids, glucose, insulin, inflammatory markers, and adipocytokines.
Plasma glucose concentrations were measured according to the hexokinase method; plasma insulin concentrations were determined by a two-site immunometric assay (Citation25,Citation26). Serum cholesterol and triglyceride concentrations were measured with the use of standard enzymatic methods (Citation27,Citation28). LDL-cholesterol concentrations were calculated using the Friedewald formula (Citation29). Hs-CRP was measured with immunoturbidimetric methods, using Konelab T-serie High Sensitivity CRP analyzer (Thermo Fisher Scientific Oy, Vantaa, Finland). High-molecular-weight adiponectin (HMW adiponectin) was analyzed with an enzyme-linked immunosorbent assay (HMW adiponectin ELISA kit, Millipore, St Charles, MO, USA). IL-6, TNF-α, and leptin were analyzed with multiplex sandwich immunoassays (Milliplex Human Metabolic Hormone Panel, Millipore).
Written informed consent was obtained from each subject before any procedures were carried out. The Ethics Committee for Epidemiology of Helsinki and Uusimaa Hospital District approved the study.
Statistical methods
For analysis we transformed variables with right-skewed distributions to normality by taking logarithms. We analyzed the data using multiple linear regression (continuous outcomes) and multiple logistic regression (binary outcomes). With clinical variables we always included age as a predictor; with neonatal measurements we included year of birth. We included sex as a predictor in pooled analyses. The analyses are based on continuous variables, but presented in groups for easier appreciation of the true effect sizes. The analyses were conducted in IBM SPSS Statistics version 21.
Results
Maternal characteristics and offspring birth size
Mean maternal age was 28.6 (SD 5.5) years, parity 2.0 (1.3), height 159.5 (5.8) cm, weight 67.5 (8.1) kg, and BMI before delivery 26.5 (2.6) kg/m2. Maternal socio-economic status was assessed according to the occupation of the offspring's fathers; 72% came from families belonging to the laborer class, 19% from lower-middle-class families, and 9% from upper-middle-class families. In a simultaneous analysis mother's BMI increased by 0.11 kg/m2 with each year of age (95% CI 0.08 to 0.13, P <0.001) () and by 0.19 kg/m2 with each additional previous birth (95% CI 0.08 to 0.31, P = 0.001).
Figure 1. Body fat percentage plotted against mother's body mass index. Men shown with solid circles and a solid regression line, and women with open triangles and a dashed regression line.
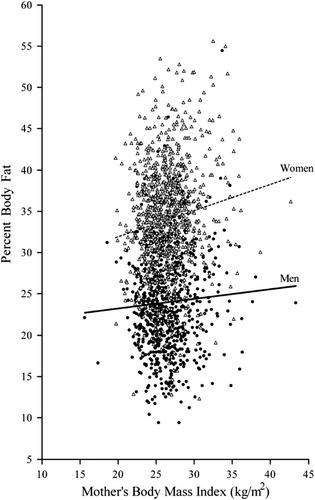
We divided the mothers into fourths according to their BMI. Higher maternal BMI was positively associated with gestational age, birth weight, length at birth, head circumference, and ponderal index in the offspring () (all P values < 0.001). These findings remained significant after adjustment for childhood socio-economic status.
Table I. Maternal and newborn characteristics (mean, standard deviation) according to fourths of maternal body mass index during late pregnancy.
Offspring anthropometrics in adult life
and show adult anthropometry and clinical characteristics at a mean age of 62 years, according to fourths of maternal BMI during late pregnancy, in men and women, respectively. Higher maternal BMI was associated with significantly higher BMI, lean body mass, and fat mass among the offspring. It was also associated with higher body fat percentage in women (P < 0.001).
Table II. Adult offspring anthropometry and clinical characteristics (mean, standard deviation) according to fourths of maternal body mass index during late pregnancy. Results for men.
Table III. Adult offspring anthropometry and clinical characteristics (mean, standard deviation) according to fourths of maternal body mass index during late pregnancy. Results for women.
We studied whether birth size interacted with maternal BMI on offspring body composition in adult life. There was a significant interaction between birth weight and maternal BMI on body fat percentage (P for interaction 0.003). Among mothers with BMI below the highest quartile, a higher offspring birth weight was associated with lower fat percentage. This association was mainly contributed to by an association observed in women. By contrast, among those with maternal BMI in the highest quartile, higher offspring birth weight predicted higher fat percentage in adult life. This again was contributed to by an association among men. Regression coefficients for adult body composition in relation to birth weight and maternal BMI during late pregnancy are shown in . There were gender differences, and the results are therefore also presented separately for men and women.
Table IV. Regression coefficients for adult offspring body composition measurements in relation to offspring birth weight according to sex and maternal body mass index during late pregnancy.
Adult cardio-metabolic risk factors ( and )
Maternal BMI was not associated with systolic or diastolic blood pressure, fasting glucose, insulin concentrations, or blood lipids in the offspring. Nor was it associated with inflammatory markers or adipocytokines.
Discussion
Maternal adiposity in pregnancy is positively associated with body size at birth among the offspring. In general, body fat percentage in adult life was higher among those born to mothers with a higher BMI. However, there was a significant interaction between birth weight and maternal BMI on offspring body fat percentage in adult life. These findings suggest that maternal body size programs offspring body composition for life. Gender-specific effects were also observed. Prenatal programming of an unfavorable body composition could be one underlying factor explaining the positive association between maternal BMI and the offspring's later cardio-metabolic morbidity.
Using data from the epidemiological part of the HBCS we have previously shown that maternal BMI during pregnancy is positively associated with several health outcomes in the offspring (Citation10). These outcomes are cardiovascular mortality and morbidity and type 2 diabetes. We reported gender differences. One factor potentially explaining these gender differences is prenatal programming of body fat percentage. A higher maternal BMI in association with higher birth weight in males was associated with a higher body fat percentage in adult life. This could be the consequence of adverse prenatal programming of body composition. A higher birth weight in offspring of mothers with higher BMI could reflect a disadvantageous body composition due to maternal obesity. A higher body fat percentage is a known risk factor for development of type 2 diabetes and related metabolic traits. Previously, sex differences in prenatal growth according to maternal body size have been reported (Citation30).
In the present study we have focused upon potential underlying mechanisms explaining the association between maternal adiposity and the offspring's later health. We propose that programming of body composition could be one plausible mechanism. Our findings suggest that a disadvantageous body composition is programmed in early life and could explain the association between maternal adiposity and the offspring's later cardio-metabolic health. The overnutrition hypothesis proposes that maternal overweight and obesity is associated with elevated maternal glucose, insulin, and free fatty acids levels (Citation12,Citation13). The long-term consequences of this can result in permanent changes in body composition of the developing fetus. This could increase the vulnerability to later obesity-related health outcomes.
It is well established that children born small- or large- for-gestational-age are at increased risk for the development of type 2 diabetes in adult life (Citation14–16). Understanding factors that are responsible for the increased risk is of major importance. Maternal body size is a marker of maternal long-term nutritional status and is strongly associated with offspring birth size. Consistent with the present findings, it has previously been shown that offspring of obese mothers have increased fat mass, but not lean mass in early life (Citation15). Children born with low birth weight have less lean body mass in adult life, also after taking adult BMI into account. This would suggest that cardio-metabolic morbidity is programmed by different mechanisms in low- and high-birth-weight children. However, maternal nutritional status and adiposity are affecting both. These findings suggest that programming of adiposity is sensitive to maternal body size and underlies long-term metabolic consequences of maternal body size.
We report, to our knowledge, for the first time that there is an interaction between maternal BMI and birth weight on body fat percentage. Males born to mothers belonging to the highest fourth of BMI and with high birth weight have a more disadvantageous body composition in adult life compared to men born to mothers with lower BMI. In both men and women lean body mass increased with higher birth weights with no major influence of maternal BMI.
However, no associations were observed between maternal BMI, fasting glucose and insulin concentrations, lipids, or systolic or diastolic blood pressure in the offspring. We have previously reported that there is a positive association between maternal BMI and type 2 diabetes and cardiovascular disease among the offspring (Citation10). The reason for the present findings could reflect differences in sample size, but it could also reflect survival bias among those participating in the clinical study.
There are limitations to our study, which have been described previously (Citation22,Citation24). The HBCS is restricted to people who were both born and attended child welfare clinics in the city of Helsinki. Most children and their parents attended these clinics, which were free. The people in our study may not be representative of all people living in Helsinki in those days. However, at birth the distribution of fathers’ occupations was similar to that in the city as a whole. The mother's BMI in late pregnancy reflects both weight gain during pregnancy and BMI before pregnancy. We do not have data on gestational weight gain, nor do we have data on maternal body composition. However, BMI in late pregnancy is highly correlated with BMI before pregnancy (Citation16). We have no data on gestational diabetes and are unable to distinguish its effects from those of maternal overweight and obesity. Gestational diabetes was recognized as a pregnancy condition only from the 1960s onwards, while screening started even later. Body composition was assessed with bio-impedance. This is not the gold standard for assessment of body composition. However, bio-impedance offers accurate estimates of body composition within a wide range of adiposity (Citation31,Citation32). The strengths of our study include the long follow-up period, and reliable measurements of maternal body size and birth size obtained from hospital records. The outcomes were based on clinical measures made by trained research nurses.
In conclusion, we have previously in the HBCS shown that increased maternal BMI in late pregnancy is an independent predictor of cardiovascular disease and type 2 diabetes among the offspring (Citation7). The long-term effect of maternal BMI is probably mediated by lifelong changes in body composition. Our study emphasizes the importance of maternal influences during pregnancy on later health. Strategies to raise awareness of the risks of overweight and obesity among women of child-bearing age are required.
Funding: HBCS has been supported by grants from Finska Läkaresällskapet, the Finnish Special Governmental Subsidy for Health Sciences, Academy of Finland, Samfundet Folkhälsan, Liv och Hälsa, the Signe and Ane Gyllenberg Foundation, and EU FP7 (DORIAN) project number 278603.
Declaration of interest: The authors report no conflicts of interest.
References
- Shai I, Jiang R, Manson JE, Stampfer MJ, Willett WC, Colditz GA, et al. Ethnicity, obesity, and risk of type 2 diabetes in women: a 20-year follow-up study. Diabetes Care. 2006;29:1585–90.
- Pedersen SD. Metabolic complications of obesity. Best Pract Res Clin Endocrinol Metab. 2013;27:179–93.
- Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115:e290–6.
- Drake AJ, Reynolds RM. Impact of maternal obesity on off spring obesity and cardiometabolic disease risk. Reproduction. 2010;140:387–98.
- Godfrey KM, Gluckman PD, Hanson MA. Developmental origins of metabolic disease: life course and intergenerational perspectives. Trends Endocrinol Metab. 2010;21:199–205.
- Reynolds RM, Osmond C, Phillips DI, Godfrey KM. Maternal BMI, parity, and pregnancy weight gain: influences on offspring adiposity in young adulthood. J Clin Endocrinol Metab. 2010;95:5365–9.
- Pirkola J, Pouta A, Bloigu A, Hartikainen AL, Laitinen J, Järvelin MR, et al. Risks of overweight and abdominal obesity at age 16 years associated with prenatal exposures to maternal prepregnancy overweight and gestational diabetes mellitus. Diabetes Care. 2010;33:1115–21.
- Reynolds RM, Allan KM, Raja EA, Bhattacharya S, McNeill G, Hannaford PC, et al. Maternal obesity during pregnancy and premature mortality from cardiovascular event in adult off spring: follow-up of 1 323 275 person years. BMJ. 2013;347:f4539.
- Forsén T, Eriksson JG, Tuomilehto J, Teramo K, Osmond C, Barker DJ. Mother's weight in pregnancy and coronary heart disease in a cohort of Finnish men: follow up study. BMJ. 1997;315:837–40.
- Eriksson JG, Sandboge S, Salonen MK, Kajantie E, Osmond C. Long-term consequences of maternal overweight in pregnancy on offspring later health: findings from the Helsinki Birth Cohort Study. Ann Med. 2014;46:434–8.
- Ramsay JE, Ferrell WR, Crawford L, Wallace AM, Greer IA, Sattar N. Maternal obesity is associated with dysregulation of metabolic, vascular, and inflammatory pathways. J Clin Endocrinol Metab. 2002;87: 4231–7.
- Catalano PM, Ehrenberg HM. The short- and long-term implications of maternal obesity on the mother and her offspring. BJOG. 2006; 113:1126–33.
- Sewell MF, Huston-Presley L, Super DM, Catalano P. Increased neonatal fat mass, not lean body mass, is associated with maternal obesity. Am J Obstet Gynecol. 2006;195:1100–3.
- Gluckman PD, Hanson MA. Developmental and epigenetic pathways to obesity: an evolutionary-developmental perspective. Int J Obes (Lond). 2008;32(Suppl 7):S62–71.
- Catalano PM, Presley L, Minium J, Hauguelde Mouzon S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care. 2009;32:1076–80.
- Li N, Liu E, Guo J, Pan L, Li B, Wang P, et al. Maternal prepregnancy body mass index and gestational weight gain on pregnancy outcomes. PLoS One. 2013;8:e82310.
- Barker DJP. Fetal origins of coronary heart disease. BMJ. 1995; 311:171–4.
- Barker DJP, Osmond C, Kajantie E, Eriksson JG. Growth and chronic disease: findings in the Helsinki Birth Cohort. Ann Hum Biol. 2009;36:445–58.
- Whincup PH, Kaye SJ, Owen CG, Huxley R, Cook DG, Anazawa S, et al. Birth weight and risk of type 2 diabetes: a systematic review. JAMA. 2008;300:2886–97.
- Yu ZB, Han SP, Zhu GZ, Zhu C, Wang XJ, Cao XG, et al. Birth weight and subsequent risk of obesity: a systematic review and meta-analysis. Obes Rev. 2011;12:525–42.
- Dabelea D, Pettitt DJ, Hanson RL, Imperatore G, Bennett PH, Knowler WC. Birth weight, type 2 diabetes, and insulin resistance in Pima Indian children and young adults. Diabetes Care. 1999;22:944–50.
- Eriksson JG, Osmond C, Kajantie E, Forsén TJ, Barker DJ. Patterns of growth among children who later develop type 2 diabetes or its risk factors. Diabetologia. 2006;49:2853–8.
- Eriksson JG. Early growth and coronary heart disease and type 2 diabetes: findings from the Helsinki Birth Cohort Study (HBCS). Am J Clin Nutr. 2011;94:1799S–802S.
- Barker DJ1, Osmond C, Forsén TJ, Kajantie E, Eriksson JG. Trajectories of growth among children who have coronary events as adults. N Engl J Med. 2005;353:1802–9.
- Kunst A, Draeger B, Ziegenhorn J. UV-methods with hexokinase and glucose-6-phosphate dehydrogenase. In: Bergmeyer HU, editor. Methods of enzymatic analysis. Weinheim, Germany: Verlag Chemie; 1983. p. 163–72.
- Sobey WJ, Beer SF, Carrington CA, Clark PM, Frank BH, Gray IP, et al. Sensitive and specific two-site immunoradiometric assays for human insulin, proinsulin, 65-66 split and 32-33 split proinsulins. Biochem J. 1989;260:535–41.
- Lie RF, Schmitz JM, Pierre KJ, Gochman N. Cholesterol oxidase-based determination, by continuous-flow analysis of total and free cholesterol in serum. Clin Chem. 1976;22:1627–30.
- Fossati P, Prencipe L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem. 1982; 28:2077–80.
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502.
- Lampl M, Gotsch F, Kusanovic JP, Gomez R, Nien JK, Frongillo EA, et al. Sex differences in fetal growth responses to maternal height and weight. Am J Hum Biol. 2010;22:431–43.
- Bedogni G, Malavolti M, Severi S, Poli M, Mussi C, Fantuzzi AL, et al. Accuracy of an eight-point tactile-electrode impedance method in the assessment of total body water. Eur J Clin Nutr. 2002;56:1143–8.
- Sartorio A, Malavolti M, Agosti F, Marinone PG, Caiti O, Battistini N, et al. Body water distribution in severe obesity and its assessment from eight-polar bioelectrical impedance analysis. Eur J Clin Nutr. 2005;59:155–6.
