Abstract
Introduction. Patients with rheumatoid arthritis (RA) have an increased cardiovascular (CV) morbidity and mortality. Pulse-wave velocity (PWV) and augmentation index (AIx) are non-invasive methods to assess arterial stiffness, a marker of CV risk. We performed a meta-analysis evaluating the impact of RA on aortic-PWV, brachial-PWV, brachial–ankle (ba-) PWV, AIx, and AIx normalized to a 75 beats/minute heart rate (AIx@75).
Materials and Methods. Studies evaluating the relationship between RA and aortic-PWV, brachial-PWV, ba-PWV, AIx, and AIx@75 were systematically searched. A total of 25 studies (1,472 RA patients, 1,583 controls) were included.
Results. Compared to controls, RA patients showed a significantly higher aortic-PWV (mean difference 1.32 m/s; 95% CI 0.77, 1.88; P < 0.00001), ba-PWV (MD 198.42 cm/s; 95% CI 45.79, 342.76; P = 0.01), AIx (MD 11.50%; 95% CI 5.15, 17.86; P = 0.0004), and AIx@75 (MD 6.99%; 95% CI 4.92, 9.06; P < 0.00001), with a trend toward a higher brachial-PWV (MD 0.34 m/s; 95% CI –0.03, 0.70; P = 0.07). When analyzing studies on early RA, the difference in aortic-PWV among RA patients and controls was even higher (MD 2.30 m/s; 95% CI 1.15, 3.45; P < 0.0001).
Conclusion. Meta-regression showed that a more severe inflammatory status impacted on aortic-PWV, AIx, and AIx@75. Arterial stiffness, a recognized marker of CV risk, is increased in RA patients. This alteration is associated with the severity of the inflammatory status and is present even in early-stage disease.
Patients with rheumatoid arthritis (RA) show increased arterial stiffness, with increased pulse-wave velocity (PWV) and augmentation index (AIx).
Arterial stiffening in RA is dependent on inflammatory status severity and is present even in the early stages of the disease.
Increased arterial stiffness may be a marker of the elevated cardiovascular risk of RA patients.
Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune disorder that affects synovial joints and leads to chronic pain, bone erosions, and progressive disability (Citation1). With a prevalence of 0.5%–1% in the general population, RA is the most common chronic inflammatory condition (Citation2,Citation3).
Beyond joint disease, RA is characterized by a high prevalence of co-morbidities, such as gastrointestinal (Citation4,Citation5), respiratory (Citation6,Citation7), and renal diseases (Citation5,Citation8). Moreover, metabolic syndrome (MetS) and its major features (obesity, hypertension, impaired fasting glucose, hyperlipidemia) have been frequently found in RA patients (Citation9). RA is also associated with shortened life expectancy (Citation1), and cardiovascular (CV) disease accounts for 35%–50% of excess mortality in this clinical setting (Citation10). Accordingly, the number of deaths from ischemic heart disease and cerebrovascular accidents is significantly higher in RA patients than in the general population (Citation11).
The increased CV morbidity and mortality in patients with RA cannot be entirely explained by traditional CV risk factors, and the underlying mechanisms leading to the increased CV risk in RA are not yet clearly understood (Citation12,Citation13). Thus, the association between RA and CV risk is still a matter of study.
Increased arterial stiffness is one of the earliest stages of the atherosclerotic process (Citation14), and pulse-wave velocity (PWV) is widely accepted as an accurate and non-invasive method to assess arterial stiffness in humans (Citation15). While PWV is a direct measure of arterial distensibility, the augmentation index (AIx) is a more complex parameter depending on vascular elasticity and peripheral resistance (Citation16). PWV (Citation17) and AIx (Citation18) are currently considered independent predictors of major CV events and all-cause mortality. Thus, these surrogate markers of subclinical atherosclerosis provide important prognostic information over and above traditional CV risk factors (Citation19).
During recent years, there has been a growing interest in the relationship between these markers of CV risk and RA. In particular, some case-control studies reported increased arterial stiffness in RA patients (Citation20,Citation21). However, contrasting results have been reported in recent studies (Citation22,Citation23), and no meta-analytical data providing overall information about this issue are currently available.
The aim of the present study is to perform a systematic review and meta-analysis of all studies evaluating the impact of RA on PWV and AIx. Moreover, we implemented some meta-regression models to evaluate the effect of some clinical and demographic variables on these outcomes.
Methods
To identify all available studies, a detailed search pertaining to RA and the markers of CV risk (i.e. PWV and AIx) was conducted according to PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines (Citation24). A systematic search was performed in the electronic databases (PubMed, Web of Science, Scopus, EMBASE), using the following search terms in all possible combinations: rheumatoid arthritis, arterial stiffness, pulse-wave analysis, pulse-wave velocity, and augmentation index. The last search was performed on 5 March 2015. The search strategy was developed without any language or study publication year restriction.
In addition, the reference lists of all retrieved articles were manually reviewed. In case of missing data, study authors were contacted by e-mail to try to retrieve original data. Two of the authors (P.A. and M.N.D.D.M.) analyzed each article and performed the data extraction independently. In case of disagreement, a third investigator was consulted (M.T.). Discrepancies were resolved by consensus. Selection results showed a high inter-reader agreement (κ = 1) and have been reported according to PRISMA flow chart (Supplementary online material to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2015.1068950).
Data extraction and quality assessment
According to the pre-specified protocol, all studies evaluating the impact of RA on the markers of CV risk were included. Case-reports, case-series without a control group, reviews, and animal studies were excluded. To be included in the analysis, a study had to provide values (means with standard deviation or standard error) of carotid–femoral PWV (aortic-PWV), carotid–radial PWV (brachial-PWV), brachial–ankle PWV (ba-PWV), AIx, and/or AIx normalized to a 75 beats/minute heart rate (AIx@75) among RA patients and controls. The included studies were classified as having a case-control design or a cohort design.
In each study, data regarding sample size, major clinical and demographic variables, values of aortic-PWV, brachial-PWV, ba-PWV, AIx, and AIx@75 in RA patients and controls were extracted.
Devices and methods for PWV and AIx assessment used in included studies are reported in the Supplementary online material to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2015.1068950.
Given the characteristics of the included studies, the evaluation of methodological quality of each study was performed with the Newcastle–Ottawa Scale (NOS), which is specifically developed to assess the quality of non-randomized observational studies (Citation25). The scoring system encompasses three major domains (selection, comparability, exposure), and the resulting score ranges between 0 and 8, a higher score representing a better methodological quality. Results of the NOS quality assessment are reported in the Supplementary online material to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2015.1068950.
Statistical analysis and risk of bias assessment
Statistical analysis was carried out using Review Manager (Version 5.2, The Cochrane Collaboration, Copenhagen, Denmark) provided by The Cochrane Collaboration.
Differences among cases and controls were expressed as mean difference (MD) with pertinent 95% confidence intervals (95% CI).
Aortic-PWV and brachial-PWV have been expressed in meters per second (m/s), ba-PWV in centimeters per second (cm/s), while AIx and AIx@75 have been expressed as percentages (%).
The overall effect was tested using Z scores, and significance was set at P < 0.05. Statistical heterogeneity between studies was assessed with chi-square Cochran's Q test and with I2 statistic, which measures the inconsistency across study results and describes the proportion of total variation in study estimates that is due to heterogeneity rather than sampling error. In detail, an I2 value of 0% indicates no heterogeneity, 25% low, 25%–50% moderate, and 50% high heterogeneity (Citation26).
Publication bias was assessed by Egger's test and represented graphically by funnel plots of the standard difference in means versus the standard error. Visual inspection of funnel plot asymmetry was performed to assess for possible small-study effects, and Egger's test was used to assess publication bias, over and above any subjective evaluation. A P < 0.10 was considered statistically significant (Citation27). In case of a significant publication bias, Duval and Tweedie's trim and fill method with the random-effect model was used to allow for the estimation of an adjusted effect size (Citation28).
In order to be as conservative as possible, the random-effect method was used for all analyses to take into account the variability among included studies.
Sensitivity analyses
We repeated sensitivity analyses by including only the studies judged as ‘high quality’ according to NOS (i.e. NOS ≥ the median value found among included studies).
In order to avoid the risk of data overlap, a sensitivity analysis was performed after excluding studies involving the same recruitment centers and enrolling patients in the same time-period as other included studies.
Subgroup analyses
Given the potential influence of disease duration on the outcomes, we planned to perform separate analyses for studies on early RA (defined by a disease duration < 12 months) and late RA (defined by a disease duration ≥ 12 months).
Meta-regression analyses
We hypothesized that differences among included studies may be affected by demographic variables (mean age, male gender) and clinical data related to disease activity [disease activity score in 28 joints (DAS28), rheumatoid factor (RF) positivity, C-reactive protein (CRP) levels, erythrocyte sedimentation rate (ESR)], anti-rheumatic treatment [therapy with non-steroidal anti-inflammatory drugs (NSAIDs), sulfasalazine (SSZ), corticosteroids (CCS), methotrexate (MTX), or TNFα-blockers], and by the coexistence of traditional CV risk factors (hypertension, smoking habit, diabetes mellitus, obesity, hyperlipidemia). To assess the possible effect of such variables in explaining different results observed across studies, we planned to perform meta-regression analyses after implementing a regression model with changes in aortic-PWV, brachial-PWV, ba-PWV, AIx, or AIx@75 as dependent variables (y) and the above mentioned co-variates as independent variables (x). This analysis was performed with Comprehensive Meta-analysis (Version 2, Biostat, 2005, Englewood, NJ, USA).
Results
After excluding duplicate results, the search retrieved 342 articles. Of these studies, 194 were excluded because they were off the topic after scanning the title and/or the abstract, 116 because they were reviews/comments/case-reports or they lacked data of interest. Of three studies the online full-length version was not available, but, for two of them, data could be extracted from the abstract. Other six studies were excluded after full-length paper evaluation.
Thus, 25 articles (on 1,472 RA patients and 1,583 healthy controls) were included in the final analysis (Supplementary online material to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2015.1068950) (Citation20–23,Citation29–49). In detail, we included nine studies reporting on aortic-PWV (421 cases and 547 controls), six studies on brachial-PWV (7 data-sets on 396 cases and 349 controls), three on ba-PWV (211 cases and 253 controls), six studies with data on AIx (214 cases and 327 controls), and seven on AIx@75 (590 cases and 571 controls).
Study characteristics
All included studies had a case-control design. Major characteristics of case and control study populations are shown in , and further data about disease activity and ongoing anti-rheumatic treatment of RA patients are reported in .
Table I. Demographic and clinical data of rheumatoid arthritis patients and controls in included studies.
Table II. Measures of disease activity and data on treatment in rheumatoid arthritis patients in included studies.
The number of patients varied from 14 to 203, the mean age from 40.1 to 60.8 years, and the prevalence of male gender from 0% to 62.5%.
The presence of hypertension was reported by 0%–65% of patients, smoking habit by 0%–30%, diabetes mellitus by 0%–14%, obesity by 0%, and hyperlipidemia by 0%–77%.
Mean body mass index (BMI) varied from 22.4 kg/m2 to 29.0 kg/m2. Mean values of total cholesterol (TC) ranged from 3.63 to 5.45 mmol/L, of LDL-cholesterol (LDLc) from 2.60 to 3.27 mmol/L, of HDL-cholesterol (HDLc) from 1.00 to 1.64 mmol/L, and of triglycerides (TGs) from 1.12 to 1.96 mmol/L.
Mean values of DAS28 varied from 3.0 to 5.8, CRP from 0.3 to 14.9 mg/dL, and ESR from 13.0 to 49.0 mm/1 h. The positivity of RF was reported by 61.9%–77.8% of patients, an ongoing treatment with NSAIDs by 0%–92.9%, with SSZ by 0%–42.4%, with CCS by 0%–83.8%, with MTX by 0%–86.1%, and with TNFα-blockers by 0%–66.7%.
In one study (Citation21), which provided separate data for patients with early and late RA, the two groups were evaluated as two different data-sets.
Definition of early RA was highly variable among included studies, being defined as newly diagnosed RA (Citation36,Citation38) or as disease during less than 1 (Citation21) or 6 years (Citation49).
The NOS for quality assessment of included studies showed a median value of 6.
Pulse-wave velocity (PWV)
In nine studies (Citation23,Citation33,Citation36,Citation39,Citation44–46,Citation48,Citation49) we found that the 421 RA patients showed a significantly higher aortic-PWV than the 547 controls (MD 1.32 m/s; 95% CI 0.77, 1.88; P < 0.00001) (). Heterogeneity among these studies was statistically significant (I2 = 89%; P < 0.00001), and it was not reduced after excluding one study at time.
Figure 1. Pulse-wave velocity (PWV) in rheumatoid arthritis (RA) patients and controls. Data on carotid–femoral PWV (Panel A), carotid–radial PWV (Panel B), and brachial–ankle PWV (Panel C).
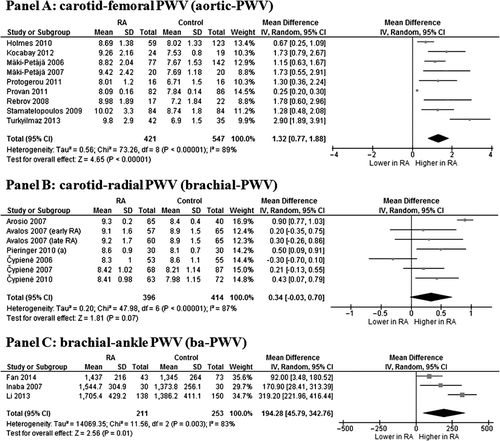
Six studies (7 data-sets) (Citation20–22,Citation30,Citation31,Citation41), evaluating a total of 396 cases and 349 controls, showed a trend toward a higher brachial-PWV in RA patients than in controls (MD 0.34 m/s; 95% CI −0.03, 0.70; P = 0.07, I2 = 87%; P < 0.00001) ().
Similarly, a total of three studies (Citation32,Citation34,Citation37) on 211 cases and 253 controls showed that RA patients have a significantly higher ba-PWV as compared to controls (MD 198.42 cm/s; 95% CI 45.79, 342.76; P = 0.01) (). These studies showed a significantly high heterogeneity (I2 = 83%; P = 0.003), but, after excluding the study by Li et al. (Citation37), similar results with no heterogeneity were found (MD 113.97 cm/s; 95% CI 38.78, 189.16; P = 0.003, I2 = 0%; P = 0.36).
Augmentation index (AIx)
Six studies (Citation23,Citation30,Citation35,Citation38,Citation46,Citation47), evaluating a total of 214 cases and 327 controls, showed a significantly higher AIx in RA subjects as compared to controls (MD 11.50%; 95% CI 5.15, 17.86; P = 0.0004) (). Significant heterogeneity among studies was found (I2 = 88%; P < 0.00001), and it was not reduced by excluding one study at time.
Figure 2. Augmentation index (AIx) in rheumatoid arthritis (RA) patients and controls. Data on AIx (Panel A) and AIx normalized to a heart rate of 75 beats per minute (Panel B).
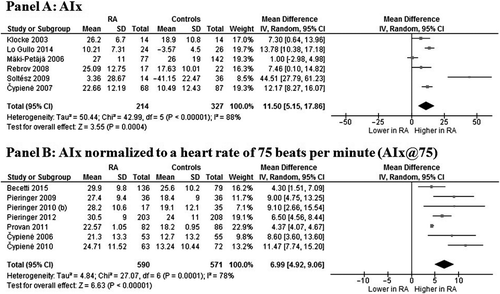
Similar results were found in seven studies (Citation22,Citation29,Citation31,Citation40,Citation42,Citation43,Citation45) evaluating AIx@75 in 590 RA patients and 571 controls. Patients showed significantly higher AIx@75 as compared to controls (MD 6.99%; 95% CI 4.92, 9.06; P < 0.00001) (), with a high heterogeneity among studies (I2 = 78%; P = 0.0001), not reduced by the one-study-at-a-time exclusion.
Publication bias
Because it is recognized that publication bias can affect the results of meta-analyses, we attempted to assess this potential bias using funnel plots analysis. Visual inspection of funnel plots of effect size versus standard error for studies evaluating aortic-PWV, brachial-PWV, and AIx@75 suggested an asymmetric distribution of studies around the mean (Supplementary online material to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2015.1068950), and Egger's test confirmed a significant publication bias (P < 0.001, P = 0.01, and P = 0.02, respectively). Interestingly, the adjusted effect size, estimated by Duval and Tweedie's trim and fill method, substantially confirmed results for aortic-PWV (MD 1.32 m/s; 95% CI 0.77, 1.88), brachial-PWV (MD 0.34 m/s; 95% CI −0.03, 0.70), and AIx@75 (MD 5.42%; 95% CI 7.28, 37.97).
In contrast, the distribution of studies evaluating ba-PWV and AIx was rather symmetrical, and no publication bias was found by Egger's test (P = 0.99 and P = 0.61, respectively).
Sensitivity analyses
The median value of NOS quality assessment was 6. Thus, the analyses were repeated by including only the 15 studies classified as ‘high quality’ (NOS ≥ 6) (Supplementary online material to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2015.1068950) (Citation21,Citation23,Citation29–31,Citation33–35,Citation37–40,Citation43,Citation48,Citation49). Of two studies (Citation20,Citation46) data of interest were extracted by the abstract, thus no quality assessment could be performed.
Of interest, after excluding studies classified as ‘low quality’ (Citation22,Citation32,Citation36,Citation41,Citation42,Citation44,Citation45,Citation47) and those with only abstract available (Citation20,Citation46), all results were substantially confirmed ().
Table III. Sensitivity analyses. Panel A: ‘high-quality’ studies (i.e. Newcastle–Ottawa Scale ≥ 6) included; Panel B: exclusion of studies potentially reporting on the same population as other included studies.
Similar results were confirmed also after excluding studies (Citation21,Citation22,Citation30,Citation39–42,Citation44) potentially reporting on the same population as other included studies () (Citation23,Citation29,Citation31,Citation43,Citation48).
Interestingly, in both sensitivity analyses, the difference between RA patients and controls became significant for brachial PWV.
Subgroup analyses
Given the potential influence of disease duration on the outcomes, we decided to perform separate subgroup analyses for studies on early RA (Citation21,Citation36,Citation38,Citation49) and late RA (Citation20–23,Citation29–35,Citation37,Citation39–48).
Interestingly, our results on aortic-PWV were confirmed in both early and late RA. However, the relatively low number of studies on early RA (n = 4) made this subgroup analysis unlikely to be performed for the other outcomes. Thus, results on brachial-PWV, ba-PWV, AIx, and AIx@75 could be confirmed only in late RA patients ().
Table IV. Subgroup analyses. Panel A: studies on early RA; Panel B: studies on late RA.
Meta-regression analyses
We assessed the possible effect of different variables in explaining different results observed across studies: demographic variables (mean age, male gender), clinical variables related to disease activity (DAS28, RF positivity, CRP levels, ESR), anti-rheumatic treatment (therapy with NSAIDs, SSZ, CCS, MTX, or TNFα-blockers), and coexistence of traditional CV risk factors (hypertension, smoking habit, diabetes mellitus, obesity, hyperlipidemia).
Regression models showed that age significantly impacted on AIx@75 (Z = −4.66; P < 0.001) (), while increasing percentage of males was associated with a low effect size for brachial-PWV (Z = −2.27; P = 0.02) () and AIx@75 (Z = −1.96; P = 0.05) ().
Figure 3. Meta-regression analyses. Effects of age on AIx@75 (Panel A), of male gender on brachial-PWV (Panel B) and AIx@75 (Panel C), of DAS28 on aortic-PWV (Panel D) and AIx (Panel E), of CRP on AIx (Panel F) and AIx@75 (Panel G), and of ESR on aortic-PWV (Panel H) in patients with rheumatoid arthritis (RA). AIx = augmentation index; AIx@75 = augmentation index normalized to a 75 beats/minute heart rate; Aortic-PWV = carotid–femoral pulse-wave velocity; brachial-PWV = carotid–radial pulse-wave velocity; CRP = C-reactive protein; DAS28 = Disease Activity Score in 28 Joints; ESR = erythrocyte sedimentation rate.
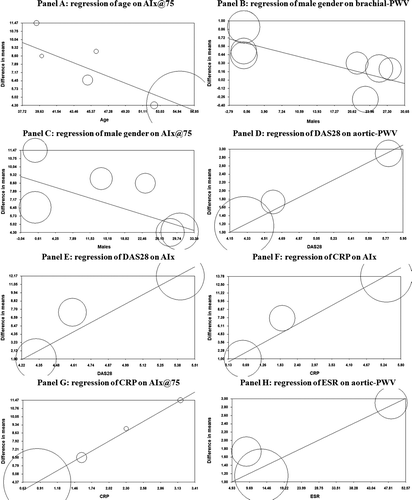
In addition, a more severe inflammatory status (as expressed by DAS28, CRP levels, and ESR) was associated with a high effect size for aortic-PWV, AIx, and AIx@75. In particular, DAS28 significantly impacted on aortic-PWV (Z = 2.95; P = 0.003) () and AIx (Z = 3.50; P = 0.0005) (), CRP levels on AIx (Z = 4.37; P = 0.00001) () and AIx@75 (Z = 4.76; P < 0.001) (), while ESR impacted on aortic-PWV (Z = 2.25; P = 0.02) (). All the other demographic and clinical data did not impact on the evaluated outcomes.
Discussion
Results of the present meta-analysis consistently show that RA is associated with increased arterial stiffness. In particular, we reported an increased aortic-PWV, ba-PWV, AIx, and AIx@75 in RA patients. A trend towards a higher brachial-PWV was also documented in RA. Our findings are strengthened by the sensitivity analyses and, particularly, by the subgroup analysis which confirmed results on aortic-PWV both in early RA and in late RA patients. Moreover, regression models were able to refine the results further, providing the evidence that age, male gender, and a more severe inflammatory status may significantly impact on the evaluated outcomes.
Arterial stiffening is one of the earliest stages of the atherosclerotic process (Citation50). PWV and AIx are widely accepted as non-invasive methods to assess clinically the peripheral and central arterial stiffness (Citation15,Citation16) and, in turn, as surrogate markers of subclinical atherosclerosis (Citation19). The clinical relevance of increased arterial stiffness lies in its ability to predict CV morbidity and mortality over and above other traditional CV risk factors in patients with hypertension (Citation51), end-stage renal disease (Citation52), elderly individuals (Citation53), and in the general population (Citation54,Citation55). This prognostic importance has also been recently confirmed in a meta-analysis (Citation17).
Thus, clinical assessment of arterial stiffness consistently suggests an elevated CV risk in RA patients, which is widely confirmed by some epidemiological studies showing an increased incidence of major CV events in this clinical setting (Citation10,Citation11). This evidence is even more significant if we consider the relatively young age of the population enrolled in included studies, with a mean age ranging between 40.1 and 60.8 years. Moreover, despite the presence of several CV risk factors, the meta-regression analysis confirmed that all results of our meta-analysis were largely independent of these co-variates.
Many CV risk factors are thought to have a causal role in the atherosclerotic process (Citation56). Although RA patients exhibit an increased prevalence of these CV risk factors (Citation9), the relationship between atherosclerosis and RA seems to be more complex, and the presence of traditional risk factors might not entirely explain the accelerated atherosclerotic process in this clinical setting. Thus, other mechanisms (i.e. inflammatory and immunological) have been proposed to explain the relationship between RA and atherosclerosis (Citation9). The improvement of the CV risk profile following the control of systemic inflammation by anti-inflammatory treatments (Citation57) argues for the possibility that systemic inflammation acts as an independent CV risk factor in RA (Citation58).
In keeping with this, the European League Against Rheumatism (EULAR) proposed the application of a 1.5 multiplier to the CV risk calculated in rheumatic patients through the scores currently used for the general population (e.g. the Framingham score) (Citation59). The results of our meta-regression analyses—showing that a more severe inflammatory status (as expressed by DAS28, CRP levels, and ESR) is associated with a higher aortic-PWV, AIx, and AIx@75—strongly supports the hypothesis that inflammation/disease activity should be considered in the CV risk profile assessment of RA patients.
Interestingly, our subgroup analyses excluded the potential influence of disease duration on aortic-PWV and confirmed results on this outcome both in early RA and in late RA patients. However, considering the relatively low number of studies on early RA (n = 4), results on brachial-PWV, ba-PWV, AIx, and AIx@75 could be confirmed only in late RA patients. Of interest, the difference in aortic-PWV between cases and controls was even greater when specifically considering the early RA subset (MD 2.30 m/s) compared with patients affected by late RA (MD 1.32 m/s). The latter result is in line with some data from our recent meta-analysis (Citation60), showing that the difference in CCA-IMT among patients and controls is higher in early RA. As previously supposed (Citation60), this may be due to the younger age of patients and controls recruited in the studies on early RA. In such a young clinical setting, the pro-atherogenic effect of RA is likely to be more significant, and this supports the hypothesis that CV risk already starts to increase as soon as the first signs of autoimmunity and inflammation appear, which is often some months/years prior to diagnosis (Citation61,Citation62).
Overall, our findings further support the hypothesis that premature atherosclerosis may be one of the main features of RA and autoimmune disorders (Citation63) and that chronic inflammation plays an important role in its pathogenesis, acting independently and/or synergistically with traditional CV risk factors. In order to provide a comprehensive overview of the relationship between RA and arterial stiffness, we evaluated all available methods for its clinical assessment. Thus, we also included studies on ba-PWV, which is a promising technique to measure arterial stiffness conveniently and more suited to routine clinical use than the other more ‘complicated’ approaches (Citation64). However, we should consider that aortic-PWV still remains the most widely used technique and the ‘gold standard’ measure of arterial stiffness with a clear predictive value (Citation18). The clinical relevance of our results can be better understood when we consider that the risk of major CV events increases by about 14% with each 1 m/s increase in aortic-PWV (Citation17). Similarly, this risk increases by 31.8% with each 10% increase in AIx (Citation18).
Further extending the hypothesis of an increased CV risk in RA patients and suggesting the need for a strict monitoring of subclinical signs of atherosclerosis in RA patients, we have recently documented an increased carotid intima-media thickness (IMT) accompanied by higher prevalence of carotid plaques in patients with RA (Citation60). Interestingly, we also demonstrated that the increase in IMT was dependent on the severity of the inflammatory process in patients with RA.
Overall, our results further support the need for large long-term interventional trials with CV end-points to investigate whether benefits in articular disease achieved by aggressive suppression of inflammation may translate into reduced CV risk in RA.
Some potential limitations of our study need to be discussed.
First, studies included in our meta-analysis have different inclusion and exclusion criteria, and most of patients included in the analysis had concomitant CV risk factors (hypertension, smoking, obesity, diabetes mellitus, hyperlipidemia) and different disease activity status. Since meta-analysis is performed on aggregate data and some information is missing in each study, the multivariate approach allowed for the adjustment for some (but not all) potential confounders. Thus, although results of meta-regression analyses were able to refine analyses by assessing the influence of most clinical and demographic variables on the observed results, caution is necessary in overall results interpretation.
Second, heterogeneity among the studies was generally significant. Although it was not possible to ascertain conclusively the sources of heterogeneity, all results were confirmed after adjustment for potential publication bias.
A further limitation is the relative lack of specific data on early RA and the high variability in the definition of early RA among studies. In the frame of a sensitivity analysis, to adjust results for the potential influence of disease duration on the outcomes, we were able to confirm our results on aortic-PWV in early RA, but we could not confirm results on brachial-PWV, ba-PWV, AIx, and AIx@75 in this clinical setting. In line with some recent data suggesting the presence of an increased subclinical atherosclerosis in patients with early RA (Citation60), further studies specifically focusing on PWV and AIx in early RA patients are needed to address the hypothesis of a significant increase in the CV risk starting from very early stages of the disease in patients with RA (Citation61).
Another potential limitation of this study is that PWV and AIx measurement may be influenced by many confounding factors, significantly limiting reproducibility of arterial stiffness assessment and, in turn, the relevance of our results. In particular, some studies (Citation65,Citation66) indicate that heart rate may affect results, and, with the only exception of AIx@75, all the other outcomes have not been standardized to a specific heart rate.
Moreover, we have to consider that differences among assessment techniques and devices, as well as the lack of comparable age-adjusted normal values may limit the validity of arterial stiffness parameters as markers of early atherosclerosis (Citation67). Thus, caution is necessary in overall results interpretation. However, in the attempt to overcome this potential limitation, we repeated the analyses by using standardized mean difference (SMD) instead of MD, this method being designed to be used when different methods of measurement are analyzed together (Citation26). Interestingly, all results were confirmed using SMD (data not shown).
Finally, results on AIx should be interpreted with caution, as AIx is a more complex parameter depending not only on arterial stiffness but also on peripheral resistance (Citation16).
In conclusion, in our meta-analysis RA appeared significantly associated with increased arterial stiffness and, in turn, with subclinical atherosclerosis and CV risk. Thus, patients with RA may benefit from a more meticulous screening for CV risk factors and more specific CV prevention strategies. However, additional and specifically designed studies are needed in order to establish the optimal management of these patients.
Supplementary material available online
Supplementary Tables I-II and Figures 1–3 to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2015.1068950
iann_a_1068950_sm4993.pdf
Download PDF (512.9 KB)Acknowledgements
The authors want to thank the members of the CaRRDs (Cardiovascular Risk in Rheumatic Diseases) study group: Matteo Nicola Dario Di Minno, Roberta Lupoli, Antonella Scalera, Alessandro Di Minno, Pasquale Ambrosino, Giovanni Tarantino, Giovanni Di Minno (Department of Clinical Medicine and Surgery, Regional Reference Centre for Coagulation Disorders, Federico II University, Naples, Italy); Rosario Peluso, Raffaele Scarpa (Department of Clinical Medicine and Surgery, Rheumatology Research Unit, Psoriatic Arthritis Clinic, Federico II University, Naples, Italy); Paolo Osvaldo Rubba (Department of Clinical Medicine and Surgery, Atherosclerosis Prevention and Vascular Medicine Unit, Federico II University, Naples, Italy); and Salvatore Iervolino (Rheumatology and Rehabilitation Research Unit, ‘Salvatore Maugeri’ Foundation, Scientific Institute of Telese Terme, Benevento, Italy).
Funding: This study has been performed in the frame of the project entitled ‘Biomarkers of Cardiovascular Risk and Disease Activity in Patients with Psoriatic Arthritis: Modifications Induced by Treatment with TNF-alpha Blockers’ (GR-2011-02352752) funded by the Italian Ministry of Health.
Declaration of interest: Matteo Nicola Dario Di Minno has acted as paid lecturer or board member and received grants and honoraria from Bayer, Biotest, Pfizer, and Novo-Nordisk in the last 36 months for researches unrelated to the present study. All the other authors have nothing to declare.
References
- Scarno A, Perrotta FM, Cardini F, Carboni A, Annibali G, Lubrano E, et al. Beyond the joint: subclinical atherosclerosis in rheumatoid arthritis. World J Orthop. 2014;5:328–35.
- Alamanos Y, Drosos AA. Epidemiology of adult rheumatoid arthritis. Autoimmun Rev. 2005;4:130–6.
- Gibofsky A. Overview of epidemiology, pathophysiology, and diagnosis of rheumatoid arthritis. Am J Manag Care. 2012;18:S295–302.
- Mitchell DM, Spitz PW, Young DY, Bloch DA, McShane DJ, Fries JF. Survival, prognosis, and causes of death in rheumatoid arthritis. Arthritis Rheum. 1986;29:706–14.
- Vandenbroucke JP, Hazevoet HM, Cats A. Survival and cause of death in rheumatoid arthritis: a 25-year prospective follow up. J Rheumatol. 1984;11:158–61.
- Monson RR, Hall AP. Mortality among arthritics. J Chronic Dis. 1976;29:459–67.
- Prior P, Symmons DPM, Scott DL, Brown R, Hawkins CF. Cause of death in rheumatoid arthritis. Br J Rheumatol. 1984;23:92–9.
- Mutru O, Laakso M, Isomaki H, Koota K. Ten year mortality and causes of death in patients with rheumatoid arthritis. Br Med J (Clin Res Ed). 1985;290:1797–9.
- Di Minno MN, Iervolino S, Lupoli R, Russolillo A, Coppola A, Peluso R, et al. Cardiovascular risk in rheumatic patients: the link between inflammation and atherothrombosis. Semin Thromb Hemost. 2012;38: 497–505.
- Minaur NJ, Jacoby RK, Cosh JA, Taylor G, Rasker JJ. Outcome after 40 years with rheumatoid arthritis: a prospective study of function, disease activity, and mortality. J Rheumatol Suppl. 2004;69:3–8.
- Aviña-Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690–7.
- La Montagna G, Cacciapuoti F, Buono R, Manzella D, Mennillo GA, Arciello A, et al. Insulin resistance is an independent risk factor for atherosclerosis in rheumatoid arthritis. Diab Vasc Dis Res. 2007;4:130–5.
- Salmon JE, Roman MJ. Subclinical atherosclerosis in rheumatoid arthritis and systemic lupus erythematosus. Am J Med. 2008;121:S3–8.
- Jadhav UM, Kadam NN. Non-invasive assessment of arterial stiffness by pulse-wave velocity correlates with endothelial dysfunction. Indian Heart J. 2005;57:226–32.
- Mackenzie IS, Wilkinson IB, Cockcroft JR. Assessment of arterial stiffness in clinical practice. QJM. 2002;95:67–74.
- Yasmin Brown MJ. Similarities and differences between augmentation index and pulse wave velocity in the assessment of arterial stiffness. QJM. 1999;92:595–600.
- Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–27.
- Vlachopoulos C, Aznaouridis K, O’Rourke MF, Safar ME, Baou K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. Eur Heart J. 2010;31:1865–71.
- Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. European Network for Non-invasive Investigation of Large Arteries. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27: 2588–605.
- Arosio E, De Marchi S, Rigoni A, Prior M, Delva P, Lechi A. Forearm haemodynamics, arterial stiffness and microcirculatory reactivity in rheumatoid arthritis. J Hypertens. 2007;25:1273–8.
- Avalos I, Chung CP, Oeser A, Gebretsadik T, Shintani A, Kurnik D, et al. Increased augmentation index in rheumatoid arthritis and its relationship to coronary artery atherosclerosis. J Rheumatol. 2007;34:2388–94.
- Cypienė A, Laucevicius A, Venalis A, Ryliškyte L, Dadoniene J, Petrulioniene Z, et al. Increased arterial stiffness in young patients with rheumatoid arthritis. Semin Cardiol. 2006;12:141–8.
- Mäki-Petäjä KM, Hall FC, Booth AD, Wallace SM, Yasmin Bearcroft PW, et al. Rheumatoid arthritis is associated with increased aortic pulse-wave velocity, which is reduced by anti-tumor necrosis factor-alpha therapy. Circulation. 2006;114:1185–92.
- Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.100009.
- Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. Ottawa Hospital Research Institute. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm.
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
- Sterne JA, Egger M, Smith GD. Systematic reviews in health care: investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323:101–5.
- Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63.
- Becetti K, Oeser A, Ormseth M, Solus JF, Raggi P, Stein CM, et al. Urinary albumin excretion is increased in patients with rheumatoid arthritis and associated with arterial stiffness. J Rheumatol. 2015;42:593–8.
- Cypienė A, Laucevicius A, Venalis A, Ryliskyte L, Dadoniene J, Petrulioniene Z, et al. Non-invasive assessment of arterial stiffness indices by applanation tonometry and pulse wave analysis in patients with rheumatoid arthritis treated with TNF-alpha blocker remicade (infliximab). Proc West Pharmacol Soc. 2007;50:119–22.
- Cypienė A, Dadonienė J, Rugienė R, Ryliškytė L, Kovaitė M, Petrulionienė Z, et al. The influence of mean blood pressure on arterial stiffening and endothelial dysfunction in women with rheumatoid arthritis and systemic lupus erythematosus. Medicina (Kaunas). 2010;46:522–30.
- Fan F, Galvin A, Fang L, White DA, Moore XL, Sparrow M, et al. Comparison of inflammation, arterial stiffness and traditional cardiovascular risk factors between rheumatoid arthritis and inflammatory bowel disease. J Inflamm (Lond). 2014;11:29.
- Holmes MV, Jiang B, McNeill K, Wong M, Oakley SP, Kirkham B, et al. Paradoxical association of C-reactive protein with endothelial function in rheumatoid arthritis. PLoS One. 2010;5:e10242.
- Inaba M, Tanaka K, Goto H, Sakai S, Yamada S, Naka H, et al. Independent association of increased trunk fat with increased arterial stiffening in postmenopausal patients with rheumatoid arthritis. J Rheumatol. 2007;34:290–5.
- Klocke R, Cockcroft JR, Taylor GJ, Hall IR, Blake DR. Arterial stiffness and central blood pressure, as determined by pulse wave analysis, in rheumatoid arthritis. Ann Rheum Dis. 2003;62:414–18.
- Kocabay G, Hasdemir H, Yildiz M. Evaluation of pulse wave velocity in systemic lupus erythematosus, rheumatoid arthritis and Behçet's disease. J Cardiol. 2012;59:72–7.
- Li P, Han CX, Ma CL, Guo JL, Liu B, Du J, et al. Determinants of brachial-ankle pulse wave velocity in Chinese patients with rheumatoid arthritis. Clin Dev Immunol. 2013;2013:342869.
- Lo Gullo A, Mandraffino G, Imbalzano E, Mamone F, Aragona CO, D’Ascola A, et al. Toll-like receptor 3 and interleukin 1β expression in CD34 + cells from patients with rheumatoid arthritis: association with inflammation and vascular involvement. Clin Exp Rheumatol. 2014;32:922–9.
- Mäki-Petäjä KM, Booth AD, Hall FC, Wallace SM, Brown J, McEniery CM, et al. Ezetimibe and simvastatin reduce inflammation, disease activity, and aortic stiffness and improve endothelial function in rheumatoid arthritis. J Am Coll Cardiol. 2007;50:852–8.
- Pieringer H, Schumacher S, Stuby U, Biesenbach G. Augmentation index and large-artery remodeling in patients with longstanding rheumatoid arthritis compared with healthy controls. Semin Arthritis Rheum. 2009;39:163–9.
- Pieringer H, Stuby U, Pohanka E, Biesenbach G. Arterial stiffness in a muscular artery in women with longstanding rheumatoid arthritis compared with healthy controls and patients with traditional cardiovascular risk factors. Rheumatol Int. 2010;30:1335–9.
- Pieringer H, Stuby U, Pohanka E, Biesenbach G. Augmentation index in patients with rheumatoid arthritis and ankylosing spondylitis treated with infliximab. Clin Rheumatol. 2010;29:723–7.
- Pieringer H, Brummaier T, Schmid M, Pichler M, Hayat-Khayyati A, Ebner S, et al. Rheumatoid arthritis is an independent risk factor for an increased augmentation index regardless of the coexistence of traditional cardiovascular risk factors. Semin Arthritis Rheum. 2012;42: 17–22.
- Protogerou AD, Zampeli E, Fragiadaki K, Stamatelopoulos K, Papamichael C, Sfikakis PP. A pilot study of endothelial dysfunction and aortic stiffness after interleukin-6 receptor inhibition in rheumatoid arthritis. Atherosclerosis. 2011;219:734–6.
- Provan SA, Semb AG, Hisdal J, Stranden E, Agewall S, Dagfinrud H, et al. Remission is the goal for cardiovascular risk management in patients with rheumatoid arthritis: a cross-sectional comparative study. Ann Rheum Dis. 2011;70:812–17.
- Rebrov AP, Nikitina NM. Risk factors of cardiovascular diseases in patients with rheumatoid arthritis. Klin Med (Mosk). 2008;86:56–9.
- Soltész P, Dér H, Kerekes G, Szodoray P, Szücs G, Dankó K, et al. A comparative study of arterial stiffness, flow-mediated vasodilation of the brachial artery, and the thickness of the carotid artery intima-media in patients with systemic autoimmune diseases. Clin Rheumatol. 2009;28:655–62.
- Stamatelopoulos KS, Kitas GD, Papamichael CM, Chryssohoou E, Kyrkou K, Georgiopoulos G, et al. Atherosclerosis in rheumatoid arthritis versus diabetes: a comparative study. Arterioscler Thromb Vasc Biol. 2009;29:1702–8.
- Turkyilmaz AK, Devrimsel G, Kirbas A, Cicek Y, Karkucak M, Capkin E, et al. Relationship between pulse wave velocity and serum YKL-40 level in patients with early rheumatoid arthritis. Rheumatol Int. 2013;33:2751–6.
- van Popele NM, Grobbee DE, Bots ML, Asmar R, Topouchian J, Reneman RS, et al. Association between arterial stiffness and atherosclerosis: the Rotterdam Study. Stroke 2001;32:454–60.
- Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–41.
- Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness on survival in end-stage renal disease. Circulation. 1999;99:2434–9.
- Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113: 657–63.
- Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–11.
- Willum-Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, et al. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113: 664–70.
- Martín-Timón I, Sevillano-Collantes C, Segura-Galindo A, Del Cañizo-Gómez FJ. Type 2 diabetes and cardiovascular disease: have all risk factors the same strength? World J Diabetes. 2014;5: 444–70.
- Jacobsson LT, Turesson C, Gülfe A, Kapetanovic MC, Petersson IF, Saxne T, et al. Treatment with tumor necrosis factor blockers is associated with a lower incidence of first cardiovascular events in patients with rheumatoid arthritis. J Rheumatol. 2005;32:1213–18.
- Kitas GD, Gabriel SE. Cardiovascular disease in rheumatoid arthritis: state of the art and future perspectives. Ann Rheum Dis. 2011;70: 8–14.
- Peters MJ, Symmons DP, McCarey D, Dijkmans BA, Nicola P, Kvien TK, et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis. 2010;69:325–31.
- Ambrosino P, Lupoli R, Di Minno A, Tasso M, Peluso R, Di Minno MN. Subclinical atherosclerosis in patients with rheumatoid arthritis. A meta-analysis of literature studies. Thromb Haemost. 2015;113: 916–30.
- van den Oever IA, van Sijl AM, Nurmohamed MT. Management of cardiovascular risk in patients with rheumatoid arthritis: evidence and expert opinion. Ther Adv Musculoskelet Dis. 2013;5:166–81.
- Peluso R, Cafaro G, Di Minno A, Iervolino S, Ambrosino P, Lupoli G, et al. Side effects of TNF-α blockers in patients with psoriatic arthritis: evidences from literature studies. Clin Rheumatol. 2013;32:743–53.
- Ambrosino P, Lupoli R, Di Minno A, Iervolino S, Peluso R, Di Minno MN. Markers of cardiovascular risk in patients with antiphospholipid syndrome: a meta-analysis of literature studies. Ann Med. 2014;46:693–702.
- Yamashina A, Tomiyama H, Arai T, Hirose K, Koji Y, Hirayama Y, et al. Brachial-ankle pulse wave velocity as a marker of atherosclerotic vascular damage and cardiovascular risk. Hypertens Res. 2003;26:615–22.
- Lantelme P, Mestre C, Lievre M, Gressard A, Milon H. Heart rate: an important confounder of pulse wave velocity assessment. Hypertension. 2002;39:1083–7.
- Imura R, Yamamoto K, Kanamori K, Mikami T, Yasuda H. Non invasive ultrasonic measurement of the elastic properties of the human abdominal aorta. Cardiovasc Res. 1986;20:208–14.
- Kerekes G, Soltész P, Nurmohamed MT, Gonzalez-Gay MA, Turiel M, Végh E, et al. Validated methods for assessment of subclinical atherosclerosis in rheumatology. Nat Rev Rheumatol. 2012;8:224–34.
