Abstract
This meta-analysis examined the effect of probiotics on the reduction of lipid components and coexisting risk factors associated with cardiovascular disease. All randomized controlled trials published in English on PubMed and Scopus from 2000 to 2014 were systematically searched. Using the PEDro scale to assess the quality of studies, a total of 15 studies with 788 subjects were selected for inclusion in the analysis. The mean difference and effect size with a 95% confidence interval (CI) were extracted from individual studies. Statistically significant pooled effects of probiotics were found on reduction of total cholesterol, low-density lipoprotein (LDL), body mass index (BMI), waist circumference, and inflammatory markers. Subgroup analysis revealed statistically significant effects of probiotics on total cholesterol and LDL when the medium was fermented milk or yogurt (P < 0.001) compared to capsule form, consumption was at least 8 weeks in duration (P < 0.001), and the probiotics consisted of multiple strains (P < 0.001) rather than a single strain. A significant reduction was found in LDL in trials which contained Lactobacillus Acidophilus strain (P < 0.001) compared to other types of strains. Our findings suggest that probiotic supplementation use is effective in lowering the lipid level and coexisting factors associated with cardiovascular disease.
Probiotics supplementation use is effective in reducing total cholesterol, LDL, weight, waist, and inflammatory markers.
Probiotics use on total cholesterol and LDL is more effective when the medium was fermented milk or yogurt compared to capsule form, consumption was at least 8 weeks in duration, and the probiotics consisted of multiple strains rather than a single strain.
A significant reduction was found in LDL in trials which contained L. acidophilus strain compared to other types of strains.
Probiotic supplementation use is effective in lowering the lipid level and coexisting factors associated with cardiovascular disease.
Introduction
Cardiovascular disease (CVD) is a leading cause of mortality worldwide, with an increasing morbidity rate in both developing and developed countries (Citation1). A number of modifiable and major risk factors, such as elevated low-density lipoprotein cholesterol (LDL-C), increased triglyceride-rich lipoproteins, and low levels of high-density lipoprotein cholesterol (HDL-C) (Citation1), are significant contributors to CVD. Additional coexisting risk factors include overweight and obesity (Citation2), inflammatory markers such as hr-C-reactive protein (CRP), and tumor necrosis factor alpha (TNF-alpha) (Citation3). If not adequately addressed, these risk factors contribute to high cardiovascular morbidity and mortality rates (Citation4,Citation5).
Adopting healthy lifestyle habits is an important part of managing CVD risk factors and reducing costs associated with the disease (Citation1). In this context, probiotics have gained recent attention as products for managing CVD. Probiotics have been defined by the World Health Organization (WHO) as ‘live microorganisms which when administered in adequate amounts confer a health benefit on the host’ (Citation6). It has been demonstrated that probiotics can modulate gut health (Citation7,Citation8) and strengthen the immune system by regulating gut microbiota (Citation9).
Although a number of clinical studies have shown that probiotics can lower CVD risk factors, this research has significant limitations (Citation10,Citation11); there is conflicting and inconclusive evidence about the effects of probiotics on improving lipid components (Citation11) and which forms, consumption duration, and dosage limits per strain are required to optimize risk reduction.
A meta-analysis conducted by Agerholm-Larsen and colleagues, which found that probiotic diary product consumption resulted in the reductions in total and LDL cholesterol, focused on a small number of pre-2000 trials with short-term (4–8 weeks) interventions (Citation10). However, to date, no systematic review or meta-analysis has been conducted on findings from recent clinical trials investigating the effect of probiotics consumption on CVD risk factors. This study addresses this issue by using a meta-analytic approach to review the evidence from randomized controlled trials (RCTs) that examined the effects of probiotics and consumption characteristics on reducing cholesterol and other coexisting CVD risk factors.
Methods
Literature search
The review protocol was registered at the Prospero International Prospective Register of Systematic Reviews (Registration ID: PROSPERO 2014:CRD42014010591; website: http://www.crd.york.ac.uk/PROSPERO_REBRANDING/display_record.asp?ID=CRD42014010591). Relevant studies published between 2000 and 2014 in the following databases were systematically identified by the two authors independently for inclusion in the meta-analysis: PubMed, Scopus, Evidence-Based Medicine (EBM) Guidelines, and the Cochrane Library. A combination of the following keywords was employed in this process: Probiotics AND cardiovascular disease risk factors OR lipid profiles OR inflammatory factors. Further identification occurred through the use of keywords related to specific CVD risk factors and types of probiotics including: Streptococcus Thermophilus, Lactobacillus Bulgaricus, Lactobacillus Acidophilus, Bifidobacterium, Lactobacillus Plantarum, and Lactobacillus Fermentum. The title and abstract were initially screened for relevance. The full text of the relevant articles was then retrieved for further reading and assessment. The literature search and presentation of results were undertaken according to Systematic Reviews and Meta-analysis (PRISMA) guidelines. Identified articles were imported into the EndNote reference management software to remove any duplicates. The reviewers then independently extracted data on patients’ characteristics and outcome measures. Where there was unreported data, the research team contacted the authors of the studies in question to obtain the data. This occurred with two included studies (Citation12,Citation13).
Inclusion criteria
The research team chose the following criteria to determine the inclusion of studies using the PICO structure: Patient problem or population (P), intervention (I), comparison (C), and outcome(s) (O) (Citation14,Citation15):
Participants were 18 years or older;
Clinical trials involved participants with CVD risk factors, including hypertension, abnormal lipid profiles, obesity, diabetes, or metabolic syndromes;
Study design was a randomized control trial in which participants were randomly allocated into intervention (probiotic consumption) and control (placebo) groups, from which similar baseline data on key outcome variables were collected;
Outcome variables included total cholesterol, HDL, LDL, triglycerides, BMI, waist circumference, hs-CRP, and TNF-alpha;
Only those papers with the largest sample and longest intervention duration were included where there were multiple publications and companion papers from the same population.
Data abstraction and quality assessment
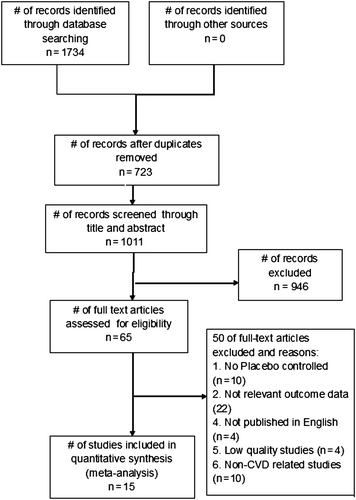
Abstraction of data and assessment of studies according to the criteria was independently conducted by two reviewers. A summary of the review is presented in a PRISMA flow chart (). A pre-piloted data form was used to extract data from the selected randomized controlled studies. Data on the effects of probiotics in at-risk groups were extracted. At-risk groups were defined as participants who had hypertension, abnormal lipid profiles, obesity, or metabolic abnormality. Outcome variables that were examined included cholesterol, LDL, HDL, triglyceride, BMI, and waist circumference, as well as inflammatory markers such as hs-CRP and TNF-alpha. Other variables examined included duration of probiotics intake, dosage of probiotics use, probiotics in fermented milk/yogurt or in capsule forms, types of strains of probiotics, single strain versus multiple strains of probiotics intake. The cut-off scores of 5.5 mmol/L and 3.5 mmol/L, respectively, for total cholesterol and LDL were taken to divide total cholesterol and LDL as elevated and normal levels, in accordance with guideline provided by Gould and colleagues (Citation16,Citation17).
The PEDro tool was used to assess the quality of the abstracted articles () (Citation18). This tool categorizes the quality of the evidence into three levels—high quality (8 or more points), moderate quality (4–7 points), and low quality (3 points or less)—based on 10 factors. Studies that were deemed to be low quality were excluded (Citation18). The 10 factors were: 1) Random allocation of subjects into groups; 2) Concealed randomization; 3) Similarity of baseline information between groups; 4) Blinding in relation to subjects and assessors; 5) Blinding in relation to researchers; 6) Blinding in relation to assessors; 7) Low attrition rate; 8) Use of ‘intention to treat’ analysis; 9) Use of variability measures such as standard deviation and/or standard error; and 10) Between-group comparison results. One point was allocated for each factor present in the study. Factors that were used to downgrade the quality of evidence in a study included the potential for bias such as non-randomization, single blinding or no blinding, lack of baseline data, no between-group comparison results, no use of an ‘intention to treat’ measure, and no use of variability measures. There were no obvious discrepancies in ratings between the two assessors relating to the quality of the selected studies (Supplementary Table 1, to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2015.1071872).
Table I. Characteristics of included clinical trials.
Statistical analysis
A mean difference with a 95% confidence interval (CI) was used. Random-effect models were used to take into account between-study variation. The heterogeneity level was estimated based on the I2 index for all studies. Studies with values of less than 30% were considered to have low heterogeneity, 30%–50% was considered moderate heterogeneity. Random-effect models were used to take into account between-study variation. The detailed effect of probiotics was further explored using subgroup analysis to assess whether there are possible sources of heterogeneity. These analyses were conducted in relation to the effects of: 1) Duration of the probiotics intake of less than 8 weeks compared with 8 weeks or longer; 2) Dosage level involving 109 colony-forming units (CFU) or more versus 108 CFU or less and fermented dairy products (109 CFU as cut-off); 3) Medium type (i.e. milk-based versus capsule-based); 4) Single strain versus multiple strain; 5) Acidophilus versus non-acidophilus strains; and 6) People with hypercholesterolemia and other CVD risk factors. Publication bias was assessed by use of a funnel plot (Citation19). Sensitivity analysis was conducted to assess whether the inferences were overly dependent on a particular study.
Results
In the initial search, 1,734 articles were identified from the key databases (1,051 from PubMed, 667 from Scopus, and 16 from Cochrane Library) () and imported into EndNote. After removing duplicate studies, the remaining 1,011 studies were screened for relevance through their title and abstract, and then assessed according to the eligibility criteria previously stated. This step resulted in 65 articles. A further review that was conducted using PEDro excluded 40 papers (22 had irrelevant outcome data, 10 lacked placebo groups, 4 used a language other than English, 4 were low quality, and 10 were trials with healthy participants). The remaining 15 papers were included in the final quantitative analysis; 14 were of high quality (8 points or more) and one was of moderate quality (5–7 points). shows the final list of included studies and their summary characteristics.
Description of the included studies
Fifteen randomized and placebo-controlled studies examining the effects of probiotics on the risk factors of CVD were included in the analysis (), representing a total sample of 788 subjects. Among these studies, all were blinded to assessors, 11 were double-blinded, and 4 were single-blinded. All studies had placebo-controlled groups as comparison groups. Placebo groups used placebo capsules that were identical to probiotics capsules in appearance except that the bacterial strains were absent (Citation20–24). Studies that used milk or yogurt as the medium had placebo products that were prepared in the same way as the probiotic milk or yogurt except the bacterial strains were absent (Citation11–13,Citation20,Citation25–30). Countries in which the studies were conducted included Canada, Australia, Brazil, Denmark, Sweden, Spain, Iran, Japan, and Korea. Subject characteristics included hypercholesterolemia (Citation11,Citation21,Citation24,Citation26,Citation27), hypertension (Citation28,Citation29), diabetes (Citation20,Citation25), overweight and obesity (Citation13,Citation22,Citation23,Citation31), metabolic abnormality (Citation12), and heavy smoking (Citation30). All studies measured one or more of the following outcome variables: cholesterol, HDL, LDL, triglyceride, waist circumference, BMI, and inflammatory biomarkers. The summary description of the study characteristics is presented in .
Effects on cholesterol and lipid profiles
Ten studies reported the effects of probiotics on total cholesterol, 10 studies on LDL, 11 studies on HDL, and 12 studies on triglyceride (). There were 464 participants in studies examining total cholesterol, 533 participants for LDL, 579 participants for HDL, and 614 participants for triglyceride. Of the 12 studies on triglyceride, 4 reported results involving 225 hypercholesterolemia participants, and 8 studies involving 389 participants reported results in other health conditions including hypertension, diabetes, and metabolic conditions.
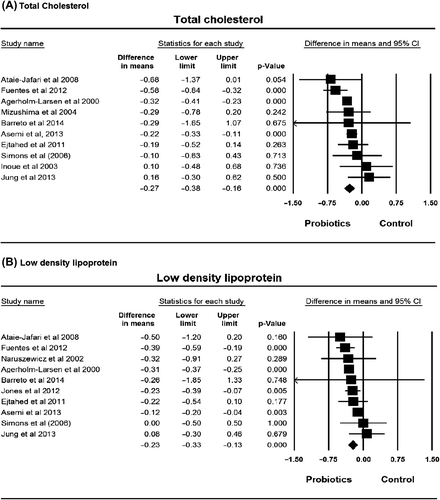
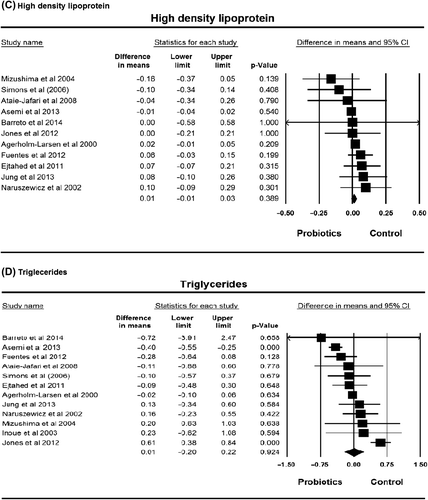
Figure 2. Lipid profile plot. (A) total cholesterol; (B) low-density lipoprotein cholesterol; (C) high-density lipoprotein cholesterol; (D) triglycerides.
Of the 10 studies on cholesterol 8 reported a reduction in this factor after consuming probiotics. The mean reduction at baseline was 0.50 mmol/L (ranging from 0.13 to –1.43 mmol/L), compared with 0.19 mmol/L (ranging from 0.36 to –0.56 mmol/L) reduction in the control group. The pooled effect of the total cholesterol was –0.27 mmol/L (95% CI –0.38 to –0.16, P < 0.00001) in the probiotics group compared with the control group. The forest plot of the effect is presented in . The heterogeneity was not significant (I2 = 35.5%, P = 0.12).
Nine of 10 studies on LDL presented changes in this factor after consuming probiotics. The mean reduction of LDL was 0.49 mmol/L (ranging 0.16 to –1.89 mmol/L). The pooled effect of the LDL was –0.23 mmol/L (95% CI –0.33 to –0.13 mmol/L, P < 0.00001) in the mean difference in the probiotics group compared with the control group (), with significant heterogeneity (I2 = 56.58%, P = 0.01).
Five of 11 studies on HDL showed an increase in this factor, while 6 studies showed a decrease in HDL after consuming probiotics. There was an average decrease of 0.03 mmol/L (ranging from 0.13 to –0.26 mmol/L). The pooled effect of the HDL was 0.01 mmol/L (95% CI –0.01 to 0.03 mmol/L, P = 0.39), with non-significant heterogeneity (I2 = 0%, P = 0.57) ().
Five of 12 studies on triglycerides showed a decrease in this factor, 6 studies showed an increase, and 1 study showed no change after consuming probiotics. The lowest decrease in triglycerides was 0.01 mmol/L and the highest decrease was 0.29 mmol/L. The meta-analysis result showed non-statistical decrease of 0.01 mmol/L (95% CI –0.20 to 0.22 mmol/L, P = 0.92), with non-significant heterogeneity (I2 = 0%, P = 0.57) ().
Effects on BMI and waist circumference
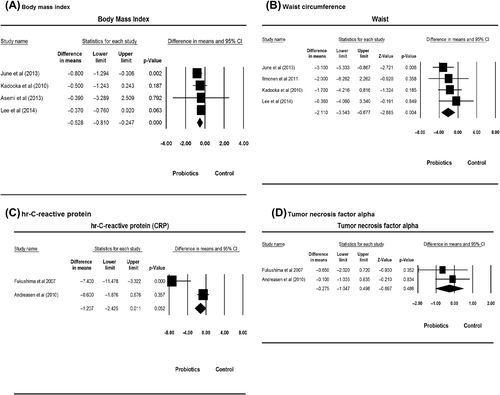
Four studies (Citation13,Citation22,Citation23,Citation25) reported BMI results among 234 subjects, and four studies (Citation13,Citation22,Citation23,Citation32) reported waist circumference results among 304 subjects (). Three of the four studies showed a decrease in BMI after consuming probiotics. The mean reduction of BMI following probiotics consumption was 0.3 kg/m2 (–0.12 to 0.50 kg/m2). The pooled effect of the BMI was –0.52 kg/m2 (95% CI –0.80 to –0.24 kg/m2, P < 0.001), with unmeasurable heterogeneity (I2 = 0%, P = 0.59) (). All four studies showed a decrease in waist circumference. The average reduction of waist circumference was 1.82 cm, and the range of decease in waist circumference was 2.0 cm to 1.56 cm. The pooled effect of the waist circumference was –2.11 cm (95% CI –3.54 to –0.68 cm), with unmeasurable heterogeneity (I2 = 0%, P = 0.63) ().
Effects on inflammatory markers
The effects of probiotics on inflammatory markers including hr-CRP and TNF-alpha were measured. Two studies (Citation20,Citation33) on hr-CRP and TNF results among 69 subjects were reported. Probiotics caused an average hr-CRP reduction of 1.67 mg/L (range 0.20 mg/L to 2.26 mg/L reduction) in the probiotics group. The pooled effect was –2.95 mg/L (95% CI –0.99 to –4.91 mg/L, P = 0.002) (). TNF-alpha had an average reduction of 3.25 pg/mL (range 0 pg/mL to 9.0 pg/mL reduction) in the probiotics group, with a pooled effect of the TNF-alpha of –2.34 pg/mL (95% CI –0.53 to –4.14 pg/mL, P = 0.486) (), with no heterogeneity for hr-CRP (I2 = 0%, P = 0.002) and TNF-alpha (I2 = 0%, P = 0.49).
Subgroup and sensitivity analysis
The subgroup analysis found that the effects of the probiotics on cholesterol were statistically significant when it was in milk/ yogurt form (P < 0.001) compared to those using probiotics in capsule form (P > 0.05). Probiotics with multiple strains (P < 0.001) and the trials that were 8 weeks or longer in duration (P < 0.001) had significant effects on total cholesterol and LDL compared to those trials with a single strain and using probiotics for less than 8 weeks. A greater reduction with clinical significance was found when trials had baseline total cholesterol of more than 5.5 mmol/L (P < 0.001) compared with trials with total cholesterol of less than 5.5 mmol/L (P < 0.05) (). The reduction in cholesterol in trials was significant across trials both with acidophilus strain and those without acidophilus strains.
Table II. The subgroup analyses of the effect of probiotics on total cholesterol and LDL-C by probiotics administration criteria.
The subgroup analysis found that the effect of the probiotics on LDL was statistically significant when it was in milk/yogurt form (P < 0.001) compared to those trials using probiotics in capsule form, when the probiotics consisted of multiple strains (P < 0.001) rather than a single strain, and when the trials contained acidophilus strain compared to trials that did not include the acidophilus strains. A greater reduction with clinical significance was also found in trials with baseline LDL of 3.5 mmol/L or more (P < 0.001) compared with trials with less than 3.5 mmol/L (P < 0.05) ().
The subgroup analysis found that the effects of the probiotics diet on HDL and triglyceride were not related to the form of probiotics, dosage, number of strains, duration of administration of the probiotics consumption, and health status of the participants ().
Table III. The subgroup analyses of the effect of probiotics on HDL-C and triglycerides by probiotics administration criteria.
Sensitivity analyses revealed that no particular study significantly affected the summary effects for total cholesterol, LDL, BMI, and waist circumference.
Publication bias
The results for all factors showed minimal asymmetry, suggesting minimal publication bias. A visual inspection of the funnel plots showed no clear evidence of publication bias with regard to effects on total cholesterol, LDL, HDL, triglyceride, BMI, and waist circumference (). Findings from Egger's test (total cholesterol: P = 0.56; LDL: P = 0.74; HDL: P = 0.97; triglyceride: P = 0.93; BMI: P = 0.94; waist circumference: P = 0.35) () supported the finding that there was no publication bias. Trim and fill did not change the overall effects for all variables. These tests did not reveal any statistically significant evidence of publication bias for any assessed outcomes.
Figure 4. Funnel plots. (A) Total cholesterol; (B) low-density lipoprotein cholesterol; (C) high-density lipoprotein cholesterol; (D) triglyceride; (E) BMI; (F) Waist.
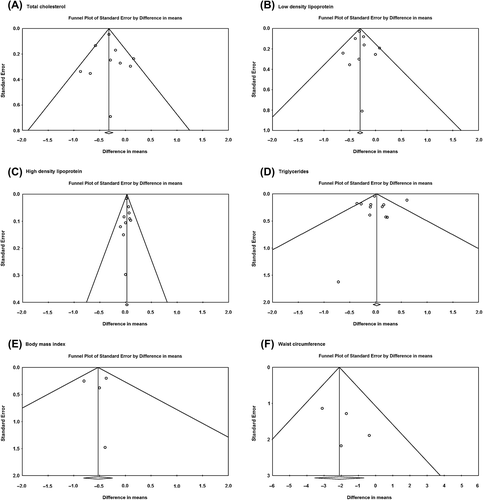
Table IV. Egger test results on lipids, BMI, and waist circumference.
Discussion
This meta-analysis systematically reviewed 15 randomized controlled trials that examined the effects of probiotics on reducing cholesterol and other coexisting risk factors associated with CVD. To our knowledge, this is the first meta-analysis of high-quality randomized controlled trials investigating this topic. The pooled effects based on the meta-analysis revealed that probiotics reduced CVD risk factors, including high blood pressure and lipid components, as well as improving BMI, waist circumference, and CRP. However, the effects of probiotics on HDL, triglycerides, and TNF were not statistically significant. The reduction in total cholesterol and LDL was similar to that reported in another meta-analysis of the effect of statin doses on reducing total cholesterol and LDL (Citation34).
Probiotics were found to provide multiple benefits—not only reducing cholesterol and LDL, but also improving coexisting risk factors such as BMI, waist circumference, and inflammatory biomarkers. Worldwide, these major risk and coexisting risk factors are often ignored in treatment regimes, yet they need to be addressed as reduction in lipid components alone does not significantly reduce the mortality and morbidity associated with CVD. Our results are consistent with previous published reviews regarding the effects of probiotics on the risk factors of CVD, including cholesterol (Citation35,Citation36) and LDL (Citation35,Citation36).
Effects of probiotics on lipid profiles and CVD risk factors
Subgroup analyses revealed that the significant effects of probiotics on the reduction of total cholesterol and LDL may be due to the effects of probiotic forms (i.e. milk/yogurt versus capsules), duration of intake, and number of strains in the products. Most of the trials (11 out of 15) used a fermented dairy product, rather than a capsule. Probiotics in the fermented dairy form had significant pooled effects on the total cholesterol and LDL, and probiotics in the capsule form had no significant reduction of total cholesterol and LDL. These findings are consistent with two clinical trials that found that probiotics using a fermented dairy product were effective in reducing total cholesterol and LDL to 10% (Citation37,Citation38).
It was found that trials using multiple probiotic strains showed a statistically significant reduction of total cholesterol, whereas trials with a single strain did not have a significant reduction in total cholesterol. This result is similar to the finding that, compared with those with a single strain, trials with multiple strains had better effects on blood pressure (Citation39). Due to a limited number of trials with a single strain, further studies are warranted to confirm this finding. In the studies we reviewed, a number of genera of bacteria were used in probiotics, including L. acidophilus, L. plantarum, Bifidobacterium, Enterococcus, S. thermophilus, L. bulgaricus, Streptococcus. We found that L. acidophilus has the strongest effect on LDL reduction. This may be because L. acidophilus can pass through the digestive tract intact and alive compared to other strains, thereby optimizing its activity level in the large intestine (Citation40).
The mechanism through which probiotics in fermented milk acts might be through the production of an enzyme called bile salt hydrolase (Citation41). This enzyme reduces the absorption of cholesterol in the bloodstream, and instead it becomes trapped in the gut and later excreted in fecal matter. The conversion of cholesterol to bile salts in the liver and subsequent secretion and fecal excretion provides the major route (∼90%) for the elimination of elevated or excess cholesterol. Another mechanism by which the lipid-lowering effects occur is through probiotics stimulating the production of short-chain fatty acids in the gut, which modulate blood lipid levels (Citation42,Citation43). This mechanism acts by removing cholesterol through the incorporation of cholesterol into cellular membranes in the intestines, thereby reducing serum cholesterol in humans (Citation44).
The impact of probiotics on cholesterol and LDL may be also explained by its effects on the reduction of coexisting risk factors, including BMI, waist circumference, and inflammatory markers such as CRP. The reduction of these factors is important because of their cumulative effect on CVD (Citation1,Citation45). Outcomes of a number of clinical trials indicate the weight-lowering and anti-oxidative properties of probiotics as manifested by the improvement in the BMI and waist circumference (Citation46). This effect may be due to the calcium in dairy products, which can reduce body fat and body mass (Citation46). In addition, probiotics can attenuate acute-phase inflammation by changing the intestinal inflammatory barrier, and impact the inflammatory process via the effect of intestinal microflora on bile and metabolism. The reduction of these risk factors through probiotics consumption has significant implications for reducing the mortality and morbidity attributable to CVD when major risk factors such as cholesterol and LDL are also controlled (Citation1).
No studies in this meta-analysis reported any adverse side effects related to the media used (fermented milk and capsules), indicating that they were safe and well tolerated. In addition, 14 of the 15 studies had less than a 15% attrition rate over the course of the intervention, suggesting that the subjects had a high level of satisfaction with the consumption of probiotics. Fourteen of the 15 included trials did not use medication. When the study using herbal extracts in both probiotics and placebo groups was excluded from the analysis (Citation23), the results remained significant. This suggests that probiotics consumption provides health benefits equivalent to medication use and has achieved significant intervention effects at both the statistical and clinical level without medication intake or controlled medication use.
Strengths, limitations, and implications
A strength of this study is that all studies published since 2000 have been included in the meta-analysis, and where there was unreported data, the authors were contacted to provide it. Therefore, all available data were included, thereby increasing the power of the study (Citation39). All included studies in this review used placebos as the control group; thus, the effect of intervention due to probiotics consumption could be examined. The results of these analyses should therefore be viewed as accurate and precise. This is the first meta-analysis study on RCTs relating to the effect of probiotics on lipid profiles. This meta-analysis suggests an avenue for future intervention programs to investigate the effects of probiotics consumption on improving lipid profiles.
A number of limitations of this study should be acknowledged. The studies did not systematically report treatment effects in clinically sensible subgroups, such as risk strata defined by the Framingham Risk Score (Citation47). A previous randomized control study suggested that the treatment effects of dietary-based interventions vary across these strata, with benefits confined to high-risk individuals who have at least two to three clinical risk factors (Citation48). Further exploration of such subgroup differences within the context of RCTs will entail an individual patient data meta-analysis. There was inconsistent use of strain types among some of the studies, with some using one strain and others multiple strains, making it difficult to assess which strain or strains contributed to the effect of probiotics. Further RCTs involving long-term interventions that consistently compare single versus multiple strains of probiotics supplement regimens are required to clarify these findings further. It is also acknowledged that we did not include studies published in languages other than English, so it is unknown whether results from these studies would have modified our findings.
The efficacy of the probiotics in reducing the risk of complications, reoccurrence of major cardiovascular events, and mortality in patients with more severe heart conditions was not examined in our study. Future research is required using a large sample size and long-term intervention RCT to investigate whether alternative nutrition regimens are effective with regard to the reduction of not only the major risk factors of CVD, but also the coexisting inflammatory factors. This will enhance our understanding of the application of probiotics in CVD prevention and treatment. Further research is also needed into whether probiotics has a superior effect to pharmaceutical drug administration in order to determine whether it should be included as a treatment option for CVD. At the very least, the field of translational cardiovascular medicine should explore probiotics as an important dietary supplement for reducing total cholesterol and LDL, as well as other coexisting risk factors of CVD.
Conclusions
The findings of this study suggest that probiotics supplementation use is effective in reducing total cholesterol, LDL, and the coexisting risk factors of CVD. Probiotics are more effective when they are taken as a fermented milk or yogurt product, consumed for at least 8 weeks, and include multiple strains. By managing multiple risk factors and coexisting risk factors associated with CVD, probiotics may provide a powerful avenue to reduce current high rates of cardiovascular morbidity and mortality.
Supplementary material available online
Supplementary Table 1 to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2015.1071872.
iann_a_1071872_sm5213.pdf
Download PDF (435.2 KB)Acknowledgements
J.S. searched the articles; extracted and analyzed and interpreted data; and drafted, edited, and proofed the paper. N.B. extracted data and reviewed and proofed the paper.
Funding: The authors have no financial disclosure.
Declaration of interest: The authors report no conflicts of interest.
References
- World Health Organization. Prevention of cardiovascular disease: guidelines for assessment and management of total cardiovascular risk. Geneva: World Health Organization; 2007.
- Hall JE, Jones DW, Kuo JJ, da Silva A, Tallam LS, Liu J. Impact of the obesity epidemic on hypertension and renal disease. Curr Hypertens Rep. 2003;5:386–92.
- Cushman M, Arnold AM, Psaty BM, Manolio TA, Kuller LH, Burke GL, et al. C-reactive protein and the 10-year incidence of coronary heart disease in older men and women: the cardiovascular health study. Circulation. 2005;112:25–31.
- Godley P, Pham H, Rohack J, Woodward B, Yokoyama K, Maue SK. Opportunities for improving the quality of hypertension care in a managed care setting. Am J Health Syst Pharm. 2001;58: 1728–33.
- Klungel OH, Seidell JC, de Boer A. Overestimation of the prevalence of hypertension in the population. J Hypertens. 1998;16:1702–3.
- Gilliland SE, Morelli L, Reid G. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Cordoba, Argentina: Organization of the United Nations/World Health Organization 2001.
- Fooks LJ, Gibson GR. Probiotics as modulators of the gut flora. Br J Nutr. 2002;88(Suppl 1):S39–49.
- Kaushik JK, Kumar A, Duary RK, Mohanty AK, Grover S, Batish VK. Functional and probiotic attributes of an indigenous isolate of Lactobacillus plantarum. PLoS One. 2009;4:e8099–e.
- Bron PA, van Baarlen P, Kleerebezem M. Emerging molecular insights into the interaction between probiotics and the host intestinal mucosa. Nat Rev Microbiol. 2012;10:66–78.
- Agerholm-Larsen L, Bell ML, Grunwald GK, Astrup A. The effect of a probiotic milk product on plasma cholesterol: a meta-analysis of short-term intervention studies. Eur J Clin Nutr. 2000;54:856–60.
- Jones ML, Martoni CJ, Di Pietro E, Simon RR, Prakash S. Evaluation of clinical safety and tolerance of a Lactobacillus reuteri NCIMB 30242 supplement capsule: a randomized control trial. Regul Toxicol Pharmacol. 2012;63:313–20.
- Barreto FM, Colado Simão AN, Morimoto HK, Batisti Lozovoy MA, Dichi I, Helena da Silva Miglioranza L. Beneficial effects of Lactobacillus plantarum on glycemia and homocysteine levels in postmenopausal women with metabolic syndrome. Nutrition. 2014;30:939–42.
- Kadooka Y, Sato M, Imaizumi K, Ogawa A, Ikuyama K, Akai Y, et al. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur J Clin Nutr. 2010;64:636–43.
- Huang X, Lin J, Demner-Fushman D. Evaluation of PICO as a knowledge representation for clinical questions. AMIA Annu Symp Proc. 2006:359–63.
- Tacconelli E. Systematic reviews: CRD’s guidance for undertaking reviews in health care. Lancet Infect Dis. 2010;10:1–.
- Gould AL, Davies GM, Alemao E, Yin DD, Cook JR. Cholesterol reduction yields clinical benefits: meta-analysis including recent trials. Clin Ther. 2007;29:778–94.
- Gould AL, Rossouw JE, Santanello NC, Heyse JF, Furberg CD. Cholesterol reduction yields clinical benefit: impact of statin trials. Circulation. 1998;97:946–52.
- Verhagen AP. The Delphi list: a criteria list for quality assessments of randomised clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol. 1998;51:1235–41.
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
- Andreasen AS, Larsen N, Pedersen-Skovsgaard T, Berg RMG, Moller K, Svendsen KD, et al. Effects of Lactobacillus acidophilus NCFM on insulin sensitivity and the systemic inflammatory response in human subjects. Br J Nutr. 2010;104:1831–8.
- Fuentes MC, Lajo T, Carrión JM, Cuñé J. Cholesterol-lowering efficacy of Lactobacillus plantarum CECT 7527, 7528 and 7529 in hypercholesterolaemic adults. Br J Nutr. 2013;109:1866–72.
- Jung SP, Lee KM, Kang JH, Yun SI, Park HO, Moon Y, et al. Effect of Lactobacillus gasseri BNR17 on overweight and obese adults: a randomized, double-blind clinical trial. Korean J Fam Med. 2013;34:80–9.
- Lee SJ, Bose S, Seo JG, Chung WS, Lim CY, Kim H. The effects of co-administration of probiotics with herbal medicine on obesity, metabolic endotoxemia and dysbiosis: a randomized double-blind controlled clinical trial. Clin Nutr. 2013;13:S0261–5614.
- Simons LA, Amansec SG, Conway P. Effect of Lactobacillus fermentum on serum lipids in subjects with elevated serum cholesterol. Nutr Metab Cardiovasc Dis. 2006;16:531–5.
- Asemi Z, Zare Z, Shakeri H, Sabihi SS, Esmaillzadeh A. Effect of multispecies probiotic supplements on metabolic profiles, hs-CRP, and oxidative stress in patients with type 2 diabetes. Ann Nutr Metab. 2013;63:1–9.
- Ataie-Jafari A, Larijani B, Alavi Majd H, Tahbaz F. Cholesterol-lowering effect of probiotic yogurt in comparison with ordinary yogurt in mildly to moderately hypercholesterolemic subjects. Ann Nutr Metab. 2009;54:22–7.
- Ejtahed HS, Mohtadi Nia J, Homayouni Rad A, Niafar M, Asghari Jafarabadi M, Mofid V. The effects of probiotic and conventional yoghurt on diabetes markers and insulin resistance in type 2 diabetic patients: a randomized controlled clinical trial. Iranian J Endocrinol Metab. 2011;13:112.
- Inoue K, Shirai T, Ochiai H, Kasao M, Hayakawa K, Kimura M, et al. Blood-pressure-lowering effect of a novel fermented milk containing gamma-aminobutyric acid (GABA) in mild hypertensives. Eur J Clin Nutr. 2003;57:490–5.
- Mizushima S, Ohshige K, Watanabe J, Kimura M, Kadowaki T, Nakamura Y, et al. Randomized controlled trial of sour milk on blood pressure in borderline hypertensive men. Am J Hypertens. 2004; 17:701–6.
- Naruszewicz M, Johansson ML, Zapolska-Downar D, Bukowska H. Effect of Lactobacillus plantarum 299v on cardiovascular disease risk factors in smokers. Am J Clin Nutr. 2002;76:1249–55.
- Agerholm-Larsen L, Raben A, Haulrik N, Hansen AS, Manders M, Astrup A. Effect of 8 week intake of probiotic milk products on risk factors for cardiovascular diseases. Eur J Clin Nutr. 2000;54:288–97.
- Ilmonen J, Isolauri E, Poussa T, Laitinen K. Impact of dietary counselling and probiotic intervention on maternal anthropometric measurements during and after pregnancy: a randomized placebo-controlled trial. Clin Nutr. 2011;30:156–64.
- Fukushima Y, Miyaguchi S, Yamano T, Kaburagi T, Iino H, Ushida K, et al. Improvement of nutritional status and incidence of infection in hospitalised, enterally fed elderly by feeding of fermented milk containing probiotic Lactobacillus johnsonii La1 (NCC533). Br J Nutr. 2007;98:969–77.
- Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–81.
- Guo C, Zhang L. Cholesterol-lowering effects of probiotics—a review. Wei Sheng Wu Xue Bao. 2010;50:1590–9.
- Guo CF, Zhang LW, Han X, Yi HX, Li JY, Tuo YF, et al. Screening for cholesterol-lowering probiotic based on deoxycholic acid removal pathway and studying its functional mechanisms in vitro. Anaerobe. 2012;18:516–22.
- Agerbaek M, Gerdes LU, Richelsen B. Hypocholesterolaemic effect of a new fermented milk product in healthy middle-aged men. Eur J Clin Nutr. 1995;49:346–52.
- Bertolami MC, Faludi AA, Batlouni M. Evaluation of the effects of a new fermented milk product (Gaio) on primary hypercholesterolemia. Eur J Clin Nutr. 1999;53:97–101.
- Khalesi S, Sun J, Buys N, Jayasinghe R. Effect of probiotics on blood pressure: a systematic review and meta-analysis of randomized, controlled trials. Hypertension. 2014;64:897–903.
- Baroutkoub A, Mehdi RZ, Beglarian R, Hassan J, Zahra S, Mohammad MS, et al. Effects of probiotic yoghurt consumption on the serum cholesterol levels in hypercholestromic cases in Shiraz, Southern Iran. Scientific Research and Essays. 2010;5:2206–9.
- Jones ML, Tomaro-Duchesneau C, Martoni CJ, Prakash S. Cholesterol lowering with bile salt hydrolase-active probiotic bacteria, mechanism of action, clinical evidence, and future direction for heart health applications. Expert Opin Biol Ther. 2013;13:631–42.
- Pereira DIA, Gibson GR. Effects of consumption of probiotics and prebiotics on serum lipid levels in humans. Crit Rev Biochem Mol Biol. 2002;37:259–81.
- Wong JM, Souza Rd, Kendall CW, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006; 40:235–43.
- Pigeon RM, Cuesta EP, Gililliand SE. Binding of free bile acids by cells of yogurt starter culture bacteria. J Dairy Sci. 2002;85:2705–10.
- Wing RR, Lang W, Wadden TA, Safford M, Knowler WC, Bertoni AG, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481–6.
- Ebringer L, Ferencík M, Krajcovic J. Beneficial health effects of milk and fermented dairy products—review. Folia Microbiol (Praha). 2008; 53:378–94.
- Anderson KM, Odell PM, Wilson PW, Kannel WB. Cardiovascular disease risk profiles. Am Heart J. 1991;121:293–8.
- Tovar J, Nilsson A, Johansson M, Ekesbo R, Aberg A, Johansson U, et al. A diet based on multiple functional concepts improves cardiometabolic risk parameters in healthy subjects. Nutr Metab (Lond). 2012;9:9–29.