Abstract
Background. The prevalence of multimorbidity (≥ 1 disease within an individual) is rapidly increasing. So far, studies on the relationship between vitamin D and morbidity are mainly focusing on effects on single disease domains only, while vitamin D biology is associated with several diseases throughout the human body.
Methods. We studied 8,726 participants from the LifeLines Cohort Study (a cross-sectional, population-based cohort study) and used the self-developed composite morbidity score to study the association between vitamin D levels and multimorbidity.
Results. Study participants (mean age 45 ± 13 years, 73% females) had a mean plasma vitamin D level of 59 ± 22 nmol/L. In participants aged between 50 and 60 years, 58% had ≥ 2 affected disease domains, while morbidity score increased with age (70–80 years: 82% morbidity score > 1; > 80 years: 89% morbidity score > 1). Each incremental reduction by 1 standard deviation (SD) of vitamin D level was associated with an 8% higher morbidity score (full model OR 0.92, 95% CI 0.88–0.97, P = 0.001). Participants with vitamin D levels < 25 nmol/L were at highest risk for increasing morbidity prevalence (versus > 80 nmol/L, OR 1.34, 95% CI 1.07–1.67, P = 0.01).
Conclusions. Low levels of vitamin D are associated with higher prevalence of multimorbidity, especially in participants with vitamin D levels < 25 nmol/L. Collectively, our results favor a general, rather than an organ-specific, approach when assessing the impact of vitamin D deficiency.
Multimorbidity is common, and its prevalence increases with age.
Low levels of vitamin D are associated with higher multimorbidity prevalence.
The (pathophysiological) effects of vitamin D deficiency on the human body should be assessed with a general rather than an organ-specific approach.
Introduction
The prevalence of multimorbidity, commonly defined as the manifestation of more than one single disease in an individual at a given point in time, is increasing rapidly. However, due to a lack of consensus as to how multimorbidity itself is defined (Citation1), the true prevalence of multimorbidity in the general population remains unclear, with estimates ranging from 17% to 90% (Citation2). It is apparent that the presence of multimorbidity is associated with a higher mortality risk, impaired physical and mental functioning, as well as a decreased quality of life (Citation3), yet resources continue to be directed towards addressing organ-specific single diseases, whilst multimorbidity attracts relatively less attention. The increasing prevalence of multimorbidity in the aging population thus urges health care professionals to address multiple medical problems in different disease domains in an orchestrated effort (Citation4).
Over the last few decades, the pathophysiological effects of vitamin D deficiency on the human body have been extensively studied, and it is now a widely accepted fact that vitamin D deficiency is a global problem (Citation5). Data from clinical and epidemiological studies suggest that low vitamin D levels are associated with a variety of acute and chronic diseases (Citation6). Traditionally, vitamin D is considered to act on calcium and phosphate homeostasis, but recent research has shown that low vitamin D levels are associated with a wide range of non-skeletal impairments as well, including depression (Citation7), cardiovascular and cerebrovascular disease (Citation8), and several types of cancers (Citation9). Despite this, however, studies on the relationship between vitamin D and disease have mainly focused on patients with a single disease domain present, even though there is sufficient evidence to suggest that vitamin D biology is associated with several diseases throughout the human body. These observations in the literature provided a rationale for us to study the association between vitamin D and multimorbidity.
Since vitamin D may influence several organ systems simultaneously, we studied the association of vitamin D levels and prevalence of multimorbidity using data from a large population-based study in the Netherlands (the LifeLines Cohort).
We hypothesized that low plasma concentrations of vitamin D are associated with increased prevalence of multimorbidity in the general population, especially in participants with vitamin D levels below 25 nmol/L.
Material and methods
This manuscript was written in adherence to the guidelines of Strengthening The Reporting of Observational Studies in Epidemiology (STROBE) (Citation10).
Study design
In this cross-sectional study, we collected data from participants of the LifeLines Cohort Study. Prior to the first visit at the LifeLines outpatient clinic, all participants were asked to complete a self-administered questionnaire, requiring them to provide information pertaining to their medical history, current diseases, use of medication, and health behavior. Simultaneously, participants were instructed to bring in a detailed list with information about their latest medication used, which was then registered in the LifeLines database (verification took place at a later time point). During the first visit to the LifeLines outpatient clinic, all LifeLines participants underwent a clinical examination, followed by blood and urine collection. In addition, current psychiatric disorders (depressive disorder, dysthymia, and anxiety disorders) were assessed with a brief standardized diagnostic interview: the Mini International Neuropsychiatric Interview (MINI) 5.0.0 (Citation11). Data from the questionnaires, medication list, clinical examination, and MINI were collectively used to determine the point prevalence of disease in this population, with a simple morbidity score as the composite end-point. More detailed information about the data collection can be found in Supplementary Appendix I to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2015.1073347.
Lifelines Cohort Study
LifeLines is a multidisciplinary population-based cohort study that examines the health and the health-related behaviors of 167,729 persons living in the North East region of the Netherlands in a unique three-generation design (Citation12). It employs a broad range of data collection including biomedical, socio-demographic, behavioral, physical, and psychological factors that contribute to the health and disease of the general population, with a special focus on multimorbidity. All LifeLines participants completed a number of questionnaires containing questions on a broad spectrum of diseases, including obesity, cardiovascular and renal diseases, pulmonary diseases and allergy, cognitive function and depression, and musculoskeletal conditions. After completion of these questionnaires, LifeLines participants were invited for clinical examination and blood sampling at the LifeLines outpatient clinic.
Study population
LifeLines participants were invited through their general practitioner. A selection of general practitioners from the three northern provinces (situated at the 53rd parallel, northern hemisphere) of the Netherlands, invited all of their listed patients between 25 and 50 years of age (index cohort). These probands were then asked to invite their family members (if present) to participate as well (parents, partners, parents-in-law, children). The inclusion phase was closed in December 2013 with a total of 167,729 participants. From this cohort, 9,109 plasma samples were randomly selected in which we determined vitamin D levels (Supplementary Figure 1 to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2015.1073347). The study protocol was approved by the medical ethics review committee of the University Medical Center Groningen (UMCG) and conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent upon enrollment.
Vitamin D assay
Plasma 25-hydroxyvitamin D3 levels were measured by solid phase extraction isotope dilution followed by liquid chromatography–tandem mass spectrometry (Spark-Holland Symbiosis system, Emmen, The Netherlands) with a detection limit of 1.2 nmol/L, and intra-assay and inter-assay coefficient of variation from 5.0% to 14.1% vitamin D levels were measured in nmol/L (2.5 nmol/L is equivalent to 1 ng/mL) and categorized using the following cut-offs (Citation14): sufficient (> 80 nmol/L), hypovitaminosis D (50–80 nmol/L), insufficient (25–50 nmol/L), and deficient (< 25 nmol/L).
Defining single morbidities, disease domains, and morbidity score
Single morbidities were scored according to the 10th edition of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) (Citation15) (for a detailed list of single morbidities see Supplementary Appendix II to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2015.1073347). Next, single morbidities were clustered into 12 different disease domains: genitourinary, renal, hematologic, dermatologic, musculoskeletal, ophthalmic and ear–nose–throat (ENT), psychiatric, endocrine, cardiovascular, respiratory, central nervous system (CNS), and gastrointestinal disease (for a detailed list of single morbidities within each disease domain see Supplementary Appendix II to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2015.1073347). Together, these 12 disease domains represent health and disease of the entire body. Scrutinizing relevant literature reveals that a standardized approach for measuring multimorbidity does not exist. Therefore, we calculated a simple morbidity score as a composite end-point, in which a disease domain is considered as ‘affected’ when at least one single disease is present within this disease domain shortly before and during the first visit at LifeLines outpatient clinic. The morbidity score thus represents the point prevalence of 12 disease domains, with a maximum score of 12. Unfortunately, this method of registration has inherent uncertainties concerning sensitivity of disease registration. Therefore, self-reported diseases were registered in this study when the use of appropriate medication was verified.
Statistical analyses
Baseline characteristics are presented according to vitamin D categories. Continuous data are represented as means ± standard deviation (SD) for normally distributed data and as medians with interquartile ranges (IQR) for non-normal distributions. Discrete and categorical data are presented as frequencies (%).
Unless specified otherwise, outcomes were compared to participants who had vitamin D levels in the sufficient category (> 80 nmol/L). For continuous data, baseline differences among the four vitamin D categories were tested using one-way ANOVA or Kruskal–Wallis tests, as appropriate. Baseline differences between different vitamin D categories for discrete and categorical variables were compared using a standard chi-square test.
It is known that vitamin D levels correlate significantly with season of blood withdrawal (Citation16). In order to confirm the relationship between vitamin D levels and season of blood withdrawal in our participants, we performed a Spearman's rho test. Additionally, as described previously (Citation16), we added two variables (R = cos([2π/365.25]× day) and S = sin([2π/365.25]× day) to correct for plasma vitamin D levels and day of blood sampling in all subsequent analyses.
To study the association between vitamin D levels and a single disease domain we used logistic regression analyses. In addition, to study the association between vitamin D levels as well as vitamin D categories with morbidity score we used ordinal logistic regression analyses. The proportional odds assumption for the ordinal logistic models were checked and met. Odds ratios (ORs) were reported with 95% confidence interval (95% CI). In the primary analyses, we studied the association between vitamin D levels and vitamin D categories with single disease domains and morbidity score without assessment for covariates. In addition to these analyses, we created a model to adjust for risk factors for morbidity, and determinants of plasma vitamin D levels. This model controlled for several determinants of both morbidity and plasma vitamin D levels (sex, age, BMI, smoking, kidney function) (Citation17), lifestyle factors related to morbidity and vitamin D levels (education level, physical activity, alcohol consumption) (Citation18,Citation19), and sole determinants of plasma vitamin D levels (albumin-corrected plasma calcium levels and dairy consumption) (Citation20). The results generated from this model were considered the main results and are presented unless otherwise stated. In our model, age, kidney function, and albumin-corrected plasma calcium levels were included as linear variables, whereas all other variables were included as categorical variables with dummy variables.
Since our method of data collection could potentially result in morbidities being under- or over-reported, we conducted a sensitivity analysis in which we randomly excluded two different disease domains for three consecutive times. Finally, as we did not score the presence of cancer as a separate disease domain, we also ran sensitivity analyses in both model 1 and 2 excluding participants who reported having cancer (n = 77).
All P values are two-tailed. A P value < 0.05 was considered statistically significant. Analyses were performed using STATA v.11SE (College Station, TX, USA).
Results
From January 2010 until October 2011 we collected and measured plasma vitamin D levels in 9,091 samples. After exclusion of participants with vitamin D assay issues, pregnant women, and non-Caucasian participants, a total of 8,726 vitamin D samples from participants were included in the final analysis (Supplementary Figure 1 to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2015.1073347). Mean age was 45 ± 13 years, with more females than males (73% versus 27%). The mean plasma vitamin D level was 59 ± 22 nmol/L, and 17% of the participants had plasma vitamin D levels above 80 nmol/L ().
Table I. Baseline characteristics of all participants in the LifeLines Cohort Study, as well as per vitamin D category.
Vitamin D levels and season of blood sampling
Plasma vitamin D levels were different among seasons of blood sampling (). We observed that participants visiting the LifeLines outpatient clinic during the winter were more likely to have lower vitamin D levels than those visiting during the summer (Spearman's rho = 0.37, n = 8726, P < 0.000).
Distribution of morbidity score
displays the baseline characteristics of all individuals categorized by morbidity score. Participants with the lowest morbidity score (= 0) were more likely to be younger, less heavy, and non-smoking. Furthermore, we found that individuals who had a higher morbidity score had an increased likelihood of having higher glucose levels, increased systolic and diastolic blood pressures, while they reported less physical activity, were less well educated, and had lower vitamin D levels. The median sum of disease domains, as well as the distribution of participants with a higher morbidity score, increased substantially with age (). In the group of participants aged 20–30 years 62% of the population scored at least one disease domain, while in the group aged 50–60 years 58% of the participants had at least two affected disease domains. Almost all participants over 70 years had a morbidity score > 1 (70–80 years: 82%, > 80 years: 89%). As shown in , the gastrointestinal (23%), central nervous system (17%), respiratory (16%), cardiovascular (15%), and endocrine (12%) disease domains were the disease domains that were most commonly reported as affected.
Figure 2. LifeLines Cohort Study disease domains. (A) Distribution of number of disease domains in different age categories of subjects from the LifeLines Cohort Study. (B) Distribution of affected disease domains in subjects from the LifeLines Cohort Study. Contribution per disease domain on total of reported disease domains (%).
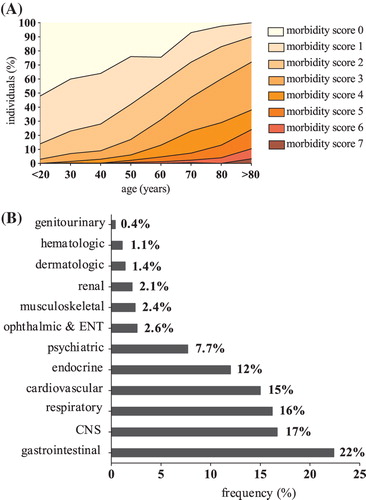
Table II. Baseline characteristics of participating subjects in the LifeLines Cohort Study, according to their morbidity score.
Vitamin D categories and baseline characteristics
also shows the baseline characteristics of the participating individuals according to their vitamin D category. Participants with lower plasma vitamin D levels were more likely to be male, had a higher BMI, systolic blood pressure, estimated glomerular filtration rate (eGFR), and plasma glucose, but lower plasma calcium and high-density lipoprotein (HDL) levels, and reported lower physical activity. In addition to these findings, we noticed some distinct differences in the baseline characteristics of the vitamin D-deficient participants. As compared to individuals with sufficient vitamin D levels, these participants were younger, more likely to be current smokers, and had a higher educational level. Furthermore, their reported dairy intake was rather low, and so was their alcohol consumption.
Association of vitamin D levels and single disease domains
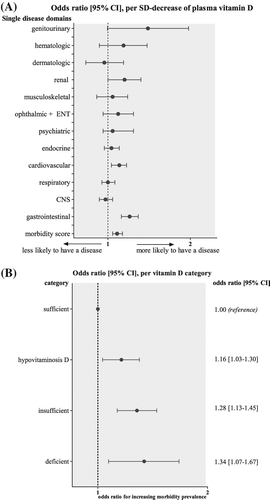
Analyzing the association between each standard deviation (SD) reduction in vitamin D level and single disease domain, we found a significant inverse relationship between vitamin D levels and prevalence of diseases within the gastrointestinal, genitourinary, and cardiovascular tract (). In contrast, none of the other domains were significantly inversely related to vitamin D levels, although a borderline significant P value of 0.061 was reported in the case of the renal disease domain. Furthermore, we found that for every 1 SD decrease of plasma vitamin D level the morbidity score increased by 13% (P < 0.001). In our full model, this effect was slightly attenuated, but still remained 8% (P = 0.001).
Figure 3. Vitamin D levels and morbidity score. (A) Full model odds ratio with 95% CI of ordinal logistic regression on vitamin D levels and morbidity score, as well as odds ratio with 95% CI of logistic regression on vitamin D levels and single morbidities. (B) Full model odds ratios with 95% CI of ordinal logistic regression on vitamin D category and morbidity score.
Association of vitamin D levels and vitamin D categories with morbidity score prevalence
Additionally, we studied the association between vitamin D category and morbidity score. displays the odds ratios of the morbidity score and vitamin D category. Vitamin D-deficient individuals, as compared to vitamin D-sufficient participants, were at the highest risk with regard to morbidity prevalence (full model: OR 1.34 [1.07–1.67], P = 0.01). Albeit less pronounced, this association remained in subjects within the hypovitaminosis D category (full model: OR 1.16 [1.03–1.30], P = 0.01) as well as the insufficient category (full model: OR 1.27 [1.13–1.45], P < 0.001), respectively.
Assessment of robustness of our results: sensitivity analysis
To assess the robustness of our results we performed sensitivity analyses. In the first part of this analysis, we randomly excluded two different disease domains for three consecutive times in both models 1 and 2. However, this did not substantially change the OR of our ordinal and logistic regression. In the second part of the sensitivity analysis, we excluded participants who reported having cancer (n = 77). Again, this did not change the overall findings (results not shown).
Discussion
We demonstrate in this cross-sectional study that, after proper adjustment for season of blood withdrawal and correction for several other confounders, low vitamin D levels are associated with an increased prevalence of multimorbidity. We report an incremental increase in multimorbidity prevalence according to a decrease in vitamin D levels, the association being strongest in participants with vitamin D levels < 25 nmol/L. Furthermore, we show that the association between low vitamin D and multimorbidity is not restricted to a specific disease or disease domain. Our findings are in line with the concept that vitamin D status influences physiological and pathophysiological processes in several organ systems within the human body (Citation21). For that reason, we suggest that vitamin D has an overall association with disease. Further, our study underscores how common multimorbidity is.
Multimorbidity in the general population
To the best of our knowledge this is the first study to show an association between vitamin D levels and prevalence of multimorbidity. Traditionally, most biomedical research is focused on single, organ-specific morbidities, although multimorbidity is a common feature in almost all individuals suffering from chronic diseases (Citation22), and as such it should be considered the norm, rather than the exception. It has been reported that people suffering from multimorbidity have impaired functional status, low quality of life, and worse health outcomes, as compared to those without multimorbidity (Citation22,Citation23). Participants who suffer from multimorbidity present a logistic and financial burden for health care systems worldwide as they use more ambulatory and clinical care (Citation24). Nevertheless, specialist medical care still mostly focuses on the treatment of single morbidities and is organized in single disease or organ-centered clinics, despite the urge to improve continuity and co-ordination of care for people with multiple diseases (Citation4). Treatment is based on single disease guidelines, and little effort has been invested in proper treatment of patients with multimorbidity (Citation25). In this study, we confirm that the number of disease domains, which we denote as morbidities, sharply increases with age (Citation26).
Recognition of the interplay between multimorbidity and vitamin D biology
Low levels of vitamin D have been associated with several single diseases from different disease domains (e.g. age-related hypertension, multiple sclerosis, periodontal disease, tuberculosis, and inflammatory bowel disease) (Citation27). Most of the reported diseases (23%) in our study are in the gastrointestinal tract, although diseases within the central nervous (17%), respiratory (17%), and cardiovascular (15%) systems are also frequently reported. Morbidities within all of these four domains have been associated with vitamin D deficiency. For example, vitamin D has been considered as a major player in pathogenesis of several gastrointestinal immune conditions (inflammatory bowel disease, Crohn's disease, and ulcerative colitis) (Citation28) and in cardiovascular diseases and heart failure (Citation29), while growing evidence suggests that low vitamin D levels are associated with the onset and severity of multiple sclerosis (Citation30). Vitamin D deficiency has also been linked to respiratory diseases like asthma and chronic obstructive pulmonary disease (COPD), although the role of vitamin D deficiency in development of COPD remains controversial (Citation31). This suggests that low vitamin D levels, or such levels in combination with a cluster of diseases, may contribute to the development of multiple diseases within different disease domains. However, results from observational and supplementation studies are non-consistent, and the role of vitamin D in disease (cause or consequence) is still under debate. Nevertheless, we believe that vitamin D deficiency should, at least, be regarded as marker of health. This is also in line with the findings from Autier et al., who suggested that that low levels of vitamin D are rather a marker of ill health than a cause or risk factor for certain diseases (Citation32). Based on these findings, we urge that future studies of vitamin D biology and deficiency should take into account the context of multiple morbidities. And although the association between vitamin D supplementation and (beneficial) effects on both skeletal and non-skeletal outcomes is less clear (Citation33,Citation34), since outcomes of large-scale clinical trials on vitamin D supplementation are eagerly awaited (Citation35), we thus emphasize the need for evaluating a wide array of outcome measures.
This study has several strengths: 1) the use of a large cohort of a relatively homogeneous community-based population, which allows extrapolation to similar populations; 2) the use of standardized definitions of specific morbidities when possible (ICD-10); and 3) the inclusion of 45 single morbidities, clustered into 12 disease domains, which represent organ systems from the entire human body. In addition, with regard to the definitions of morbidity and disease domains, we have explicitly reported the backbone of our approach (Supplementary Appendix I to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2015.1073347), which promotes the reproducibility of this analysis.
Our study shares the limitations of other studies in the field of multimorbidity, as it partly relies on the data from the self-reported questionnaires. Although clinical examination was included, some of the diseases were self-reported, and this may have caused under- or over-reporting of these morbidities. However, in the current framework of the LifeLines Cohort Study we considered this approach most feasible, although we acknowledge limitations inherent to this approach. Another limitation of this study may be the simplified composite end-point of morbidity score. Although we recognize that the effect of multimorbidity on individuals will vary with the combination and severity of morbidities, we chose to weight all morbidities equally, which resulted in a simplified count to define disease domains. This approach is different from other scores, like the Charlons co-morbidity score (Citation36) that also takes into account the severity of the co-morbidity. Of note, we are not the first ones to use such an unweighted score: Boeckxstaens and colleagues recently reported on a comparison of different multimorbidity measures (weighted versus unweighted) and found that there was no clear advantage in using a weighted score over an unweighted score (Citation37). Furthermore, this strategy enabled us to include morbidities that have a low prevalence. Nevertheless, we believe this strategy to be the most feasible and reproducible way to study multimorbidity since it takes into account the effects of multiple morbidities within disease domains rather than being restricted to single morbidities only. This study is a cross-sectional study: therefore these analyses do not provide information about possible causality of vitamin D deficiency and morbidity prevalence. Another limitation of this study is its observational nature: despite the fact that we adjusted for several potential confounders, we cannot exclude a possible effect of any unmeasured factors on the observed association. In addition, residual confounding by variables that are measured by a higher degree of detail (such as education) is possible, although we do not consider them a likely explanation for the clear association between vitamin D levels and morbidity presence.
In this study we found that morbidity increases with age and that multimorbidity is common. Furthermore, we showed that low levels of vitamin D are associated with an increased prevalence of morbidity, an association that is strongest in participants with vitamin D levels below 25 nmol/L. However, this association is not disease- nor disease domain-specific. In light of this, our results favor a general, rather than an organ-specific, approach when assessing the impact of vitamin D deficiency.
Supplementary material available online
Supplementary Appendix I–II and Figure 1 to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2015.1073347
iann_a_1073347_sm0549.zip
Download Zip (255.5 KB)Funding: This work was supported by the Netherlands Heart Foundation (grant 2007T046) and the Innovational Research Incentives Scheme program of the Netherlands Organization for Scientific Research (NWO VENI, grant 916.10.117), and the Innovational Research Incentives Scheme program of the Netherlands Organization for Scientific Research (NWO VIDI, grant 917.13.350).
Declaration of interest: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest; I.P.K. has a patent related to vitamin D measurement (PCT/NL2012/050849) pending; all other authors declare that they have no conflict of interest.
References
- de Groot V, Beckerman H, Lankhorst GJ, Bouter LM. How to measure comorbidity. A critical review of available methods. J Clin Epidemiol. 2003;56:221–9.
- Agborsangaya CB, Ngwakongnwi E, Lahtinen M, Cooke T, Johnson JA. Multimorbidity prevalence in the general population: the role of obesity in chronic disease clustering. BMC Public Health. 2013;13:1161.
- Diederichs C, Berger K, Bartels DB. The measurement of multiple chronic diseases—a systematic review on existing multimorbidity indices. J Gerontol A Biol Sci Med Sci. 2011;66:301–11.
- Sorensen HT. Multimorbidity and cancer outcomes: a for more research. Clin Epidemiol. 2013;5(Suppl 1):1–2.
- Hilger J, Friedel A, Herr R, Rausch T, Roos F, Wahl DA, et al. A systematic review of vitamin D status in populations worldwide. Br J Nutr. 2014;111:23–45.
- Hossein-nezhad A, Holick MF. Vitamin D for health: a global perspective. Mayo Clin Proc. 2013;88:720–55.
- Pan A, Lucas M, Sun Q, van Dam RM, Franco OH, Manson JE, et al. Bidirectional association between depression and type 2 diabetes mellitus in women. Arch Intern Med. 2010;170:1884–91.
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81.
- Giovannucci E. Epidemiology of vitamin D and colorectal cancer. Anticancer Agents Med Chem. 2013;13:11–19.
- von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495–9.
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33; quiz 34–57.
- Stolk RP, Rosmalen JG, Postma DS, de Boer RA, Navis G, Slaets JP, et al. Universal risk factors for multifactorial diseases: LifeLines: a three-generation population-based study. Eur J Epidemiol. 2008;23: 67–74.
- KDIGO 2012Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. 2012. Available at: http://www.kdigo.org/clinical_practice_guidelines/pdf/CKD/KDIGO_2012_CKD_GL.pdf (accessed April 2015).
- Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int. 2005;16:713–16.
- World Health Organization. International Classification of Diseases. 2010. Available at: http://apps.who.int/classifications/icd10/browse/2010/en (accessed April 2015).
- Sachs MC, Shoben A, Levin GP, Robinson-Cohen C, Hoofnagle AN, Swords-Jenny N, et al. Estimating mean annual 25-hydroxyvitamin D concentrations from single measurements: the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr. 2013;97:1243–51.
- Guessous I, McClellan W, Kleinbaum D, Vaccarino V, Zoller O, Theler JM, et al. Comparisons of serum vitamin d levels, status, and determinants in populations with and without chronic kidney disease not requiring renal dialysis: a 24-hour urine collection population-based study. J Ren Nutr. 2014;24:303–12.
- Annweiler C, Schott AM, Rolland Y, Beauchet O. Vitamin D deficiency is associated with orthostatic hypotension in oldest-old women. J Intern Med. 2014;276:285–95.
- Schottker B, Jorde R, Peasey A, Thorand B, Jansen EH, Groot L, et al. Vitamin D and mortality: meta-analysis of individual participant data from a large consortium of cohort studies from Europe and the United States. BMJ. 2014;348:g3656.
- Gonzalez-Rodriguez LG, Estaire P, Penas-Ruiz C, Ortega RM; UCM Research Group VALORNUT (920030). Vitamin D intake and dietary sources in a representative sample of Spanish adults. J Hum Nutr Diet. 2013;26(Suppl 1):64–72.
- Wang Y, Zhu J, DeLuca HF. Where is the vitamin D receptor? Arch Biochem Biophys. 2012;523:123–33.
- Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012; 380:37–43.
- Gijsen R, Hoeymans N, Schellevis FG, Ruwaard D, Satariano WA, van den Bos GA. Causes and consequences of comorbidity: a review. J Clin Epidemiol. 2001;54:661–74.
- Wolff JL, Starfield B, Anderson G. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Arch Intern Med. 2002;162:2269–76.
- Schoen C, Osborn R. 2010 Commonwealth Fund International health policy survey. Available at: www.commonwealthfund.org/surveys/2010/Nov/2010-international-survey.aspx (accessed April 2015).
- Divo MJ, Martinez CH, Mannino DM. Ageing and the epidemiology of multimorbidity. Eur Respir J. 2014;44:1055–68.
- Peterlik M. Vitamin D insufficiency and chronic diseases: hype and reality. Food Funct. 2012;3:784–94.
- Ghaly S, Lawrance I. The role of vitamin D in gastrointestinal inflammation. Expert Rev Gastroenterol Hepatol. 2014;8:909–23.
- Meems LM, van der Harst P, van Gilst WH, de Boer RA. Vitamin D biology in heart failure: molecular mechanisms and systematic review. Curr Drug Targets. 2011;12:29–41.
- Mandia D, Ferraro OE, Nosari G, Montomoli C, Zardini E, Bergamaschi R. Environmental factors and multiple sclerosis severity: a descriptive study. Int J Environ Res Public Health. 2014;11:6417–32.
- Zhang LL, Gong J, Liu CT. Vitamin D with asthma and COPD: not a false hope? A systematic review and meta-analysis. Genet Mol Res. 2014;13:7607–16.
- Autier P, Boniol M, Pizot C, Mullie P. Vitamin D status and ill health: a systematic review. Lancet Diabetes Endocrinol. 2014;2:76–89.
- Amrein K, Schnedl C, Holl A, Riedl R, Christopher KB, Pachler C, et al. Effect of high-dose vitamin D3 on hospital length of stay in critically ill patients with vitamin D deficiency: the VITdAL-ICU randomized clinical trial. JAMA. 2014;312:1520–30.
- Cauley JA, Chlebowski RT, Wactawski-Wende J, Robbins JA, Rodabough RJ, Chen Z, et al. Calcium plus vitamin D supplementation and health outcomes five years after active intervention ended: the Women's Health Initiative. J Womens Health (Larchmt). 2013;22:915–29.
- Manson JE, Bassuk SS, Lee IM, Cook NR, Albert MA, Gordon D, et al. The VITamin D and OmegA-3 TriaL (VITAL): rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp Clin Trials. 2012;33:159–71.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83.
- Boeckxstaens P, Vaes B, Van Pottelbergh G, De Sutter A, Legrand D, Adriaensen W, et al. Multimorbidity measures were poor predictors of adverse events in patients aged >/ = 80 years: a prospective cohort study. J Clin Epidemiol. 2015;68:220–7.

![Figure 1. Relationship between season of blood sampling and vitamin D levels. Data are presented in Tukey boxplots (median [IQR]). *** P < 0.001 as compared to previous season. Data are analyzed using one-way ANOVA (post hoc testing with Bonferroni). Outliers are not presented.](/cms/asset/ce2f90ac-f8bb-4ed4-b4f2-80c3464ed3f5/iann_a_1073347_f0001_b.gif)