Abstract
Practical management of stroke prevention in patients with non-valvular atrial fibrillation (AF) requires physicians to find the optimal balance between maximizing prevention of ischaemic stroke and minimizing the risk of bleeding. Vitamin K antagonists have traditionally been used for stroke prevention in patients with AF; however, they have been associated with increased risk of bleeding, particularly intracranial haemorrhage. New oral anticoagulants (OACs) have shown similar efficacy to the vitamin K antagonist warfarin but with a reduced risk of bleeding, particularly life-threatening bleeding such as intracranial haemorrhage. Decisions about which new OAC therapy to use may be influenced by patient characteristics such as age, renal function, co-medication use, and bleeding risk. This review uses a case-based approach to highlight the practical management issues to be considered by the physician when selecting a new OAC for stroke prevention in patients with non-valvular AF.
The new oral anticoagulants apixaban, dabigatran, and rivaroxaban are approved for the prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation; this approval is a result of their favourable benefit–risk profiles compared with that of warfarin, the current standard of care.
All of the new oral anticoagulants have demonstrated non-inferiority to warfarin in reducing the risk of stroke and systemic embolism and in reducing the risk of major bleeding, particularly intracranial haemorrhage or fatal bleeding.
Practical management of stroke prevention in patients with atrial fibrillation is no longer about whether to choose between warfarin and a new oral anticoagulant, but rather which new oral anticoagulant is appropriate for use in a particular patient and what its limitations are.
Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia, with an estimated prevalence of 10 million in Europe and 3 million in the United States (Citation1,Citation2). By 2050, this prevalence is expected to increase in both regions to approximately 25–30 million and 8 million, respectively (Citation1). The risk of AF increases with age, from 0.3% in those aged 18–27 years to 2.1% and 17.6% in those aged 50–55 years and ≥ 80 years, respectively (Citation3). Patients with AF can have an increased risk of stroke up to seven times that of those without AF, resulting in an incidence of approximately 5% (Citation4). The risk of stroke attributable to AF also increases with age, from 1.5% in those aged 50–59 years to 23.5% in those aged 80–89 years (Citation5). Overall, approximately 15% of strokes are due to AF (Citation6), and these are often associated with more severe outcomes in terms of morbidity and mortality than non-AF-related strokes.
Vitamin K antagonists (VKAs), such as warfarin, have traditionally been the oral anticoagulants (OACs) used for stroke prevention in patients with AF, with warfarin specifically shown to reduce this risk by 64% compared with placebo or no treatment (Citation7). However, VKAs are associated with substantial challenges. Most notable is the difficulty in achieving adequate time spent in therapeutic range, defined by the international normalized ratio (INR; target range 2.0–3.0). In controlled clinical trials, VKA-treated patients have been within this INR range only 55%–65% of the time (Citation8–10), but this falls to below 50% in routine clinical practice, which may offset the benefits of VKAs (Citation11). VKAs also have significant interactions with food, alcohol, and drugs (Citation11). New OACs, such as apixaban, dabigatran, and rivaroxaban, have all shown efficacy and safety profiles similar to those of warfarin, but are uniformly more easily and dependably used. (Citation12–15) shows a comparison of the basic pharmacological properties of warfarin with those of the new OACs. This review will use a case-based approach to focus on the practical management of stroke prevention in patients with AF using the currently licensed new OACs.
Table I. Comparison of pharmacological properties of oral anticoagulants.
Patient case study
introduces our patient case study, with various additional scenarios detailed in . This will provide a context to highlight the practical management issues and challenges associated with stroke prevention in patients with AF using the new OACs.
Figure 1. A: Patient case study. B: Different scenario of patient case study (changes shown in italics). AF = atrial fibrillation; ASA = acetylsalicylic acid; BP = blood pressure; bpm, beats per minute; CAD = coronary artery disease; CBC = complete blood count; CrCl = creatinine clearance; CV = cardiovascular; ECG = electrocardiogram; GI = gastrointestinal; HR = heart rate; ICH, intracranial haemorrhage; LA = left atrial; LV = left ventricle; RV = right ventricle; TSH = thyroid stimulating hormone.

Balancing stroke prevention and bleeding risk
What does this all mean?
Anticoagulant therapy for any indication, including stroke prevention in patients with AF, is associated with an increased risk of bleeding. Therefore, it is important to weigh treatment benefit versus hazard, and risk stratification schemes have been developed to support this objective.
The original risk predictor for stroke was the CHADS2 score (Citation16); however, this score fails to identify many patients at intermediate risk. For example, the patient described in would have a CHADS2 score of 0 and, therefore, would not previously have been considered as an obvious candidate for anticoagulation (although the patient's nominal annual stroke risk would be 1.9%, the confidence interval ranges from a low risk of 1.2% to a risk as high as 3.0%). The CHA2DS2-VASc score was subsequently developed to incorporate additional risk factors known to contribute to the overall risk of stroke, including female sex and vascular disease. Most importantly, however, it emphasized the relevance of increasing age as a risk factor, awarding 1 point for an age of 65–74 years and 2 points for an age ≥ 75 years. This has improved stroke risk stratification in patients with AF (Citation17). Assessing our patient () using CHA2DS2-VASc results in a score of 3 (vascular disease, age 65–74 years, and female sex) and a nominal annual stroke risk of 3.2%, which is sufficient to require an OAC for stroke prevention according to all guidelines (Citation11,Citation18–20).
Bleeding risk calculators have also been created. The HAS-BLED score was specifically developed to estimate the 1-year risk of major bleeding in patients with AF (Citation21). This score has an advantage over other bleeding risk scores, such as HEMORR2HAGES, in that it does not require any laboratory or genetic testing and can be assessed from medical records or routine testing in patients newly diagnosed with AF (Citation21). Referring back to our patient (), we can see that she has had a prior gastrointestinal (GI) bleeding event, is aged > 65 years, and is currently taking acetylsalicylic acid (ASA). The HAS-BLED score defines abnormal renal function as the presence of chronic dialysis, renal transplantation, or serum creatinine ≥ 200 μmol/L (Citation21). However, the new OAC clinical trials and label recommendations all used creatinine clearance (CrCl) to assess renal function (Citation22). Our patient has a CrCl of 40 mL/min, which is classified as moderate renal impairment. If an additional point is given for this, then our patient has a HAS-BLED score of 4, putting her at high risk of an estimated 8.7 bleeding events per 100 patient-years (Citation21). Because this is almost three times the patient's apparent stroke risk, the appropriateness of antithrombotic therapy might be questioned.
Bleeding risk is often cited as one of the main reasons not to prescribe anticoagulants to patients with AF (Citation23). However, stroke risk is often underestimated, whereas the risk of bleeding with anticoagulants such as VKAs and new OACs is often overestimated. In general, the benefits of stroke prevention in most cases outweigh the risk of bleeding. A Canadian survey of physicians treating patients with AF and patients at high risk of developing AF found that, compared with physicians, patients placed more value on stroke avoidance than bleeding risk. The mean thresholds for the minimum number of strokes to be prevented and the maximum increase in the risk of acceptable excess bleeding in 100 patients treated with warfarin over 2 years were 2.5 and 10.3 in physicians, respectively, and 1.8 and 17.4 in patients, respectively (Citation24).
How to reduce the risk of bleeding in patients receiving OAC therapy
It is important to recognize that the risk of bleeding in patients receiving OACs can often be reduced, whereas the risk of stroke cannot be improved. For example, the nominally high bleeding risk of our patient can be positively modified by simply withdrawing ASA therapy, thereby reducing the HAS-BLED score to 3 and putting our patient at the lower estimated risk of 3.74 bleeding events per 100 patient-years (Citation21). The HAS-BLED risk score can also be modified, as applicable, with adequate control of hypertension (itself an important contributor to stroke risk), reducing alcohol consumption, and monitoring renal function. Other methods to reduce bleeding risk include the provision of balance and mobility aids to patients with a history of falls and the use of proton pump inhibitors in patients at risk of a GI bleeding event.
The major concern with warfarin is the increased risk of bleeding—in particular, intracranial haemorrhage (ICH); the risk of death is significantly increased in any patient treated with warfarin who suffers an ICH (Citation25). All of the new OACs have been associated with significantly lower rates of ICH compared with warfarin in phase III clinical studies. Increasing the intensity of warfarin treatment (from INR < 2.0 towards INR > 3.0) is also strongly associated with an increased risk of death (P < 0.01 for trend) (Citation25). As a result, VKAs tend to be under-used in patients requiring anticoagulation. Aggravating matters is the fact that patient adherence to warfarin therapy is poor, with one population-based cohort study in Canadian patients from 1997 to 2008 finding a discontinuation rate of 31.8% within 1 year of treatment commencement, which rises to 61.3% within 5 years (Citation26).
Selecting a new oral anticoagulant
What has been demonstrated in clinical studies?
Clinical trials of the new OACs have shown efficacy and safety profiles that are similar to, and in some cases better than, those of warfarin. Key data from these clinical trials are presented in (Citation8–10,Citation27–31). In the ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) trial, apixaban was found to reduce the rates of stroke and systemic embolism compared with warfarin (1.27% versus 1.60%, respectively; P = 0.01 for superiority) (Citation8). Major bleeding was also reduced with apixaban in comparison with warfarin (2.13% versus 3.09% per year, respectively; P < 0.001) (Citation8).
Table II. Key data from new oral anticoagulant phase III trials.
In the RE-LY (Randomized Evaluation of Long-Term Anticoagulation Therapy) trial, two doses of dabigatran (110 mg and 150 mg twice daily [bid]) were compared with warfarin (Citation9). Both doses of dabigatran were found to be non-inferior to warfarin in the prevention of stroke in patients with AF (P < 0.001), but only the dabigatran 150 mg bid dose was found to be superior to warfarin (P < 0.001) (Citation9). Rates of major bleeding were 2.71% and 3.11% per year for the dabigatran 110 mg and 150 mg bid doses, respectively, compared with 3.36% per year with warfarin (Citation9). There were significantly higher rates of GI bleeding with dabigatran 150 mg bid compared with warfarin (P < 0.001) (Citation9).
Results from ROCKET AF (Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation) found rivaroxaban to be non-inferior to warfarin. In the intention-to-treat analysis, the primary efficacy end-point of stroke or systemic embolism occurred in 269 patients in the rivaroxaban group (2.1% per year) and 306 patients in the warfarin group (2.4% per year; P < 0.001 for non-inferiority; P = 0.12 for superiority) (Citation10). Major and non-major clinically relevant bleeding occurred in 14.9% and 14.5% of patients per year in the rivaroxaban- and warfarin-treated groups, respectively (Citation10). A significant increase in the rate of GI bleeding was noted with rivaroxaban compared with warfarin (3.2% versus 2.2% per year, respectively; P < 0.001)
The major clinical recommendations for antithrombotic management in patients with AF are presented in (Citation11,Citation20,Citation22) and are similar. Patients who are eligible for, but not currently on, any anticoagulant should be immediately started on such therapy, preferably one of the new OACs as per international practice guideline recommendations. Switching patients already on warfarin to a new OAC may be considered, especially in those who are poorly maintained on warfarin. In this instance, the directions given in the relevant product monographs are that warfarin must be stopped and the patient's INR must be < 2.0 before starting therapy with apixaban or dabigatran (Citation13,Citation14) or ≤ 3.0 when switching to rivaroxaban. Switching from a parenteral anticoagulant to a new OAC can be done 0–2 hours before the next scheduled dose of the parenteral anticoagulant or at the time of discontinuation for continuous parenteral anticoagulants (e.g. unfractionated heparin) (Citation14,Citation15). In the event of a missed dose, patients treated with rivaroxaban should take a dose immediately and carry on with the schedule as normal (Citation13,Citation15). Missed apixaban and dabigatran doses can be taken up to 6 hours before the next scheduled dose (Citation14). However, the dose should be omitted if there is < 6 hours left before the next scheduled dose. Double dosing is not recommended for any of the new OACs (Citation14,Citation15).
Table III. Major clinical recommendations for antithrombotic management in patients with atrial fibrillation.
No international guideline recommends any specific new OAC over the others; however, it is acknowledged that patient characteristics, drug compliance, and tolerability, as well as cost, may influence which new OAC is chosen (Citation22). The recommended doses of apixaban and dabigatran for stroke prevention in patients with AF are 5 mg and 150 mg, respectively; both are taken orally bid (Citation13,Citation14). However, the dose of dabigatran is reduced to 110 mg bid in patients aged ≥ 80 years. In patients aged between 75 and 80 years, or in those with an increased risk of bleeding, moderate renal impairment, gastritis, oesophagitis, or gastro-oesophageal reflux, either the 150 mg or 110 mg bid dose of dabigatran can be given at the discretion of the physician (Citation14), with the lower dose tending to be favoured. The 110 mg dose of dabigatran is not approved in the United States for the prevention of stroke in patients with AF. Instead, the recommended dose for patients with CrCl 15–30 mL/min is 75 mg bid orally (Citation20), but this is based on pharmacological assumptions because this dose has not been tested in patients with AF. In Europe, dabigatran is contraindicated in patients with AF who have severe renal impairment (CrCl < 30 mL/min) (Citation14). The usual apixaban dose is 5 mg bid, although it should be reduced to 2.5 mg bid if two or more of the following risk factors are present: age ≥ 80 years, weight ≤ 60 kg, or creatinine ≥ 133 μmol/L. Rivaroxaban has a recommended dose of 20 mg, but differs from apixaban and dabigatran in that it has a once-daily (od) dosing regimen (Citation15). With CrCl 15–< 50 mL/min, the rivaroxaban dose is reduced to 15 mg od. To ensure high bioavailability, rivaroxaban at either the 15 mg or the 20 mg dose should be taken with food (Citation15). Note that a full meal is not a requirement in this regard, nor does the meal need to be dinner.
Ultimately, there is an evolving treatment paradigm for stroke prevention in patients with AF. Increasingly, the issue is not whether to choose warfarin or a new OAC, but rather which new OAC to select for treatment. A suggested approach is summarized in .
Table IV. Summary of choice of oral anticoagulants based on patient characteristics. From reference (Citation36) with permission.
Adherence to new OACs
Compliance with new OAC regimens is crucial because the anticoagulant effect fades after 12–24 hours (Citation32).
A retrospective analysis in four therapeutic classes of drugs (antidiabetic, antihyperlipidaemic, antiplatelet, and cardiac) found that adherence with od regimens was generally higher than for bid regimens, by approximately 14% (Citation33). Results of another study showed lower persistence with warfarin compared with dabigatran therapy in patients with newly diagnosed AF at 6 months: 50% and 64%, respectively, falling to 24% and 41%, respectively, at 12 months (Citation34). New data from a prospective registry of new OACs have shown even higher rates of adherence to dabigatran therapy, as well as high rates of adherence to rivaroxaban therapy: after 6 months of follow-up, 74.3% of patients were still on dabigatran (bid) therapy, and 90.9% were still on rivaroxaban (od) therapy (Citation32,Citation35). The issue of whether a missed dose with the once-daily regimen of rivaroxaban is more consequential than missing a dose of one of the new OACs with bid dosing has often been raised; however, to put matters in perspective, enoxaparin is generally dosed once daily for thromboembolic prevention or treatment, yet its half-life is somewhat shorter than that of rivaroxaban (Citation36).
Although the new OACs are generally well tolerated, both dabigatran doses in the RE-LY trial were associated with significantly higher levels of dyspepsia compared with warfarin (11.8% and 11.3% with 110 mg and 150 mg dabigatran, respectively, compared with 5.8% for warfarin; P < 0.001 for both comparisons) (Citation9). This may affect adherence to dabigatran. However, dyspepsia with dabigatran therapy may be eased by taking the drug with food or using proton pump inhibitors (Citation22), although this presents a further pill burden for patients. Patient education is necessary to ensure compliance particularly at each prescription renewal (Citation37).
Patients with declining renal function
Treating patients with impaired renal function
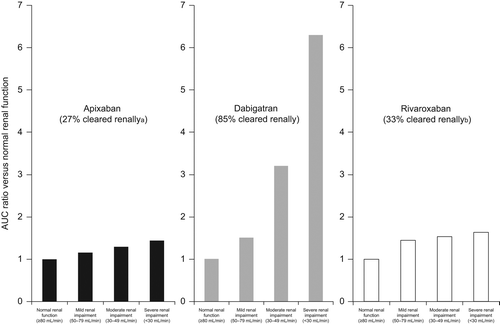
shows that our patient has moderate renal impairment (CrCl 35 ml/min), which could easily develop into severe renal impairment. This patient may be at an increased risk of bleeding according to the HAS-BLED score. One way to reduce bleeding risk is to monitor renal function at least yearly in patients with normal renal function or mild renal impairment (CrCl > 50 mL/min) and ensure that new OACs are being appropriately dosed. If a patient has moderate renal impairment (CrCl 30–50 mL/min), such as our patient case, monitoring should be conducted at least every 6 months, with a further increased frequency of monitoring in patients with a CrCl of < 30 mL/min (every 3 months) (Citation22). Additionally, renal function must be monitored at the time of any acute, especially dehydrating, illness. Results from the key new OAC trials related to renal impairment are shown in . Overall, the results show that patients with renal impairment treated with new OACs experienced fewer primary efficacy end-point events than those treated with warfarin. shows the total drug exposure with the new OACs in patients with declining renal function (Citation13–15). None of the European, Canadian, or Australian guidelines recommend new OACs for use in patients with a CrCl < 30 mL/min (Citation19,Citation22,Citation38), although in Europe both rivaroxaban and apixaban may be used with caution in patients with CrCl 15–29 mL/min (Citation13,Citation15) (use in patients with CrCl < 15 mL/min is not recommended).
Figure 2. Total drug exposure with declining renal function with new oral anticoagulant therapy. aActive drug. bFactoring in the absolute bioavailability of apixaban, ∼50% of the systemically available dose is eliminated in urine (Citation13). AUC = area under the concentration–time curve.
Stroke prevention in elderly patients with atrial fibrillation
Challenges associated with treating elderly patients with AF
Findings from a literature review showed that physicians are reluctant to recommend warfarin for elderly patients with AF, despite evidence of increased benefit in these patients versus younger patients (Citation23). The risk of falls and a history of bleeding events were also shown to be disproportionate barriers to warfarin prescription such that antiplatelet drugs were more commonly the antithrombotic agents used (Citation23). Elderly patients are more likely to have renal impairment, other co-morbidities, and frequent co-medications that increase the risk of stroke and/or the risk of bleeding. Sub-analyses carried out for different age groups help to clarify the current clinical data for the physician ().
Imagine that the case study were revised such that our patient is now 88 years of age, with kyphosis and a risk of falls (). This clearly puts her at increased risk of bleeding. However, as mentioned previously, the risk of stroke also increases with age such that any hazard related to falls is generally exceeded—often markedly so—by the stroke risk. Indeed, the average elderly patient would need to fall almost 300 times a year before the stroke-related risk of a subdural haematoma outweighed the risk of stroke with AF (Citation39). Warfarin therapy in the elderly is specifically associated with an increased risk of ICH (Citation25); this risk of ICH is significantly reduced with the new OACs, as shown in .
Stroke prevention in very elderly patients
Given that the incidence of cognitive impairment increases with age and global populations are living longer, very elderly patients with at least some cognitive impairment and a multitude of medical problems, including atrial fibrillation, are becoming ever more commonly seen in medical practice. In the past, using warfarin in such patients raised concerns about their inherent bleeding risk (Citation40), but also about their adherence to both the drug and its regular monitoring, such that its use in this clinical context was particularly limited. An advantage of the new OACs in this specific patient population is their relative ease of use and, as such, should allow greater comfort with their being prescribed to the very elderly, including those with cognitive impairment. Clearly, it is preferable to have a cognitively impaired elderly patient still able to ambulate and follow basic directions than one who is bedridden and wholly dependent on others after a major disabling stroke.
New oral anticoagulant use in patients with atrial fibrillation undergoing elective or emergency surgery
How to deal with temporary interruptions in new OAC therapy
After successfully beginning treatment with a new OAC, our patient develops cholecystitis, which requires surgery. The perioperative management of patients receiving new OACs must take into account various factors, such as renal function and the drug half-life. The European Heart Rhythm Association (EHRA) practical use document states that anticoagulant therapy should be temporarily interrupted in this situation. If the patient is undergoing a surgical procedure with ‘no clinically important bleeding risk’ (e.g. cataract surgery), the procedure can be carried out, preferably 18–24 hours after the last intake, but not at peak concentration (Citation37). The new OACs can then be restarted 6 hours later. Patients undergoing surgical procedures with a minor bleeding risk should discontinue therapy 24 hours before the procedure, whereas 48 hours is preferable for surgery with a major risk of bleeding. provides a summary of the EHRA recommendations for the discontinuation of new OACs before elective surgery. In patients requiring urgent surgery, it is recommended to discontinue a new OAC and defer surgery by 12–24 hours after the last intake, if possible (Citation36). A sub-analysis of the RE-LY trial found that urgent surgery was associated with a 5–6-fold higher risk of bleeding than elective surgery (P < 0.001). However, neither dose of dabigatran was associated with higher bleeding risks than warfarin (Citation41). Although bridging may be required for patients on warfarin, this may not be the case for patients on new OACs unless restarting anticoagulation must be delayed or the patient is unable to ingest the drug orally. Note that although rivaroxaban—and potentially apixaban (although no supportive data have been published)—can be crushed and given by nasogastric tube, dabigatran cannot (Citation14,Citation15).
Table V. Recommended times for discontinuing new OACs prior to elective surgery. From reference (Citation74) with permission.
When to restart oral anticoagulation to minimize bleeding risk
According to the EHRA practical use document, patients can resume new OAC therapy approximately 6–8 hours after a procedure that is associated with immediate and complete haemostasis (Citation36). In a different scenario, rivaroxaban is recommended 6–10 hours after elective hip or knee replacement surgery, provided haemostasis has been achieved (Citation15). This applies to a prophylactic dose of 10 mg and in patients on long-term anticoagulation for AF (20 mg or 15 mg od). Similar considerations apply to the use of the other new OACs in the perioperative setting. For many major operations, the risk of bleeding outweighs the risk of cardioembolism within the first 48–72 hours post-surgery (Citation37). For procedures associated with immobilization, low-molecular-weight heparins at intermediate or reduced doses are recommended 6–8 hours after surgery, if haemostasis has been achieved (Citation37). One important point to note is that many surgeons restart warfarin therapy soon after surgery. However, warfarin takes approximately 3–4 days to take effect (Citation12). Instead, starting a patient on a new OAC will result in a complete anticoagulant effect within 2–3 hours of dosing, which is the same time-frame in which parenteral anticoagulants act (Citation13–15). Therefore, a simple rule of thumb would be to start new OACs on the earliest day that the surgeon feels the wound is no longer at risk of major bleeding. Data suggest that the benefit of resuming OAC therapy will outweigh the risks for many patients who have experienced warfarin-associated bleeding (Citation42).
Suppose that our patient develops a postoperative GI bleeding event. Regardless of whether GI bleeding is temporally related to surgery (as in our case) or not, OAC therapy can be safely resumed once the initial bleeding has undergone effective management and it is considered safe to do so. One cohort study found that in patients treated with warfarin who experienced a GI bleeding event resumption of warfarin therapy within 90 days of the event was associated with a lower risk of thrombosis and death without a significant increase in the risk of recurrent GI bleeding (Citation42). Note that apixaban has been associated with lower rates of GI bleeding than the other new OACs and is a possible treatment option for patients at particular risk of this outcome (Citation8–10), as in our patient with her history of such events.
The issue of reversing anticoagulation
Imagine that our patient has sustained an ICH after a fall. Currently, there are no specific reversal agents available for any of the new OACs (Citation22,Citation37). However, there are also no immediate antidotes for warfarin. The currently used antidote for warfarin is vitamin K, which takes 12–24 hours to take effect (Citation43). This is too long for a patient with a serious bleeding event, such as an ICH (Citation37). Although prothrombin complex concentrate therapy has been shown to correct the INR in warfarin-treated patients with ICH, it is still associated with poor prognosis. Indeed, patients who experience an ICH on a new OAC do no worse, and possibly better, than those who experience an ICH while taking warfarin (Citation44,Citation45). For the treatment of a serious bleeding event, the EHRA practical use document recommends discontinuation of new OAC therapy and provision of supportive therapy instead (Citation37). Further studies into the use of prothrombin complex concentrate for severe bleeding events in patients treated with new OACs are required. There are several reversal agents in development for the new OACs. The investigational reversal agent andexanet alfa (PRT064445) has shown benefit with both rivaroxaban and apixaban in phase II studies (Citation46,Citation47). The reversal agent in development for direct thrombin inhibitors, aDabi-Fab, has also shown benefit in a phase I study (Citation48).
Management of stroke in a patient taking oral anticoagulants
Suppose that, instead of an ICH, the patient is brought to hospital with difficulty speaking and right-sided weakness. Normally, patients presenting with acute ischaemic stroke (AIS) should be considered for urgent thrombolytic therapy with alteplase (Citation49). However, effective anticoagulation is considered a contraindication to thrombolysis because of the risk of haemorrhagic transformation (HT). For patients taking warfarin, guidelines suggest that thrombolysis can be considered if the INR is ≤ 1.7, but recommend against it if the INR is higher (Citation50). Currently, validated commercial kits for rapid assessment of new OAC levels and their haemostatic effects are not widely available. As such, consensus is lacking among vascular neurologists regarding thrombolysis of a patient with AIS on a new OAC, even where qualitative measures such as prothrombin time or partial thromboplastin time might be normal (Citation49). Two case reports of successful thrombolysis in patients on rivaroxaban (Citation49,Citation51) provide insufficient reassurance for broader use. Pending access to measures of the haemostatic effects of new OACs, it might be prudent for patients taking such drugs not to be routinely given thrombolysis, in the event that they present with an AIS. Resumption of OACs is possible for most patients with AIS, specifically when the risk of a recurrent cardioembolic event exceeds that of HT. A time-frame of 3–21 days is generally suggested, with the specific time that an OAC is started within that range being dependent on the neuroimaging and clinical findings for a given patient (Citation49). Note that initiation of a new OAC might be relatively delayed compared with warfarin, given their more rapid onset of action. Indeed, if an OAC is to be restarted, a new OAC might even be preferred, given animal studies suggesting that they might be associated with a lower risk of HT compared with warfarin (Citation52–54). As for patients surviving an ICH during OAC treatment, it is uncertain whether any antithrombotic therapy, VKA, new OAC, or antiplatelet should be restarted. Neurologist consultation should be sought as needed.
Management of patients with atrial fibrillation and coronary artery disease
The case-study patient has a prior history of coronary artery disease (CAD), which is present in 28%–36% of patients with AF (Citation55,Citation56); and this combination is associated with increased hospitalization, 30-day mortality, and 1-year mortality rates (Citation57). Separately, AF manifests in 2%–21% of patients presenting with an acute coronary syndrome (ACS), and the combination of AF with myocardial infarction (MI) has especially adverse prognostic implications (Citation58). The prevalence of both AF and CAD increases with age, and the two conditions require antithrombotic therapy; specifically, an OAC for AF and most typically single or dual antiplatelet therapy for CAD. However, combining OACs with antiplatelet drugs increases the risk of major and fatal bleeding (Citation59). There has yet to be a major randomized controlled trial that has addressed antithrombotic therapy for patients with AF and CAD, although PIONEER-AF PCI will compare the safety of two rivaroxaban treatment strategies and a dose-adjusted VKA treatment strategy after percutaneous coronary intervention (PCI; with stent placement) in subjects with non-valvular AF, along with various antiplatelet regimens (Citation60).
In patients with AF who present with acute MI, guidelines list OAC use as a relative contraindication to thrombolysis (Citation61,Citation62). If possible, primary PCI, ideally using a radial approach, is the preferred strategy to reduce the risk of bleeding (Citation63). Thrombolysis is associated with an increased risk of bleeding, specifically in older patients and those with a higher INR (Citation64). Given that thrombolysis with alteplase can be considered in ischaemic stroke in a patient on warfarin whose INR is < 1.7, perhaps this can serve as a cut-off point at which to weigh the risks versus benefits of proceeding with tenecteplase in MI in such patients. No clear direction exists with regard to the patient presenting with acute MI on a new OAC (Citation49). Whatever the acute treatment, the OAC should be discontinued, the patient bridged to parenteral anticoagulation as per local protocols, and dual antiplatelet therapy started. Thereafter, guidelines recommend triple therapy—the combination of an OAC and dual antiplatelet therapy (ASA and clopidogrel)—for patients with AF and ACS or stent placement (Citation11,Citation65). It may be possible to reduce the risk of bleeding without increasing thromboembolic complications in patients with AF by restricting triple therapy to those at higher stroke risk and using dual antiplatelet therapy for those at a lower stroke risk (CHADS2 ≤ 1 or CHA2DS2-VASc ≤ 1) (Citation66). Another option is the use of warfarin and clopidogrel without ASA; however, this is based on a small trial of patients on long-term OAC requiring PCI (WOEST) (Citation67) and has therefore not been widely adopted. For now, triple therapy seems best limited to warfarin (with, additionally, a lower target INR of 2.0–2.5) plus ASA and clopidogrel; we currently have no randomized controlled trial data to support the substitution of warfarin with a new OAC, or clopidogrel with prasugrel or ticagrelor. Although there have been several trials examining the use of new OACs in ACS (Citation68), all of these specifically excluded patients with AF; in the only trial in which a potential benefit of a new OAC existed in patients with ACS (with rivaroxaban, in ATLAS ACS-TIMI 46) (Citation69), the dose of drug used was substantially lower than recommended for stroke prevention in AF (rivaroxaban 2.5 mg bid in ACS versus 15 mg or 20 mg od in stroke prevention in patients with AF). Again, PIONEER-AF PCI will be insightful because it will additionally compare the safety of rivaroxaban and a VKA in conjunction with various antiplatelet regimens and drugs, including ticagrelor and prasugrel (Citation60).
Whatever the antithrombotic regimen of combined OAC and antiplatelet agent(s) ultimately chosen for the patient with AF and ACS, its duration should be minimized. Afterwards, there should be prompt transitioning to a single antithrombotic agent (generally an OAC alone) for long-term stroke prevention when possible, essentially once the condition has been considered stable. Our patient () has stable CAD and is therefore at an increased risk of MI. The efficacy of warfarin, by itself, for secondary prevention of CAD, has been confirmed previously (Citation70). The new OACs have not as yet been specifically assessed with respect to their benefit in the secondary prevention of CAD, although one large trial addressing this medical need is underway (Rivaroxaban for the Prevention of Major Cardiovascular Events in Coronary or Peripheral Artery Disease [COMPASS]) (Citation71). Nonetheless, it has been assumed that the new OACs are more or less interchangeable in terms of providing adequate protection, in lieu of warfarin, for patients with AF and stable CAD; international guidelines suggest as much (Citation11,Citation19), although academic debate persists as to whether the Factor Xa inhibitors might be preferred over dabigatran. Specifically, initial subgroup analysis of the RE-LY data suggested that dabigatran therapy might be associated with an elevated risk of MI, with risks of 0.72% and 0.74% with dabigatran 110 mg and 150 mg bid, respectively, compared with 0.53% with warfarin (P = 0.07 and P = 0.048, respectively) (Citation9). A detailed analysis of the RE-LY trial suggests that, compared with warfarin, the benefit of dabigatran on stroke reduction and bleeding outweighs the potential increased risk of MI (Citation30). Additionally, it was demonstrated in a revised sub-analysis that there was no significant increase in MI with dabigatran compared with warfarin; the rates were 0.82% and 0.81% with dabigatran 110 mg and 150 mg bid, respectively, compared with 0.64% with warfarin (P = 0.09 and P = 0.12, respectively) (Citation9). Debate persists around whether dabigatran protects against MI sufficiently, with systematic reviews of randomized controlled trials of dabigatran (Citation72) as well as a recent Danish cohort study (Citation73) continuing to raise concerns, contrasting with a recent FDA review (Citation74), which noted no increased risk of myocardial ischaemic events with dabigatran in a review of 134,000 Medicare patients. There has not been any concern raised about the safety and use of any of the Factor Xa inhibitors in patients with known CAD. Although there are no randomized control trials to support it, a recent European position statement has proposed that, if a new OAC is to be used in a patient with an ACS or recent PCI, it should be given at the lower tested dose for stroke prevention (that is, dabigatran 110 mg bid, rivaroxaban 15 mg od, or apixaban 2.5 mg bid). The adjuvant antiplatelet treatment should consist only of clopidogrel and/or low-dose aspirin, with none of the new P2Y12 inhibitors, prasugrel or ticagrelor, being used given the likely increased risk of bleeding (Citation75).
Conclusions
The three new OACs have been shown to be at least as effective as warfarin in preventing stroke and systemic embolism, and they are associated with a significantly lower rate of serious bleeding events of the type that particularly concern physicians, such as ICH. Challenges involved in their use persist, as this case-based overview has attempted to highlight; however, many of these challenges also apply to warfarin. There are challenges with the use of any anticoagulant in specific clinical scenarios. Experience with these compounds is increasing, and treatment protocols are emerging to deal with special circumstances such that, on the whole, use of the new OACs is no more complicated than use of warfarin, and is indeed often less so. An interesting point to consider is that warfarin would probably not have been approved if the direct OACs had been established first, given its relative lack of predictable effect, slow onset/offset of action, requirement for close coagulation monitoring and dose adjustment, numerous drug–food and drug–drug interactions, and association with relative increases in ICH and mortality. In conclusion, patients such as those described here will benefit from new OACs, which should be initiated more readily at the earliest opportunity, not only by cardiologists but also (and indeed especially) by the primary care and emergency physicians who are often the ones making the initial diagnosis of AF.
Acknowledgements
The author would like to thank Parveena Laskar, who had funding from Bayer HealthCare Pharmaceuticals and Janssen Scientific Affairs, LLC, for providing editorial assistance.
Declaration of interest: Dr Cox has served on advisory boards for AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Pfizer, and Sanofi-Aventis; has participated in research funded by Bayer, Merck, Pfizer, and Sanofi-Aventis; has served as/is a consultant to the Nova Scotia Department of Health, the New Brunswick Department of Health, the Public Health Agency of Canada, and the Canadian Agency for Drugs and Technologies in Health; is a member of the Canadian Cardiovascular Society's Atrial Fibrillation Guidelines Panel and Chair of its Atrial Fibrillation Quality Indicator Subcommittee.
References
- Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol. 2009; 104:1534–9.
- Stefansdottir H, Aspelund T, Gudnason V, Arnar DO. Trends in the incidence and prevalence of atrial fibrillation in Iceland and future projections. Europace. 2011;13:1110–17.
- Barrios V, Calderon A, Escobar C, de la Figuera M. Patients with atrial fibrillation in a primary care setting: Val-FAAP study. Rev Esp Cardiol. 2012;65:47–53.
- Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e257–354.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–8.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation: a major contributor to stroke in the elderly. The Framingham Study. Arch Intern Med. 1987;147:1561–4.
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–67.
- Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92.
- Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51.
- Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–91.
- Camm AJ, Kirchhof P, Lip GYH, Schotten U, Savelieva I, Ernst S, et al. Guidelines for the management of atrial fibrillation: The Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010;31:2369–429.
- Bristol-Myers Squibb. Coumadin (warfarin sodium) Prescribing Information. 2011. Available at: http://packageinserts.bms.com/pi/pi_coumadin.pdf (accessed 6 October 2014).
- Bristol-Myers Squibb, Pfizer EEIG. Eliquis® (apixaban) Summary of Product Characteristics. 2013. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002148/WC500107728.pdf (accessed 15 July 2014).
- Boehringer Ingelheim International GmbH. Pradaxa® (dabigatran etexilate) Summary of Product Characteristics. 2013. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000829/WC500041059.pdf (accessed 2 May 2014).
- Bayer Pharma AG. Xarelto® (rivaroxaban) Summary of Product Characteristics. 2013. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000944/WC500057108.pdf (accessed 1 July 2014).
- Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–70.
- Lip GYH, Nieuwlaat R, Pisters R, Lane D, Crijns H. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor based approach: The Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263–72.
- January CT, Wann LS, Alpert JS, Calkins H, Cleveland JC Jr, Cigarroa JE, et al. 2014 AHA/ACC/HRS Guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1–76.
- Skanes AC, Healey JS, Cairns JA, Dorian P, Gillis AM, McMurtry MS, et al. Focused 2012 update of the Canadian Cardiovascular Society atrial fibrillation guidelines: recommendations for stroke prevention and rate/rhythm control. Can J Cardiol. 2012;28:125–36.
- You JJ, Singer DE, Howard PA, Lane DA, Eckman MH, Fang MC, et al. Antithrombotic therapy for atrial fibrillation: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141: e531S–75S.
- Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–100.
- Camm AJ, Lip GYH, De Caterina R, Savelieva I, Atar D, Hohnloser SH, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33: 2719–47.
- Pugh D, Pugh J, Mead GE. Attitudes of physicians regarding anticoagulation for atrial fibrillation: a systematic review. Age Ageing. 2011;40: 675–83.
- Devereaux PJ, Anderson DR, Gardner MJ, Putnam W, Flowerdew GJ, Brownell BF, et al. Differences between perspectives of physicians and patients on anticoagulation in patients with atrial fibrillation: observational study. Br Med J. 2001;323:1218–22.
- Rosand J, Eckman MH, Knudsen KA, Singer DE, Greenberg SM. The effect of warfarin and intensity of anticoagulation on outcome of intracerebral hemorrhage. Arch Intern Med. 2004;164:880–4.
- Gomes T, Mamdani MM, Holbrook AM, Paterson JM, Juurlink DN. Persistence with therapy among patients treated with warfarin for atrial fibrillation. Arch Intern Med. 2012;172:1687–9.
- Halvorsen S, Atar D, Yang H, De Caterina R, Erol C, Garcia D, et al. Efficacy and safety of apixaban compared with warfarin according to age for stroke prevention in atrial fibrillation: observations from the ARISTOTLE trial. Eur Heart J. 2014;35:1864–72.
- Eikelboom JW, Wallentin L, Connolly SJ, Ezekowitz M, Healey JS, Oldgren J, et al. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. Circulation. 2011;123:2363–72.
- Halperin JL, Bloomgarden Z, Hellkamp A, Lokhnygina Y, Patel M, Becker R, et al. Rivaroxaban compared with warfarin in patients with atrial fibrillation and diabetes: a subgroup analysis of the ROCKET AF trial. American Heart Association Scientific Sessions 2012. Los Angeles, United States, 3–7 November 2012; Abstract A15544.
- Hohnloser SH, Oldgren J, Yang S, Wallentin L, Ezekowitz M, Reilly P, et al. Myocardial ischemic events in patients with atrial fibrillation treated with dabigatran or warfarin in the RE-LY (Randomized Evaluation of Long-Term Anticoagulation Therapy) trial. Circulation. 2012;125:669–76.
- Hijazi Z, Hohloser SH, Oldgren J, Andersson U, Connolly S, Ezekowitz MD, et al. Efficacy and safety of dabigatran compared with warfarin in relation to baseline renal function in patients with atrial fibrillation - a RE-LY trial analysis. American Heart Association Scientific Sessions 2013. Dallas, USA, 16–20 November 2013; Abstract 13190.
- Gelbricht V, Koehler C, Werth S, Haensel U, Schreier T, Eulitz M, et al. Real life efficacy and safety of rivaroxaban for acute VTE treatment – first results of the Prospective Noac Registry (NCT01588119). Blood (ASH Annual Meeting Abstracts). 2012;120. Abstract 1159.
- Bae JP, Dobesh PP, Klepser DG, Anderson JD, Zagar AJ, McCollam PL, et al. Adherence and dosing frequency of common medications for cardiovascular patients. Am J Manag Care. 2012;18:139–46.
- Francis KM, Siu K, Yu C, Alvrtsyan H, Rao Y, Walker DR, et al. Persistence among patients with non-valvular atrial fibrillation beginning dabigatran or warfarin. Blood (ASH Annual Meeting Abstracts). 2012;120. Abstract 365.
- Werth S, Koehler C, Gelbricht V, Haensel U, Tittl L, Beyer-Westendorf I, et al. Real life efficacy and safety of dabigatran for stroke prevention in atrial fibrillation – first results of the prospective NOAC registry (NCT01588119). Blood (ASH Annual Meeting Abstracts). 2012;120. Abstract 502.
- Kreutz R. A clinical and pharmacological assessment of once-daily versus twice-daily dosing for rivaroxaban. J Thromb Thrombolysis. 2014;38:137–49.
- Heidbuchel H, Verhamme P, Alings M, Antz M, Hacke W, Oldgren J, et al. European Heart Rhythm Association Practical Guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation. Europace. 2013;15:625–51.
- NPS RADAR. Rivaroxaban (Xarelto) for stroke prevention in non- valvular atrial fibrillation. 2012. Available at: http://www.nps.org.au/__data/assets/pdf_file/0020/218144/Rivaroxaban-NVAF.pdf (accessed 17 July 2014).
- Man-Son-Hing M, Nichol G, Lau A, Laupacis A. Choosing antithrombotic therapy for elderly patients with atrial fibrillation who are at risk for falls. Arch Intern Med. 1999;159:677–85.
- Jacobs JM, Stessman J. New anticoagulant drugs among elderly patients is caution necessary?: Comment on “The use of dabigatran in elderly patients”. Arch Intern Med. 2011;171:1287–8.
- Healey JS, Eikelboom J, Douketis J, Wallentin L, Oldgren J, Yang S, et al. Periprocedural bleeding and thromboembolic events with dabigatran compared with warfarin: results from the randomized evaluation of long-term anticoagulation therapy (RE-LY) randomized trial. Circulation. 2012;126:343–8.
- Witt DM, Delate T, Garcia DA, Clark NP, Hylek E, Ageno W, et al. Risk of thromboembolism, recurrent hemorrhage, and death after warfarin therapy interruption for gastrointestinal tract bleeding. Arch Intern Med. 2012;172:1484–91.
- Goodnough LT, Shander A. How I treat warfarin-associated coagulopathy in patients with intracerebral hemorrhage. Blood. 2011;117:6091–9.
- Hankey GJ. Intracranial hemorrhage and novel anticoagulants for atrial fibrillation: what have we learned? Curr Cardiol Rep. 2014;16:480.
- Hagii J, Tomita H, Metoki N, Saito S, Shiroto H, Hitomi H, et al. Characteristics of intracerebral hemorrhage during rivaroxaban treatment: comparison with those during warfarin. Stroke. 2014;45:2805–7.
- Crowther M, Kitt M, Lorenz T, Mathur V, Lu G, Hutchaleelaha A, et al. A phase 2 randomized, double-blind, placebo-controlled trial of PRT064445, a novel, universal antidote for direct and indirect Factor Xa inhibitors. XXIV Congress of the International Society on Thrombosis and Haemostasis. Amsterdam, Netherlands, 29 June–4 July 2013; Abstract AS 20.1.
- Crowther M, Mathur A, Kitt M, Genmin L, Conley PB, Hollenbach S, et al. A phase 2 randomized, double-blind, placebo-controlled trial demonstrating reversal of rivaroxaban-induced anticoagulation in healthy subjects by andexanet alfa (PRT064445), an antidote for FXa inhibitors. Blood (ASH Annual Meeting Abstracts). 2013;122. Abstract 3636.
- Schiele F, van Ryn J, Canada K, Newsome C, Sepulveda E, Park J, et al. A specific antidote for dabigatran: functional and structural characterization. Blood. 2013;121:3554–62.
- Hankey GJ. Unanswered questions and research priorities to optimise stroke prevention in atrial fibrillation with the new oral anticoagulants. Thromb Haemost. 2014;111:808–16.
- Adams HP Jr, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38:1655–711.
- Kawiorski MM, Alonso-Canovas A, de Felipe Mimbrera A, Sainz de la Maza S, Alvarez-Velasco R, Zarza B, et al. Successful intravenous thrombolysis in acute ischaemic stroke in a patient on rivaroxaban treatment. Thromb Haemost. 2014;111:557–8.
- Pfeilschifter W, Bohmann F, Baumgarten P, Mittelbronn M, Pfeilschifter J, Lindhoff-Last E, et al. Thrombolysis with recombinant tissue plasminogen activator under dabigatran anticoagulation in experimental stroke. Ann Neurol. 2012;71:624–33.
- Bohmann F, Mirceska A, Pfeilschifter J, Lindhoff-Last E, Steinmetz H, Foerch C, et al. No influence of dabigatran anticoagulation on hemorrhagic transformation in an experimental model of ischemic stroke. PLoS One. 2012;7:e40804.
- Sun L, Zhou W, Ploen R, Zorn M, Veltkamp R. Anticoagulation with dabigatran does not increase secondary intracerebral haemorrhage after thrombolysis in experimental cerebral ischaemia. Thromb Haemost. 2013;110:153–61.
- Nieuwlaat R, Capucci A, Camm AJ, Olsson SB, Andresen D, Davies DW, et al. Atrial fibrillation management: a prospective survey in ESC member countries: the Euro Heart Survey on Atrial Fibrillation. Eur Heart J. 2005;26:2422–34.
- Nabauer M, Gerth A, Limbourg T, Schneider S, Oeff M, Kirchhof P, et al. The Registry of the German Competence NETwork on Atrial Fibrillation: patient characteristics and initial management. Europace. 2009;11:423–34.
- Hersi A, Alhabib KF, Alsheikh-Ali AA, Sulaiman K, Alfaleh HF, Alsaif S, et al. Prognostic significance of prevalent and incident atrial fibrillation among patients hospitalized with acute coronary syndrome: Findings from the Gulf RACE-2 Registry. Angiology. 2012;63:466–71.
- Schmitt J, Duray G, Gersh BJ, Hohnloser SH. Atrial fibrillation in acute myocardial infarction: a systematic review of the incidence, clinical features and prognostic implications. Eur Heart J. 2009;30:1038–45.
- Sørensen R, Gislason G. Triple antithrombotic therapy: risky but sometimes necessary. Rev Esp Cardiol (Engl Ed). 2014;67:171–5.
- Janssen Scientific Affairs L. A study exploring two strategies of rivaroxaban (JNJ39039039; BAY-59-7939) and one of oral vitamin K antagonist in patients with atrial fibrillation who undergo percutaneous coronary intervention (PIONEER AF-PCI). 2014. Available at: http://clinicaltrials.gov/ct2/show/NCT01830543 (accessed 28 July 2014).
- O’Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e78–140.
- Steg PG, James SK, Atar D, Badano LP, Lundqvist CB, Borger MA, et al. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC). Eur Heart J. 2012;33:2569–619.
- Lip GYH, Huber K, Andreotti F, Arnesen H, Airaksinen KJ, Cuisset T, et al. Management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous coronary intervention/stenting. Thromb Haemost. 2010; 103:13–28.
- Stanley AG, Fletcher S, Tan A, Barnett DB. Is warfarin a contraindication to thrombolysis in acute ST elevation myocardial infarction? Heart. 2006;92:1145–6.
- Lip GYH, Huber K, Andreotti F, Arnesen H, Airaksinen JK, Cuisset T, et al. Antithrombotic management of atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing coronary stenting: executive summary—a Consensus Document of the European Society of Cardiology Working Group on Thrombosis, endorsed by the European Heart Rhythm Association (EHRA) and the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2010;31:1311–18.
- Paikin JS, Wright DS, Crowther MA, Mehta SR, Eikelboom JW. Triple antithrombotic therapy in patients with atrial fibrillation and coronary artery stents. Circulation. 2010;121:2067–70.
- Dewilde WJ, Oirbans T, Verheugt FW, Kelder JC, de Smet BJ, Herrman JP, et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet. 2013;381:1107–15.
- Obonska K, Navarese EP, Lansky A, Tarantini G, Rossini R, Kozinski M, et al. Low-dose of oral Factor Xa inhibitors in patients with a recent acute coronary syndrome: a systematic review and meta-analysis of randomized trials. Atherosclerosis. 2013;229:482–8.
- Mega JL, Braunwald E, Mohanavelu S, Burton P, Poulter R, Misselwitz F, et al. Rivaroxaban versus placebo in patients with acute coronary syndromes (ATLAS ACS-TIMI 46): a randomised, double-blind, phase II trial. Lancet. 2009;374:29–38.
- Hurlen M, Abdelnoor M, Smith P, Erikssen J, Arnesen H. Warfarin, aspirin, or both after myocardial infarction. N Engl J Med. 2002;347:969–74.
- ClinicalTrials.gov. A study exploring two strategies of rivaroxaban and one of oral vitamin K antagonist in patients with atrial fibrillation who undergo percutaneous coronary intervention (PIONEER AF-PCI). 2013. Available at: http://clinicaltrials.gov/ct2/show/NCT01830543 (accessed 26 September 2014).
- Mak KH. Coronary and mortality risk of novel oral antithrombotic agents: a meta-analysis of large randomised trials. BMJ Open. 2012;2:e001592.
- Larsen TB, Rasmussen LH, Gorst-Rasmussen A, Skjøth F, Rosenzweig M, Lane DA, et al. Myocardial ischemic events in ‘real world’ patients with atrial fibrillation treated with dabigatran or warfarin. Am J Med. 2014;127:329–36.
- Food and Drug Administration. FDA Drug Safety Communication: FDA study of Medicare patients finds risks lower for stroke and death but higher for gastrointestinal bleeding with Pradaxa (dabigatran) compared to warfarin. 2014. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm396470.htm (accessed 16 July 2014).
- Lip GYH, Windecker S, Huber K, Kirchhof P, Marin F, Ten Berg JM, et al. Management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous coronary or valve interventions: a joint consensus document of the European Society of Cardiology Working Group on Thrombosis, European Heart Rhythm Association (EHRA), European Association of Percutaneous Cardiovascular Interventions (EAPCI) and European Association of Acute Cardiac Care (ACCA) endorsed by the Heart Rhythm Society (HRS) and Asia-Pacific Heart Rhythm Society (APHRS). Eur Heart J. 2014;35:3155–79.
- Weitz JI, Gross PL. New oral anticoagulants: which one should my patient use? Hematology Am Soc Hematol Educ Program. 2012;2012: 536–40.
