Abstract
Introduction. This pooled analysis investigated the effects of gabapentin enacarbil (GEn) on clinical correlates of sleep disturbance in adults with moderate-to-severe primary restless legs syndrome (RLS) and no-to-moderate or severe-to-very severe baseline sleep disturbance.
Methods. Co-primary end-points were mean change from baseline to week 12 in International Restless Legs Scale (IRLS) total score and proportion of responders (‘much’/‘very much’ improved) on the investigator-rated Clinical Global Impression–Improvement (CGI-I) scale (week 12). Pain, mood, individual IRLS items, and safety were assessed.
Results. The modified intent-to-treat population was 671 adults randomized to GEn 600 mg (n = 161), GEn 1200 mg (n = 266), or placebo (n = 244). GEn significantly improved least squares mean change in IRLS total score from baseline versus placebo for no-to-moderate (GEn 600 mg,− 12.3; 1200 mg, − 11.3; placebo, − 7.7) and severe-to-very severe (− 16.6; − 17.0; − 12.7) groups (all P < 0.01). Significantly more GEn-treated patients (both doses) were CGI-I responders (week 12) versus placebo in both sleep subgroups (all P < 0.01). GEn substantially improved mood and pain scores for both sleep subgroups versus placebo. The most frequent treatment-emergent adverse events were somnolence and dizziness.
Conclusion. GEn (600 mg and 1200 mg) was effective and well tolerated in adults with moderate-to-severe primary RLS regardless of baseline sleep disturbance level.
GEn (600 mg and 1200 mg) was effective and well tolerated in adult patients with moderate-to-severe primary RLS who reported either no-to-moderate or severe-to-very severe baseline sleep disturbances.
RLS symptoms, mood, and pain scores, as well as sleep quality and quantity, improved significantly in these patient subgroups.
While sleep disturbance is the most common complaint in RLS, other clinical manifestations, including pain, mood, and impact on quality of life, should be considered.
Introduction
Restless legs syndrome (RLS), also known as Willis–Ekbom disease, is a common neurological disorder characterized by an irresistible urge to move the legs and is usually associated with unpleasant sensations in the legs. These symptoms arise or worsen during rest and in the evening; they are temporarily relieved by movement (Citation1,Citation2). Approximately 2% to 3% of the world's adult population suffers from primary moderate-to-severe RLS, and women are twice as likely as men to be affected by this disease (Citation3–9). RLS occurs more often in white North American and Northern European populations than in African, Asian, and Southeastern European populations (Citation9,Citation10).
Patients with RLS frequently experience limb dysesthesias, which results in delayed sleep onset and reduced sleep quality (Citation1,Citation2,Citation11,Citation12). Sleep complaints are the most common reason that patients seek treatment, and sleep disturbance can significantly compromise mental and physical health, thereby affecting patients’ overall quality of life (Citation7). Along with sleep impairments, many patients with RLS report great psychological discomfort, which can lead to distress and psychiatric illness (Citation13). Several population-based studies have found that symptoms of anxiety, depression, and panic disorder are significantly greater among patients with RLS than in control subjects (Citation14–16). In addition, patients with RLS frequently describe their dysesthesias as being painful, which can further reduce their quality of life (Citation3,Citation17).
Currently, the alpha-2-delta ligand gabapentin enacarbil (GEn), as well as the dopamine agonists (DAs) ropinirole, pramipexole, and rotigotine, are approved by the United States Food and Drug Administration to treat adult patients with moderate-to-severe primary RLS (Citation2). However, dopamine agonists can be associated with intolerable adverse events (AEs), including impulse control disorders and augmentation (Citation18–21). Augmentation is a paradoxical earlier onset and increase in the intensity and duration of RLS symptoms during which symptoms also spread to other parts of the body. Augmentation has been reported for 7% to 50% of patients with RLS following DA treatment (Citation18,Citation22). Treatment with a DA is also associated with symptoms of withdrawal and an increase in RLS symptoms after treatment is tapered off, as well as the need for larger doses to maintain the treatment effect (tolerance or augmentation). While a patient is still on DA treatment, recurrence and worsening of symptoms can occur as the effects of the medication wear off between doses, particularly in the morning (early morning rebound) (Citation23,Citation24). Treatment guidelines suggest that alternative agents, including alpha-2-delta ligands, may benefit patients who experience DA-related adverse events (Citation2,Citation25,Citation26).
GEn is an actively transported prodrug of gabapentin that is absorbed by high-capacity nutrient transporters in the small and large intestines. After absorption, GEn is rapidly and extensively hydrolyzed to gabapentin, thereby providing predictable and sustained gabapentin exposure (Citation27,Citation28). In the United States, GEn is approved at a dose of 600 mg once daily for the treatment of adults with moderate-to-severe primary RLS (Citation29). In three well-controlled, multicenter, randomized, double-blind, placebo-controlled, 12-week studies of adults with moderate-to-severe primary RLS, GEn significantly improved RLS symptoms, compared with placebo, and was well tolerated (XP052, XP053, XP081) (Citation30–32).
GEn treatment has improved pain and mood in adult patients with primary moderate-to-severe RLS. In a phase IIb, double-blind, randomized, placebo-controlled, 14-day trial (XP045), mood and pain at day 14 were improved in adult patients with moderate-to-severe primary RLS who received GEn 1200 mg compared with those who received placebo (Citation33). RLS pain was improved with GEn 600 mg in studies XP053 and XP081 and with GEn 1200 mg in studies XP052, XP053, and XP081 (Citation30–32). In study XP081, patients who were treated with GEn (600 mg, 1200 mg, 1800 mg, and 2400 mg) had better improvements in scores for Profile of Mood States (POMS) total mood and all POMS subscale domains compared with placebo at week 12. In addition, the proportion of patients who reported that their mood was ‘much improved’ or ‘very much improved’ from baseline was numerically greater in all GEn treatment groups compared with placebo (Citation32). GEn has previously been shown to improve subjective sleep outcomes in subjects with moderate-to-severe primary RLS, regardless of the severity of their sleep disturbance at baseline (Citation34). Here we build on these findings by investigating the effects of GEn treatment on clinical correlates of sleep disturbance. Using a pooled analysis of studies XP052, XP053, and XP081, we focus on patient subgroups with no-to-moderate or severe-to-very severe sleep disturbance at baseline.
Materials and methods
Study design and patients
The study designs and patient populations of XP052, XP053, and XP081 were described previously (ClinicalTrials.gov: NCT00298623, NCT00365352, and NCT01332305) (Citation30–32). Briefly, these were double-blind, randomized, placebo-controlled studies that enrolled adult patients with a diagnosis of moderate-to-severe primary RLS, according to the International Restless Legs Syndrome Study Group diagnostic criteria (Citation1). Eligible patients were ≥ 18 years and had RLS symptoms for ≥ 15 days during the month before screening (or, if on treatment, similar symptom frequency to that seen prior to treatment initiation), symptoms for at least 4 of 7 nights during the baseline period, and an International Restless Legs Scale (IRLS) total score of at least 15 at the beginning and end of the baseline period. Patients were excluded if they had secondary RLS, iron deficiency, body mass index ≥ 34 kg/m2, moderate to severe depression, a sleep or movement disorder other than RLS, a neurological disease, or were pregnant.
For this analysis, data from the three primary studies were pooled for each treatment group: placebo (XP052, XP053, XP081), GEn 600 mg (XP053, XP081), and GEn 1200 mg (XP052, XP053, XP081). Within the pooled populations, patients were divided into two subgroups on the basis of their baseline level of sleep disturbance, which was assessed according to the score (0–4) they provided for item 4 of the IRLS: no-to-moderate sleep disturbance (score of 0–2) or severe-to-very severe sleep disturbance (score of 3–4). All patients provided written informed consent prior to participation. The study was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. The protocol was reviewed and approved by a local or regional institutional review board, depending on study site requirements.
Efficacy and tolerability assessments
The co-primary end-points of the original parent studies were mean change of the IRLS total score from baseline to week 12 and the proportion of responders (rated ‘much’ or ‘very much’ improved) on the investigator-rated Clinical Global Impression– Improvement (CGI-I) scale at week 12 for GEn 1200 mg compared with placebo. Secondary end-points of the parent studies included the effect of GEn 600 mg on change from baseline to week 12 in the IRLS and proportion of responders on the investigator-rated CGI-I. The IRLS consists of 10 questions that assess the intensity and frequency of RLS symptoms such as the need to move and discomfort in the arms and legs, as well as the consequences of RLS, including changes to mood, sleep quality, day-time tiredness, and quality of life (Citation35). The CGI-I scale provides the clinician with an assessment of the patient's global functioning before and after starting medication (Citation36).
To understand better the clinical implications of RLS symptoms and sleep disturbance, we also assessed the average daily RLS pain score, POMS subscale scores, and scores for the IRLS items 4 (severity of sleep disturbance), 9 (daily affairs), and 10 (severity of mood disturbance). The RLS pain score was assessed for 7 days prior to baseline and at predefined study visits using an 11-point numeric pain rating scale. Using this scale, patients were asked to rate their pain from a scale of 0 (no pain) to 10 (most intense pain imaginable). Scores were then averaged over the 7 days. We report least squares (LS) mean changes from baseline per week for the average daily RLS pain score as well as for IRLS scores for items 4, 9, and 10. POMS subscale scores for anger–hostility, confusion–bewilderment, depression–dejection, fatigue–inertia, tension–anxiety, vigor–activity, and total mood disturbance were analyzed as the treatment differences in the change from baseline to each visit. The correlations between POMS subscores and the IRLS scores for items 4, 9, and 10 were also examined. Safety outcomes were assessed.
Statistical analyses
The safety population was composed of all patients who received at least one dose (or portion of a dose) of study medication, and the modified intent-to-treat (mITT) population included all patients in the safety population who also had a baseline IRLS assessment and at least one post-baseline IRLS assessment. To measure the change from baseline for the continuous end-points according to the IRLS, POMS, and RLS pain score, treatment effects were analyzed using mixed-effect models for repeated measures in conjunction with unstructured covariance matrices. These analyses included treatment group, visit day (categorical), and the treatment-by-visit interaction as effects and the baseline score as a covariate. The proportions of CGI-I responders were analyzed using logistic regression models, with treatment as the effect with last observation carried forward for imputation of missing data. Treatments were compared at each day, and analyses were performed by sleep disturbance level at baseline. No adjustment was made for multiple comparisons because all of these analyses were considered exploratory. Correlations between each POMS subscore and the IRLS scores for items 4, 9, and 10 were assessed by Spearman's rank correlation coefficients. These correlations included GEn and placebo groups combined for each sleep subgroup, and were based on results of the change from baseline to week 12. Safety data were summarized for the safety population.
Results
Patients
In the mITT population, 671 adult patients with moderate-to-severe RLS were enrolled and randomized to GEn 600 mg (n = 161), GEn 1200 mg (n = 266), or placebo (n = 244). Of these, 86% (138/161) of patients in the GEn 600-mg group, 86% (229/266) of patients in the GEn 1200-mg group, and 82% (200/244) of patients in the placebo group completed their respective study. At baseline across dose groups, 372 (55%) patients reported no-to-moderate sleep disturbance, and 299 (45%) patients reported severe-to-very severe sleep disturbance on item 4 of the IRLS. Patient demographics and baseline characteristics are shown in . RLS pain and IRLS scores were generally lower for patients with no-to-moderate sleep disturbance than for patients with severe-to-very severe sleep disturbance. Baseline scores for most POMS subscale items were numerically higher in patients with severe-to-very severe sleep disturbance compared with patients with no-to-moderate sleep disturbance ().
Table I. Baseline characteristics of the mITT population.
Table II. Baseline POMS subscale scores of the mITT population.
Efficacy—co-primary end-points
At week 12, the LS mean (standard error [SE]) change from baseline in total IRLS score was significantly greater with GEn 600 mg (− 12.3 [0.79]) and GEn 1200 mg (− 11.3 [0.61]) compared with placebo (− 7.7 [0.65]) in patients with no-to-moderate sleep disturbance (P < 0.001 for both doses compared with placebo). GEn 600 mg and 1200 mg also significantly improved LS mean (SE) change from baseline in IRLS total score for the severe-to-very severe sleep disturbance subgroup (GEn 600 mg, − 16.6 [1.06]; GEn 1200 mg, − 17.0 [0.84]; placebo, − 12.7 [0.88]; P < 0.01 for both doses versus placebo). For both subgroups, the LS mean change from baseline in the IRLS total score significantly improved with GEn 600 mg and 1200 mg at each time point compared with placebo (P < 0.05 for both doses compared with placebo) ().
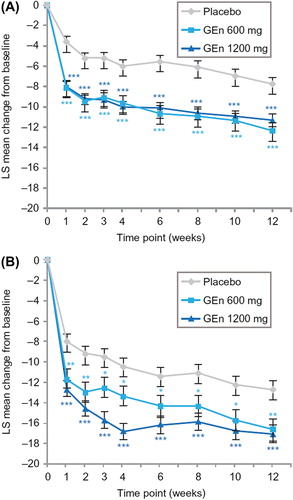
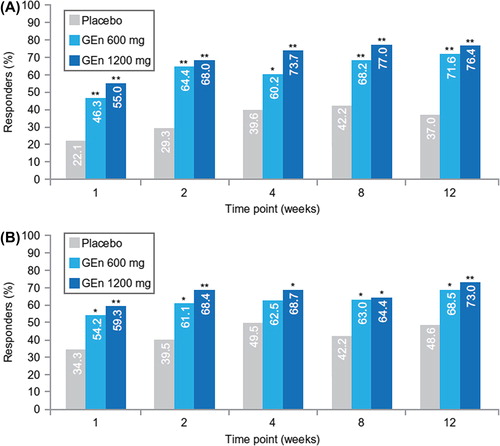
At week 12, the odds ratios for investigator-rated CGI-I responders in the no-to-moderate sleep disturbance subgroup were 4.3 (95% confidence interval [CI] 2.4–7.7; P < 0.001) for the GEn 600-mg group and 5.5 (95% CI 3.3–9.2; P < 0.001) for the GEn 1200-mg group. In the severe-to-very severe sleep disturbance subgroup, the odds ratios at week 12 were 2.3 (95% CI 1.2–4.3; P = 0.009) for the GEn 600-mg group and 2.9 (95% CI 1.6–5.0; P < 0.001) for the GEn 1200-mg group. According to the investigator-rated CGI-I, significantly more patients treated with GEn (600 mg or 1200 mg) were responders at each time point compared with those treated with placebo ().
Efficacy—improvements in scores for pain, mood, and individual items on the IRLS
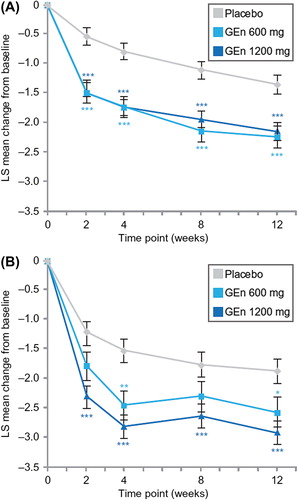
LS mean changes in average daily RLS pain scores improved at all time points from baseline in patients with no-to-moderate sleep disturbance who were treated with either GEn dose compared with those treated with placebo (). In the severe-to-very severe subgroup, GEn 600 mg improved the average daily RLS pain score from baseline to weeks 4 and 12, while improvements were observed at all time points with GEn 1200 mg (). Numerically, these improvements were greater with GEn 1200 mg than with GEn 600 mg at each time point, although the differences were not significant.
Patients in both sleep subgroups experienced significant improvements in mood following treatment with GEn, as assessed by POMS subscale scoring (). For patients with no-to-moderate sleep disturbance at baseline, the LS mean treatment difference at week 12 was greater with both GEn doses than with placebo for depression–dejection and total mood disturbance. Furthermore, GEn 1200 mg improved the LS mean treatment difference for fatigue–inertia and vigor–activity compared with placebo at week 12. GEn 1200 mg significantly improved LS mean treatment differences (SE) for total mood disturbance and vigor–activity in the severe-to-very severe subgroup according to POMS subscale scores; in contrast, GEn 600 mg did not result in significant treatment differences in the same subgroup.
Table III. POMS subscale score treatment differences in the change from baseline to week 12.
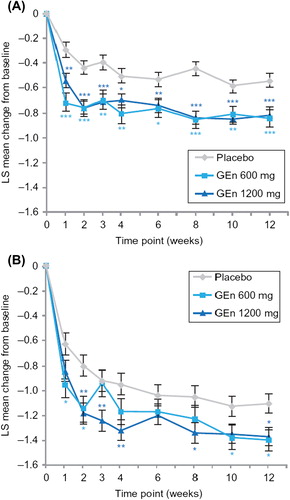
For item 4 (severity of sleep disturbance) of the IRLS, both GEn doses significantly improved LS mean change from baseline at week 12 in both sleep subgroups (). These improvements occurred at all time points assessed for the no-to-moderate sleep disturbance subgroup (). For the severe-to-very severe sleep disturbance subgroup, improvements were observed at the majority of time points ().
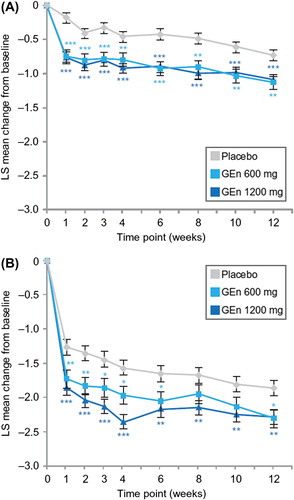
GEn (600 mg or 1200 mg) treatment also significantly improved LS mean change from baseline for individual scores of item 9 (daily affairs) of the IRLS at week 12 in both sleep subgroups (). In addition, patients with no-to-moderate sleep disturbance showed significant improvements in the scoring of item 9 of the IRLS with both GEn doses at all time points examined (). For patients with severe-to-very severe sleep disturbance, GEn 600 mg and GEn 1200 mg significantly improved LS mean change from baseline for the score of item 9 of the IRLS at most time points ().
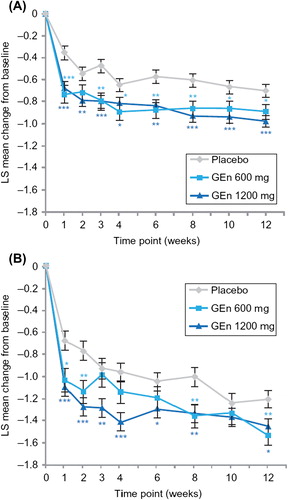
For item 10 (severity of mood disturbance) of the IRLS, GEn 600 mg and 1200 mg significantly improved LS mean change from baseline at week 12 in both sleep subgroups (). For the no-to-moderate sleep disturbance subgroup, these improvements occurred at all time points, with the exception of week 2 for GEn 600 mg (). Among patients with severe-to-very severe sleep disturbance, GEn 600 mg significantly improved LS mean change from baseline in the score of item 10 of the IRLS at several time points; significant improvements occurred at all time points, except week 10 for GEn 1200 mg ().
By combining these data for all treatment groups, correlations were made between POMS subscores and IRLS scores for items 4, 9, and 10. Variables with the highest correlations included total mood disturbance (0.26–0.49), fatigue–inertia (0.26–0.49), confusion–bewilderment (0.20–0.36), and vigor–activity (− 0.22 to − 0.39) for both sleep subgroups. The correlation coefficients generally showed moderately positive (0.40–0.60) or weakly positive (0.20–0.40) correlations (Citation37). Nevertheless, all correlations reached statistical significance (P < 0.05 for all items).
Tolerability
In the pooled analysis, somnolence, dizziness, and headache were the most commonly reported treatment-emergent AEs within both sleep subgroups in the GEn and placebo treatment arms (). Twenty-one patients in the no-to-moderate sleep disturbance subgroup (GEn 600 mg, n = 5 [6%]; GEn 1200 mg, n = 10 [7%]; placebo, n = 6 [4%]) and 23 patients in the severe-to-very severe subgroup (GEn 600 mg, n = 6 [8%]; GEn 1200 mg, n = 13 [11%]; placebo, n = 4 [4%]) withdrew owing to an AE. The majority of AEs were rated as mild or moderate in intensity.
Table IV. Most frequent treatment-emergent adverse events reported in at least 5% of the safety population.
A total of six patients experienced serious AEs (GEn 600 mg, n = 2; GEn 1200 mg, n = 1; placebo, n = 3). None of these events were considered to be treatment-related, and the three patients with serious AEs who had been receiving GEn recovered. In the no-to-moderate sleep disturbance subgroup, one patient in the GEn 600-mg group reported cellulitis. Five patients from the severe-to-very severe subgroup reported serious AEs: herniated disc (GEn 600 mg), worsening cholelithiasis (GEn 1200 mg), appendicitis (placebo), cholelithiasis (placebo), and worsened peripheral arterial disease (placebo).
Discussion
In this pooled analysis of three placebo-controlled studies of 671 adult patients in the mITT population with moderate-to-severe primary RLS, GEn (600 mg and 1200 mg) significantly improved RLS symptoms compared with placebo in subgroups with either no-to-moderate or severe-to-very severe sleep disturbance at baseline. Improvements were observed in both co-primary end-points: mean change from baseline total score of the IRLS and proportion of responders on the investigator-rated CGI-I. Both GEn doses were generally well tolerated during 12 weeks of treatment. The most commonly reported treatment-emergent AEs were somnolence, dizziness, and headache, and most AEs were rated as mild or moderate in intensity.
RLS-related pain has been reported as one of the most bothersome symptoms of RLS (Citation3) and can be the main symptom that leads patients to see a physician (Citation7). Though the intensity of the pain varies, it is of note that, in our analyses, patients in both sleep subgroups had an average baseline pain score of approximately 4 points. In a randomized trial that investigated the efficacy and tolerability of GEn in patients with post-herpetic neuralgia, patients were required to have a minimum pain intensity numeric scale score of at least 4 to qualify for study participation (Citation38). As the same scale was used in these RLS trials, a pain score of 4 would be considered significant for RLS sufferers as well. Pain can greatly impact a patient's perception of his or her disease and quality of life. Thus pain control should also be considered an important aspect of managing RLS. Our analyses showed that GEn effectively reduced pain scores across treatment groups and levels of sleep disturbance.
Significant improvements in mood disturbances were observed with GEn 600 mg and 1200 mg compared with placebo for patients with or without severe sleep disturbance. Our analysis also found that patients with severe-to-very severe sleep disturbance had clinical issues with mood compared to the no- to-moderate sleep disturbance subgroup at baseline. Furthermore, patients with severe-to-very severe sleep disturbance had incrementally worse RLS symptoms with a greater impact on other clinical manifestations.
In general, improvements with GEn compared with placebo in average daily RLS pain score, POMS scores, and IRLS scores for items 4, 9, and 10 were numerically greater in the no-to-moderate sleep disturbance subgroup than in the severe-to-very severe subgroup. Patients with no-to-moderate sleep disturbance who were treated with GEn 600 mg showed significant improvements at all time points examined for average daily RLS pain score, as well as for IRLS scores for items 4, 9, and 10 (except at week 2 for item 10 of the IRLS). At week 12, depression–dejection and total mood disturbance from the POMS scale also improved significantly following treatment with GEn 600 mg. For patients with no-to-moderate sleep disturbance treated with GEn 1200 mg, there were significant improvements at all time points examined for average daily RLS pain score and for IRLS scores for items 4, 9, and 10; several POMS items also improved significantly. Patients with severe-to-very severe sleep disturbance treated with GEn 600 mg showed significant improvements at week 12 and some earlier time points for average daily RLS pain score and for IRLS scores for items 4, 9, and 10; significant improvements were not observed for POMS items. For patients with severe-to-very severe sleep disturbance treated with GEn 1200 mg, significant improvements occurred at all time points for average daily RLS pain score and at most time points for IRLS scores for items 4, 9, and 10; several POMS items also improved significantly at week 12. These results demonstrate that GEn can improve pain and mood outcomes, even in patients with greatly impaired sleep, and highlight the need for clinicians to recognize, quantify, and manage the impact of RLS on these clinical manifestations of RLS.
Within the severe-to-very severe sleep disturbance subgroup, GEn 1200 mg showed numerically greater treatment effects compared with GEn 600 mg for these end-points. For average daily RLS pain score and IRLS item 4, statistically significant changes from baseline were observed with GEn 1200 mg versus placebo at all time points, whereas some of these time points did not show significant changes with GEn 600 mg versus placebo. For IRLS items 9 and 10, significant changes from baseline occurred at more time points with GEn 1200 mg versus placebo than with GEn 600 mg versus placebo. However, the comparison between GEn 600 mg and 1200 mg was not the primary comparison of interest for this analysis. Importantly, this analysis was not powered to detect treatment differences between GEn 600 mg and 1200 mg. In addition, although GEn 600 mg is currently the only dose approved by the United States Food and Drug Administration for the treatment of moderate-to-severe primary RLS in adults (Citation29), our results suggest that GEn 1200 mg could be an appropriate dose to consider in some cases.
Among the validated scales used to quantify RLS severity, the IRLS is currently considered the gold standard (Citation39). In addition to assessing primary RLS diagnostic features, the IRLS also includes questions regarding daily affairs (item 9) and mood disturbance (item 10), a feature that distinguishes this scale from comparator scales, such as the RLS-6 and the Johns Hopkins RLS severity scale (Citation35,Citation39). Responses to these items are clinically relevant, as patients with RLS experience overall reduced quality of life and higher rates of depression, anxiety, and other mood disturbances compared with controls (Citation1,Citation11,Citation14,Citation15). Although clinical trials have reported improvements in primary diagnostic features assessed by the IRLS following various treatments for RLS (Citation31,Citation40–43), there is little evidence to show improvements in mood and daily functioning after treatment. In our analysis, GEn 600 mg and 1200 mg significantly improved daily affairs and the severity of mood disturbance in both sleep subgroups. These data suggest GEn can effectively treat the day-time sleep, pain, and mood-related disturbances of RLS regardless of sleep disturbance severity.
Our findings are consistent with previous studies that have investigated the effectiveness of GEn in improving RLS-related quality-of-life outcomes. For example, a phase III, placebo-controlled, randomized withdrawal study (XP060) of GEn in adult patients with moderate-to-severe primary RLS showed improvements in the RLS quality-of-life overall impact score relative to baseline (Citation44). A phase III, randomized, double-blind polysomnography study showed that GEn 1200 mg significantly reduced wake time during sleep and periodic limb movements associated with arousal compared with placebo (Citation45). In a pooled analysis of studies XP052 and XP053, adult patients with moderate-to-severe primary RLS experienced improvements in sleep outcomes with GEn treatment regardless of baseline sleep disturbance severity (Citation34). Our analysis further expands upon these findings by demonstrating improvements in mood and pain outcomes following GEn treatment in adult patients with either no-to-moderate or severe-to-very severe sleep disturbance.
This pooled analysis rigorously examined the effects of GEn on mood and pain outcomes in adult patients with moderate-to-severe primary RLS who had varying levels of sleep disturbance. As this was not a formal meta-analysis, additional covariates, including site effect, study effect, and other baseline variables, were not examined. As noted previously, this analysis was not powered to compare the GEn 600-mg and 1200-mg doses. This analysis did not include RLS quality-of-life or Medical Outcomes Study data, as these outcomes were not assessed in all three studies. The categorization of patients into no-to-moderate or severe-to-very severe sleep subgroups was based on subjective measures (item 4 of the IRLS), but further examination of potential correlations between day-time functioning and objectively assessed sleep impairments could prove useful.
In summary, GEn (600 mg and 1200 mg) was effective and well tolerated in adult patients with moderate-to-severe primary RLS who reported either no-to-moderate or severe-to-very severe baseline sleep disturbance. RLS symptoms, mood, and pain scores, as well as sleep quality and quantity, improved significantly in these patient subgroups. Given the importance of improving day-time function, pain, and mood outcomes in patients with RLS, our analysis can assist clinicians in understanding the potential benefit of treatment with GEn in patients with or without severe sleep disturbance resulting from RLS symptoms.
Declaration of interest: R.K.B. reports the following disclosures: shareholder, Chief Medical Officer, and employee of SleepMed Inc.; Board of Directors of SleepMed Inc., First Community Corporation, SC and National Sleep Foundation; consultant to Aerial, Jazz, ApniCure, UCB, Teva; Speakers Bureau for Teva, Jazz, UCB, and XenoPort, Inc.; industry-funded research for GSK, Jazz, Vanda, Merck, Novo Nordisk, Pfizer, XenoPort, Eisai, Philips, ResMed, Apnicure, Sensory Medical, J&J, Apnex, and Fisher Paykel. D.O.L. is a consultant to GSK, Lundbeck, and XenoPort, Inc. M.J.B. is consultant and speaker for XenoPort, Inc. and UCB Pharma, and conducts research for Xenon. M.J.J. is a paid consultant of XenoPort, Inc. R.K. and G.S. are employees of and own stock in XenoPort, Inc. These studies and this analysis were conducted by XenoPort, Inc., Santa Clara, CA, USA. Medical writing support was provided by Sachi Yim from CodonMedical, an Ashfield Company, and was funded by XenoPort, Inc.
References
- Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisir J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–19.
- Garcia-Borreguero D, Kohnen R, Silber MH, Winkelman JW, Earley CJ, Högl B, et al. The long-term treatment of restless legs syndrome/Willis-Ekbom disease: evidence-based guidelines and clinical consensus best practice guidance: a report from the International Restless Legs Syndrome Study Group. Sleep Med. 2013;14:675–84.
- Allen RP, Walters AS, Montplaisir J, Hening W, Myers A, Bell TJ, et al. Restless legs syndrome prevalence and impact: REST general population study. Arch Intern Med. 2005;165:1286–92.
- Allen RP, Stillman P, Myers AJ. Physician-diagnosed restless legs syndrome in a large sample of primary medical care patients in western Europe: prevalence and characteristics. Sleep Med. 2010;11: 31–7.
- Berger K. What is clinically significant RLS and who decides about its treatment? Sleep Med. 2010;11:9–10.
- Allen RP, Bharmal M, Calloway M. Prevalence and disease burden of primary restless legs syndrome: results of a general population survey in the United States. Mov Disord. 2011;26:114–20.
- Hening W, Walters AS, Allen RP, Montplaisir J, Myers A, Ferini-Strambi L. Impact, diagnosis and treatment of restless legs syndrome (RLS) in a primary care population: the REST (RLS epidemiology, symptoms, and treatment) primary care study. Sleep Med. 2004;5:237–46.
- Berger K, Luedemann J, Trenkwalder C, John U, Kessler C. Sex and the risk of restless legs syndrome in the general population. Arch Intern Med. 2004;164:196–202.
- Ohayon MM, O’Hara R, Vitiello MV. Epidemiology of restless legs syndrome: a synthesis of the literature. Sleep Med Rev. 2012;16: 283–95.
- Innes KE, Selfe TK, Agarwal P. Prevalence of restless legs syndrome in North American and Western European populations: a systematic review. Sleep Med. 2011;12:623–34.
- Nagandla K, De S. Restless legs syndrome: pathophysiology and modern management. Postgrad Med J. 2013;89:402–10.
- Reese JP, Stiasny-Kolster K, Oertel WH, Dodel RC. Health-related quality of life and economic burden in patients with restless legs syndrome. Expert Rev Pharmacoecon Outcomes Res. 2007;7:503–21.
- Scholz H, Benes H, Happe S, Bengel J, Kohnen R, Hornyak M. Psychological distress of patients suffering from restless legs syndrome: a cross-sectional study. Health Qual Life Outcomes. 2011;9:73.
- Sevim S, Dogu O, Kaleagasi H, Aral M, Metin O, Camdeviren H. Correlation of anxiety and depression symptoms in patients with restless legs syndrome: a population based survey. J Neurol Neurosurg Psychiatry. 2004;75:226–30.
- Lee HB, Hening WA, Allen RP, Kalaydjian AE, Earley CJ, Eaton WW, et al. Restless legs syndrome is associated with DSM-IV major depressive disorder and panic disorder in the community. J Neuropsychiatry Clin Neurosci. 2008;20:101–5.
- Cho SJ, Hong JP, Hahm BJ, Jeon HJ, Chang SM, Cho MJ, et al. Restless legs syndrome in a community sample of Korean adults: prevalence, impact on quality of life, and association with DSM-IV psychiatric disorder. Sleep. 2009;32:1069–76.
- Hornyak M, Sohr M, Busse M; 604 and 615 Study Groups. Evaluation of painful sensory symptoms in restless legs syndrome: experience from two clinical trials. Sleep Med. 2011;12:186–9.
- Ondo W, Romanyshyn J, Vuong KD, Lai D. Long-term treatment of restless legs syndrome with dopamine agonists. Arch Neurol. 2004;61:1393–7.
- Winkelman JW, Johnston L. Augmentation and tolerance with long-term pramipexole treatment of restless legs syndrome (RLS). Sleep Med. 2004;5:9–14.
- Cornelius JR, Tippmann-Peikert M, Slocumb NL, Frerichs CF, Silber MH. Impulse control disorders with the use of dopaminergic agents in restless legs syndrome: a case-control study. Sleep. 2010; 33:81–7.
- Moore TJ, Glenmullen J, Mattison DR. Reports of pathological gambling, hypersexuality, and compulsive shopping associated with dopamine receptor agonist drugs. JAMA Intern Med. 2014;174: 1930–3.
- Allen RP, Ondo WG, Ball E, Calloway MO, Manjunath R, Higbie RL, et al. Restless legs syndrome (RLS) augmentation associated with dopamine agonist and levodopa usage in a community sample. Sleep Med. 2011;12:431–9.
- Benbir G, Guilleminault C. Pramipexole: new use for an old drug—the potential use of pramipexole in the treatment of restless legs syndrome. Neuropsychiatr Dis Treat. 2006;2:393–405.
- Nirenberg MJ. Dopamine agonist withdrawal syndrome: implications for patient care. Drugs Aging. 2013;30:587–92.
- Garcia-Borreguero D, Stillman P, Benes H, Buschmann H, Chaudhuri KR, Gonzalez Rodríguez VM, et al. Algorithms for the diagnosis and treatment of restless legs syndrome in primary care. BMC Neurol. 2011; 11:28.
- Silber MH, Becker PM, Earley C, Garcia-Borreguero D, Ondo WG; Medical Advisory Board of the Willis-Ekbom Disease Foundation. Willis-Ekbom Disease Foundation revised consensus statement on the management of restless legs syndrome. Mayo Clin Proc. 2013;88: 977–86.
- Cundy KC, Annamalai T, Bu L, De Vera J, Estrela J, Luo W, et al. XP13512 [(+/-)-1-([(alpha-isobutanoyloxyethoxy)carbonyl] aminomethyl)-1-cyclohexane acetic acid], a novel gabapentin prodrug: II. Improved oral bioavailability, dose proportionality, and colonic absorption compared with gabapentin in rats and monkeys. J Pharmacol Exp Ther. 2004; 311:324–33.
- Cundy KC, Sastry S, Luo W, Zou J, Moors TL, Canafax DM. Clinical pharmacokinetics of XP13512, a novel transported prodrug of gabapentin. J Clin Pharmacol. 2008;48:1378–88.
- HORIZANT US [2013 prescribing information]. Available at: http://www.horizant.com/docs/Horizant_PrescribingInformation.pdf (accessed 23 January 2014).
- Kushida CA, Becker PM, Ellenbogen AL, Canafax DM, Barrett RW; XP052 Study Group. Randomized, double-blind, placebo-controlled study of XP13512/GSK1838262 in patients with RLS. Neurology. 2009;72:439–46.
- Lee DO, Ziman RB, Perkins AT, Poceta JS, Walters AS, Barrett RW; XP053 Study Group. A randomized, double-blind, placebo-controlled study to assess the efficacy and tolerability of gabapentin enacarbil in subjects with restless legs syndrome. J Clin Sleep Med. 2011;7:282–92.
- Lal R, Ellenbogen A, Chen D, Zomorodi K, Atluri H, Luo W, et al. A randomized, double-blind, placebo-controlled, dose-response study to assess the pharmacokinetics, efficacy, and safety of gabapentin enacarbil in subjects with restless legs syndrome. Clin Neuropharmacol. 2012;35:165–73.
- Walters AS, Ondo WG, Kushida CA, Becker PM, Ellenbogen AL, Canafax DM, et al.;XP045 Study Group Gabapentin enacarbil in restless legs syndrome: a phase 2b, 2-week, randomized, double-blind, placebo-controlled trial. Clin Neuropharmacol. 2009;32:311–20.
- Bogan RK, Ellenbogen A, Becker PM, Kushida C, Ball E, Ondo WG, et al. Gabapentin enacarbil in subjects with moderate to severe primary restless legs syndrome with and without severe sleep disturbance: an integrated analysis of subjective and novel sleep endpoints from two studies. J Parkinsonism Restless Legs Syndr. 2013;3:31–40.
- Walters AS, LeBrocq C, Dhar A, Hening W, Rosen R, Allen RP, et al.; International Restless Legs Syndrome Study Group. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 2003;4:121–32.
- Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont). 2007;4:28–37.
- Dancey CP, Reidy J. Statistics without maths for psychology: Using SPSS for Windows, 3rd ed. London: Pearson, Prentice Hall; 2004.
- Zhang L, Rainka M, Freeman R, Harden RN, Bell CF, Chen C, et al. A randomized, double-blind, placebo-controlled trial to assess the efficacy and safety of gabapentin enacarbil in subjects with neuropathic pain associated with postherpetic neuralgia (PXN110748). J Pain. 2013;14:590–603.
- Kohnen R, Allen RP, Benes H, Garcia-Borreguero D, Hening WA, Stiasny-Kolster K, et al. Assessment of restless legs syndrome— methodological approaches for use in practice and clinical trials. Mov Disord. 2007;22(Suppl 18):S485–94.
- Inoue Y, Uchimura N, Kuroda K, Hirata K, Hattori N. Long-term efficacy and safety of gabapentin enacarbil in Japanese restless legs syndrome patients. Prog Neuropsychopharmacol Biol Psychiatry. 2012; 36:251–7.
- Inoue Y, Shimizu T, Hirata K, Uchimura N, Ishigooka J, Oka Y, et al. Efficacy and safety of rotigotine in Japanese patients with restless legs syndrome: a phase 3, multicenter, randomized, placebo-controlled, double-blind, parallel-group study. Sleep Med. 2013;14:1085–91.
- Kushida CA, Walters AS, Becker P, Thein SG, Perkins AT, Roth T, et al.; XP021 Study Group. A randomized, double-blind, placebo-controlled, crossover study of XP13512/GSK1838262 in the treatment of patients with primary restless legs syndrome. Sleep. 2009;32:159–68.
- Zhang W, Wang Y, Cong SY, Nao JF, Feng J, Bi GR. Efficacy and tolerability of pramipexole for the treatment of primary restless leg syndrome: a meta-analysis of randomized placebo-controlled trials. Neuropsychiatr Dis Treat. 2013;9:1035–43.
- Bogan RK, Bornemann MA, Kushida CA, Trân PV, Barrett RW; XP060 Study Group. Long-term maintenance treatment of restless legs syndrome with gabapentin enacarbil: a randomized controlled study. Mayo Clin Proc. 2010;85:512–21. Erratum in: Mayo Clin Proc. 2010; 85:693–4.
- Winkelman JW, Bogan RK, Schmidt MH, Hudson JD, DeRossett SE, Hill-Zabala CE. Randomized polysomnography study of gabapentin enacarbil in subjects with restless legs syndrome. Mov Disord. 2011;26:2065–72.
