Abstract
Background. Tendinotoxicity of glucocorticoids (GC) has been shown, but evidence on how this translates into clinical practice remains scarce.
Objectives. To explore the association between oral or inhaled GC use and the risk of Achilles or biceps tendon rupture (ATR/BTR).
Methods. We identified patients aged 18 to 89 years with incident ATR or BTR (1995–2013) for a matched (1:4) case-control analysis using the UK-based Clinical Practice Research Datalink. We stratified oral GC use by indication, timing and duration of use, continuous versus intermittent use, cumulative dose, and average daily dose. We stratified inhaled GC use by timing and number of prescriptions.
Results. Among 8,202 cases, we observed increased odds ratios (ORs) around 3.0 for continuous oral GC use, which declined shortly after therapy cessation (similarly across indications). Odds ratios increased with average daily dose (≥ 10 mg/day, OR 4.05, 95% CI 2.32–7.08) and were elevated after one cycle of high-dose oral GC (≥ 20 mg/day). There was no effect of inhaled GC at any level of exposure.
Conclusion. Our results provide evidence that oral GC therapy increases the risk of tendon rupture in a dose–response relationship. A single short-term high-dose GC treatment course may be sufficient transiently to increase the risk of tendon rupture.
Oral, but not inhaled, glucocorticoid therapy was associated with an increased risk of tendon rupture.
Risk increase started shortly after treatment initiation and intensified with increasing daily doses.
One short course of high-dose glucocorticoid therapy may transiently increase the risk of tendon rupture.
Introduction
Achilles and biceps tendon ruptures are the two most frequent types of tendon rupture. Achilles tendon ruptures (ATR) often occur in middle-aged men during sports activity, while biceps tendon ruptures (BTR) typically occur spontaneously in elderly patients, mainly as result of chronic shoulder tendinopathy (Citation1–4). Although tendon ruptures are usually preceded by trauma, pre-existing tendon damage may predispose to rupture at little extrinsic stress (Citation5–8). Scarce evidence exists on factors that compromise tendon integrity, though over-use, advanced age, male gender, obesity, genetic factors, diabetes mellitus, dyslipidemia, adrenal disorders, chronic kidney disease, and rheumatic diseases have been considered (Citation9,Citation10).
Use of glucocorticoids (GC) applied systemically, locally injected, or via inhalation has been anecdotally reported to be associated with tendinopathies for more than 45 years (Citation11–15). In vitro and in vivo studies have demonstrated a negative influence of GC on biomechanical properties of tendons, causing pathological changes in tendinocytes shortly after GC application (Citation14,Citation16–20). Two previous observational studies quantified the association between GC use and ATR, although these were only reported as secondary issues within studies of fluoroquinolone-induced ATR (Citation5,Citation13,Citation14). Underlying indication, daily GC dose (the presumed main predictive factor for GC-induced fractures) (Citation21), treatment duration, or differentiation between continuous versus intermittent GC use were not addressed (Citation5,Citation13). Considering the abundant use of GC, in-depth pharmacoepidemiological studies are needed to identify patients at risk of GC-induced tendon ruptures (Citation11,Citation15,Citation19,Citation22,Citation23). In a large observational case-control analysis using data from the UK-based primary-care database Clinical Practice Research Datalink (CPRD), we analysed the risk of ATR/BTR in patients exposed to oral or inhaled GC.
Material and methods
Data source
The CPRD is an anonymous primary-care database comprising approximately 8 million active patients enrolled with selected general practitioners (GPs). In the UK, GPs hold a gatekeeper role within the National Health System. After referrals, consultants are required to send information on diagnoses and treatments to the GP who enters this information into the database. GPs provide information on patient demographics and characteristics (e.g. age, sex, height, weight, smoking status), symptoms or diagnoses, lab test results, and referrals to secondary care. Drug prescriptions are generated via computer, ensuring a complete drug history. Patients are representative of the UK population, and extensive validation has documented high validity of the database (Citation24,Citation25). The study protocol was approved by the Independent Scientific Advisory Committee (ISAC) for Medicines and Healthcare products Regulatory Agency (MHRA) database research.
Study population
Cases
We identified patients aged 18 to 89 years with an incident Read code (Citation24) for ATR or BTR between January 1995 and December 2013, and with ≥ 3 years of recorded active history in the database prior to the ATR/BTR diagnosis (‘index date’). We excluded patients with recorded alcoholism, other substance abuse, cancer (except non-melanoma skin cancer), or HIV before the index date (increased risk of bias and confounding), as well as patients with a recorded previous rupture of, or manipulation of, the Achilles or biceps tendons, or with a record of acquired or congenital malformation of the respective tendon. We further excluded patients with a record of a major accident (e.g. vehicle accident, fall from roof or ladder) within 30 days before the index date, to exclude patients with tendon ruptures caused by exceptional extrinsic stress. Cases with recorded trauma, for example during sporting accidents, were kept in. We did not exclude patients with previous Achilles or biceps tendinitis as this may precede tendon rupture and thus lies in the causal pathway (Citation2,Citation5,Citation10,Citation11).
Controls
We randomly matched four ATR/BTR-free controls to each case on age (year of birth), sex, GP, calendar time (index date), and number of years of recorded history in the database prior to the index date. We applied the same exclusion criteria to controls as to cases.
Exposure
We defined exposure as ≥ 1 recorded prescription for any oral (see WHO-ATC H02AB) or inhaled (see WHO-ATC R03BA) GC prior to the index date, classified as new use (i.e. ≥ 365 days of GC-free history before the first GC prescription) or prevalent use (existing GC use at the start of the record). We compared new oral GC use to never use, stratified by timing of use (current, recent, or past, i.e. last prescription ≤ 180, 180–364, or ≥ 365 days before the index date, respectively). New current oral GC use was further stratified by time since GC treatment start (< 1 month, 1–3 months, 3–6 months, 6 months–1.5 years, 1.5–5 years, ≥ 5 years) prior to the index date, and sub-stratified by number of recorded prescriptions prior to the index date as proxy for continuity of GC use. In a sub-analysis we assessed time since last prescription within patients with < 6 months treatment duration with ≤ 2 prescriptions recorded. We further sub-stratified the analysis on oral GC use by time since treatment start by gender and age (≥/< 65 years).
We classified new current oral GC use according to the presumed underlying indication by capturing previous Read codes of diseases associated with GC use, i.e. asthma or chronic obstructive pulmonary disease (COPD) only, connective tissue disease (CTD) only (rheumatoid arthritis, polymyalgia rheumatica, systemic lupus erythematosus, other CTD), vasculitis only, inflammatory bowel disease (IBD) only, ≥ 2 of these diseases, or none of them). We stratified the two largest subgroups (asthma/COPD and CTD) by timing and by time since oral GC treatment start, as well as by number of prescriptions. We also stratified new current oral GC use by cumulative GC dose (total mg prednisone equivalents) prior to the index date. We reviewed records of current users of prednisone/prednisolone-only over 2 years prior to the index date and decided for each patient whether prednisone was used continuously or not, taking into account frequency of prescriptions, number of tablets, underlying indication, disease duration, and dosage instructions, where available. Within the selected subset of continuous prednisone-only users we calculated the average daily prednisone dose by dividing the total mg prescribed by the number of oral prednisone-exposed days during this 2-year period.
We compared new users of inhaled GC to never users, stratified by timing (current, recent, past use) and duration of drug use (number of prescriptions) prior to the index date. In a sensitivity analysis we excluded users of inhaled GC with concomitant (< 180 days) oral GC use.
Small sample size precluded analysis of intravenously administered GC which occurs mostly in inpatients. Locally injected GC is contraindicated for Achilles tendinopathies but is used for pain relief in patients with shoulder tendinopathies (Citation26,Citation27). As manual profile review revealed that 70% of patients with a local GC injection within 180 days before BTR diagnosis had a record for pre-existing shoulder disorders, we did not assess this association due to likely protopathic bias.
Statistical analysis
We conducted multivariable conditional logistic regression analyses to evaluate the association between GC exposure and ATR/BTR using SAS 9.4 (SAS Institute, Cary, NC, US). We calculated relative risk estimates as odds ratios (ORs) with 95% confidence intervals (CIs). Based on clinical knowledge (Citation28), we defined a priori the potential confounders for the multivariable model and adjusted all ORs for smoking (non, current, ex, unknown), alcohol consumption (</≥ 15 units per week, or unknown), obesity, and for the co-morbidities diabetes mellitus, chronic kidney disease (including hemodialysis), osteoporosis, osteoarthritis, infectious arthritis, and gout. We adjusted for concomitant use (last prescription < 180 days before the index date) of fluoroquinolones, hormone replacement therapy, statins, fibrates, and locally injected no more query fields (Citation29–31). We adjusted all models, except the analysis on GC indications, for systemic lupus erythematosus, rheumatoid arthritis, inflammatory bowel disease, Crohn's disease, and polymyalgia rheumatic. We further adjusted analyses of inhaled GC for concomitant oral GC use.
Diagnostic validity
While ATR is a relatively straightforward diagnosis (Citation7), BTR is more complicated to diagnose as it frequently coexists with other shoulder pathologies. In a sensitivity analysis we identified ATR/BTR patients whose diagnosis was preceded within 180 days by an established diagnostic imaging procedure (ultrasound, magnetic resonance imaging, arthrogram, or X-ray) (Citation2,Citation12,Citation32) or by a consultation with a specialist (Citation5,Citation7). Within this sample we repeated the analyses stratified by timing and time since treatment start, and stratified by timing of use and by number of prescriptions. We also stratified the study population into ATR versus BTR cases (and their controls), and reassessed use of oral or inhaled GCs in the two subpopulations separately.
Results
Of 8,202 cases (32,808 controls), 5,027 (61.3%) cases were diagnosed with ATR, and 3,175 (38.7%) with BTR. Demographics, characteristics, and selected co-morbidities are displayed in . ATR and BTR occurred more frequently in men (approximately 70%), and BTR patients were on average older (mean age 65.1 years) than ATR patients (mean age 50.3 years).
Table I. Demographics, life-style factors and co-morbidities of ATR and BTR cases and controls separately.
Of all ATR/BTR cases, 405 cases (4.9%) were currently exposed to oral GC (new use), which resulted in an overall OR of 2.07 (95% CI 1.79–2.38) compared to never use. We observed increased ORs around 4.0 in patients with continuous oral GC use between < 1 month and 1.5 years, where ORs remained around 2.0 during extended continuous use. Current intermittent oral GC use yielded substantially lower ORs throughout (). Record review revealed that most new current oral GC users with a treatment duration of < 6 months and ≤ 2 prescriptions likely received short-term high-dose GC treatment; of 183 patients 106 were for asthma/COPD exacerbation(s) or respiratory tract infections, 13 for acute skin conditions, 7 for new-onset CTD, 4 for IBD exacerbations, and 3 for acute gout. Within these 183 short-term high-dose patients, ORs closely correlated with the time since last prescription, with the highest OR in patients with a prescription within 30 days prior to the index date (< 30 days: OR 3.58 [1.87–6.86]; 30–59 days: OR 2.07 [1.04–4.13]; 60–89 days: OR 1.22 [0.45–3.28]; 90–180 days: OR 1.37 [0.83–2.26].
Table II. Oral GC use stratified by timing of use, time since treatment start, and continuous versus intermittent use.
Odds ratios increased with increasing average daily dose in the subset of current continuous prednisone-only users (311 patients); the OR was 4.05 (95% CI 2.32–7.08) in patients with ≥ 10 mg/day (, ). On the other hand, ORs plateaued at around 2.0 with cumulative oral GC dose ≥ 1,000 mg prednisone equivalent (2,500–5,000 mg prednisone equivalent, highest OR of 3.37, 95% CI 2.45–4.62) (, ). Most new current oral GC users had an underlying respiratory disorder only (137 cases, 34.3%, OR 1.87, 95% CI 1.51–2.31), followed by CTD only (103 cases, 25.4%, OR 3.26, 95% CI 2.49–4.26) (). Most CTD patients had continuous treatment, whereas about half of all respiratory patients had intermittent GC treatment. Risks were similar in the two subgroups. Stratified analyses revealed slightly higher ORs in women than in men. Current long-term GC use was more frequent in patients aged ≥ 65 years, and relative risks were slightly higher (Supplementary Table I, to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2015.1074272). We observed similar results for ATR and BTR separately (Supplementary Tables 2 and 3, available online).
Figure 1. Average daily oral GC dose (continuous prednisone-only users) within 2 years prior to the index date. Adjusted for smoking, obesity, alcohol consumption, diabetes, gout, chronic kidney disease, osteoarthritis, infectious arthritis, osteoporosis, rheumatoid arthritis, lupus, polymyalgia rheumatica, inflammatory bowel disease, vasculitis, concomitant use of fluoroquinolones, hormone replacement therapy, statins, fibrates, and locally injected GC. CI = confidence interval; GC = glucocorticoids; OR = odds ratio.
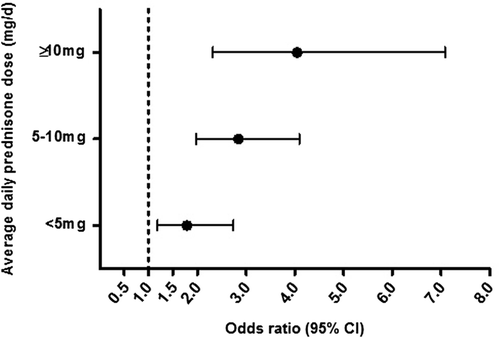
Figure 2. Total cumulative oral GC dose prior to the index date. Adjusted for smoking, obesity, alcohol consumption, diabetes, gout, chronic kidney disease, osteoarthritis, infectious arthritis, osteoporosis, rheumatoid arthritis, lupus, polymyalgia rheumatica, inflammatory bowel disease, vasculitis, concomitant use of fluoroquinolones, hormone replacement therapy, statins, fibrates, and locally injected GC. CI = confidence interval; GC = glucocorticoids; OR = odds ratio.
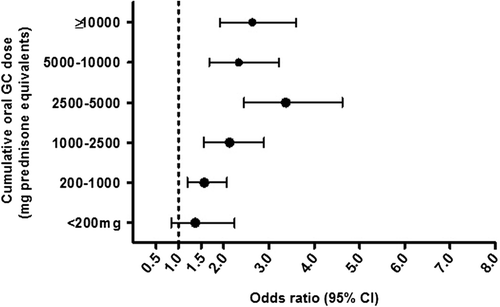
Table III. Current oral GC use stratified by average daily dose (continuous prednisone-only users) and by total cumulative dose (in prednisone equivalents).
Table IV. Current oral GC use stratified by indication, timing, duration, and continuity of GC treatment.
Use of inhaled GCs was not associated with an altered risk of ATR or BTR at any level of duration or timing (), nor after excluding patients with concomitant oral GC use (data not displayed). The sensitivity analysis of patients with an ATR/BTR diagnosis preceded by referrals or diagnostic procedures revealed no material change in ORs for inhaled or oral GCs (Supplementary Table 4 to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2015.1074272).
Table V. Use of inhaled GC stratified by timing of use and by number of prescriptions.
Discussion
This case-control study provides evidence that oral GC therapy increases the risk of tendon rupture in a dose–response relationship, with increasing ORs in patients with high average daily doses. Odds ratios increased shortly after therapy initiation and declined quickly after treatment cessation. One short-term course of high-dose oral GC may be sufficient to transiently increase the risk of tendon rupture. Inhaled GC use was not associated with an altered risk of ATR/BTR.
A previous CPRD study reported a crude OR of 2.6 (95% CI 2.2–3.1) for ever use of oral GCs and ATR (Citation13). Another study using US health plan data reported an adjusted OR of 1.4 (95% CI 1.0–1.8) in patients with current oral GC use (within 6 months) and ATR (Citation5). We observed increased ORs of around 4.0 for current continuous oral GC use between < 1 month and 1.5 years of duration (somewhat lower in current use ≥ 1.5 years). The effect decreased within 180 days after the drug was discontinued. Seeger et al. previously reported an association between cumulative oral GC dose and the risk of ATR among people exposed within 6 months before diagnosis (maximum category ≥ 301 mg prednisone equivalents) (Citation5). In our data set, ORs plateaued at cumulative GC dose ≥ 1,000 mg prednisone equivalents, while ORs linearly increased with increasing average daily dose in the subset of continuous prednisone-only users. While the effect of continuous use was evaluated only in a subset of current prednisone-only users, prednisone is the primary chronic GC therapy and is thus likely representative of all continuous GC use, an evaluation of which was otherwise not practical. Most short-term intermittent oral GC users (< 6-month treatment duration, ≤ 2 prescriptions) likely received daily GC doses of ≥ 20 mg (mostly for acute respiratory conditions), and time since last prescription was strongly associated with the risk of ATR/BTR (highest OR of 3.7 in patients with a prescription < 30 days). Thus, a single cycle of high-dose GC therapy (≥ 20 mg/d) may be sufficient may be sufficient the risk for tendon rupture, which then declines rapidly after treatment cessation. This may explain the lower ORs in current intermittent oral GC users; although current users had a recorded prescription within 180 days before the index date, intermittent users likely have a time-lag between the last ingested dose and the index date. Our results are consistent with a previously suggested risk for Achilles tendinopathies in association with high-dose oral GC use (median daily dose ≥ 80 mg prednisone) in analyses of pharmacovigilance data and case reports (Citation14).
Vasculitis, CTD, and IBD have been independently associated with tendon rupture (Citation13,Citation19,Citation33). We observed increased ORs across all strata of indication including approximately 3-fold increased ORs in continuous oral GC users with asthma/COPD only, which to our knowledge has not been independently associated with tendon rupture (we found a null result in users of inhaled GC). The somewhat higher ORs in the CTD subgroup may be due to inflammatory activity, but differences in average daily GC doses may also play a role.
Our results may be explained by mechanisms recently described by Muto et al. who reported apoptotic tendinocytes, increased MMP-3 activity, and impaired biomechanical properties of rats’ Achilles tendons within 1 week after local GC injection, an effect that resolved within 3 weeks (Citation20). A similar risk pattern has been shown for GC-induced fractures, which increased rapidly after oral GC treatment initiation and declined shortly after treatment cessation (Citation21,Citation34,Citation35). Similar to our results, high daily dose was presumed to be a strong risk factor for GC-induced fractures, an effect which is likely due to an interaction with increasing therapy duration/cumulative dose, and a higher risk in continuous GC users (Citation35–37).
Previous studies have shown a preponderance of males among ATR patients, although women were more likely to have pre-existing predisposing factors when an acute ATR occurred (Citation3,Citation4,Citation8). In concordance, within our predominantly male study population, 8.2% of female cases and only 3.8% of male cases were currently exposed to oral GC (slightly higher ORs in women than in men). We found a decreased OR for ATR/BTR in smokers, which has been reported in a previous observational study and which may be explained by the presumption that smokers engage less in sports activity (Citation10). Sports and leisure activities are not captured in the CPRD, but we adjusted our analyses for smoking, alcohol consumption, and obesity, all proxies for a patient's life-style.
We did not observe an increased risk for ATR/BTR across all strata of timing and duration of inhaled GC use in our data set, which is consistent with the only previous multivariable analysis on ATR in association with inhaled GC (Citation5).
The sensitivity analysis restricted to ATR/BTR cases with a previously recorded diagnostic imaging work-up or a referral/discharge to/from a specialist, i.e. cases with little risk for misclassification, yielded virtually the same results for patients with exposure to oral or inhaled GC. Furthermore, the fact that results were similar within separate analyses on ATR and BTR patients suggests a GC effect irrespective of the tendon rupture site.
This observational case-control study is based on a large and extensively validated primary-care database, but several limitations nevertheless have to be considered. First, our study population may include some ATR/BTR events caused by extreme extrinsic stress. However, such outcome misclassification would dilute our result toward the null rather than producing spuriously high associations. Second, we cannot rule out the possibility that some patients had ATR/BTR before database entry, which in theory may have affected the likelihood of getting GC therapy later on. Third, the CPRD neither routinely captures sports activities nor does it completely capture conditions such as misalignment of extremities, discrepancies in limb length, muscular insufficiencies, or inappropriate footwear, unless they were clearly diagnosed and recorded; thus, we could not control for such factors in the analyses on ATR (Citation10). Finally, we cannot rule out residual confounding and chance, since we could not control for ethnic background, socio-economic status, profession, or nutrition, as these parameters are not routinely recorded in the CPRD. Despite these limitations, this is, to our knowledge, the first in-depth analysis of the association between GC use and tendon rupture in a large observational study.
In summary, our findings suggest that oral, but not inhaled, GC therapy increases the risk of tendon rupture, an effect that starts shortly after treatment initiation and declines shortly after treatment cessation. One single course of high-dose GC therapy (daily doses > 20 mg/day) may be sufficient to increase the risk of tendon rupture. This association is closely similar to one reported for GC-induced fractures. A transient effect of GC use on tendons through apoptosis and increasing MMP-3 activity may support the etiologic plausibility of our findings (Citation20).
Supplementary material available online
Supplementary Table I–IV, to be found online at http://informahealthcare.com/doi/abs/10.3109/07853890.2015.1074272.
iann_a_1074272_sm8115.pdf
Download PDF (125.4 KB)Acknowledgements
We thank Michael Bodmer, MD, who served as clinical consultant during data analysis. We also thank Pascal Egger for the data withdrawal and processing, as well as for his technical support.
Funding: No funding was received for the conduct of this study.
Declaration of interest: None of the authors have any conflicts of interest to declare.
References
- Elser F, Braun S, Dewing CB, Giphart JE, Millett PJ. Anatomy, function, injuries, and treatment of the long head of the biceps brachii tendon. Arthroscopy. 2014;27:581–92.
- Snyder GM, Mair D, Lattermann C. Tendinopathy of the long head of the biceps. Med Sport Sci. 2012;57:76–89.
- Raikin SM, Garras DN, Krapchev PV. Achilles tendon injuries in a United States population. Foot Ankle Int. 2013;34:475–80.
- Lantto I, Heikkinen J, Flinkkilä T, Ohtonen P, Leppilahti J. Epidemiology of Achilles tendon ruptures: increasing incidence over a 33-year period. Scand J Med Sci Sports. 2014;25:1–6.
- Seeger JD, West WA, Fife D, Noel GJ, Johnson LN, Walker AM. Achilles tendon rupture and its association with fluoroquinolone antibiotics and other potential risk factors in a managed care population. Pharmacoepidemiol Drug Saf. 2006;15:784–92.
- Longo UG, Loppini M, Marineo G, Khan WS, Maffulli N, Denaro V. Tendinopathy of the tendon of the long head of the biceps. Sports Med Arthrosc. 2011;19:321–32.
- Cook J, Khan K, Purdam C. Achilles tendinopathy. Man Ther. 2002;7:121–30.
- Vosseller JT, Ellis SJ, Levine DS, Kennedy JG, Elliott AJ, Deland JT, et al. Achilles tendon rupture in women. Foot Ankle Int. 2013;34:49–53.
- Magnan B, Bondi M, Pierantoni S, Samaila E. The pathogenesis of Achilles tendinopathy: a systematic review. Foot Ankle Surg. 2014; 20:154–9.
- Kraemer R, Wuerfel W, Lorenzen J, Busche M, Vogt PM, Knobloch K. Analysis of hereditary and medical risk factors in Achilles tendinopathy and Achilles tendon ruptures: a matched pair analysis. Arch Orthop Trauma Surg. 2012;132:847–53.
- Kirchgesner T, Larbi A, Omoumi P, Malghem J, Zamali N, Manelfe J, et al. Drug-induced tendinopathy: from physiology to clinical applications. Joint Bone Spine. 2014;81:485–92.
- Thevendran G, Sarraf KM, Patel NK, Sadri A, Rosenfeld P. The ruptured Achilles tendon: a current overview from biology of rupture to treatment. Musculoskelet Surg. 2013;97:9–20.
- Van der Linden PD, Sturkenboom MCJM, Herings RMC, Leufkens HMG, Rowlands S, Stricker BHC. Increased risk of Achilles tendon rupture with quinolone antibacterial use, especially in elderly patients taking oral corticosteroids. Arch Intern Med. 2013;163:1801–7.
- Blanco I, Krähenbühl S, Schlienger RG. Corticosteroid-associated tendinopathies. An analysis of the published literature and spontaneous pharmacovigilance data. Drug Saf. 2005;28:633–43.
- Singh D, Pandit D, Doherty M. High dose inhaled corticosteroids can cause Achilles tendonitis. Respir Med CME. 2009;2:15–17.
- Dean BJF, Lostis E, Oakley T, Rombach I, Morrey ME, Carr AJ. The risks and benefits of glucocorticoid treatment for tendinopathy: a systematic review of the effects of local glucocorticoid on tendon. Semin Arthritis Rheum. 2014;43:570–6.
- Dean BJF, Franklin SL, Murphy RJ, Javaid MK, Carr AJ. Glucocorticoids induce specific ion-channel-mediated toxicity in human rotator cuff tendon: a mechanism underpinning the ultimately deleterious effect of steroid injection in tendinopathy? Br J Sports Med. 2014;48:1620–6.
- Poulsen RC, Watts AC, Murphy RJ, Snelling SJ, Carr AJ, Hulley PA. Glucocorticoids induce senescence in primary human tenocytes by inhibition of sirtuin 1 and activation of the p53/p21 pathway: in vivo and in vitro evidence. Ann Rheum Dis. 2014;73:1405–13.
- Newnham D, Douglas J, Legge JS, Friend J. Achilles tendon rupture: an underrated complication of corticosteroid treatment. Thorax. 1991;46:853–4.
- Muto T, Kokubu T, Mifune Y, Inui A, Harada Y, Yoshifumi, et al. Temporary inductions of matrix metalloprotease-3 (MMP-3) expression and cell apoptosis are associated with tendon degeneration or rupture after corticosteroid injection. J Orthop Res. 2014;32:1297–304.
- Van Staa TP, Laan RF, Barton IP, Cohen S, Reid DM, Cooper C. Bone density threshold and other predictors of vertebral fracture in patients receiving oral glucocorticoid therapy. Arthritis Rheum. 2003;48:3224–9.
- Fok JS, Yong TY, Li JY. Spontaneous unilateral Achilles tendon rupture with corticosteroid use for microscopic polyangiitis. J Med Cases. 2013;4:304–6.
- Kotnis RA, Halstead JC, Hormbrey PJ. Atraumatic bilateral Achilles tendon rupture: an association of systemic steroid treatment. J Accid Emerg Med. 1999;16:378–9.
- Khan NF, Harrison SE, Rose PW. Validity of diagnostic coding within the General Practice Research Database: a systematic review. Br J Gen Pract. 2010;60:e128–36.
- Walley T, Mantgani A. The UK General Practice Research Database. Lancet. 1997;350:1097–9.
- Cardone DA, Tallia AF, Johnson RW. Joint and soft tissue injection. Am Fam Physician. 2002;66:283–8.
- Khazzam M, George MS, Churchill RS, Kuhn JE. Disorders of the long head of biceps tendon. J Shoulder Elbow Surg. 2012;21:136–45.
- Hernán MA, Hernández-díaz S, Werler MM, Mitcheil AA. Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. Am J Epidemiol. 2002;155:176–84.
- Marie I, Delafenêtre H, Massy N, Thuillez C, Noblet C. Tendinous disorders attributed to statins: a study on ninety-six spontaneous reports in the period 1990–2005 and review of the literature. Arthritis Rheum. 2008;59:367–72.
- Beri A, Dwamena FC, Dwamena BA. Association between statin therapy and tendon rupture: a case-control study. J Cardiovasc Pharmacol. 2009;53:401–4.
- Maron DJ, Fazio S, Linton MF. Current perspectives on statins. Circulation. 2000;101:207–13.
- Ejnisman B, Monteiro GC, Andreoli CV, de Castro Pochini A. Disorder of the long head of the biceps tendon. Br J Sports Med. 2010;44:347–54.
- Benthien JP, Delling G, Rüther W.[Spontaneous Achilles tendon rupture in granulomatous vasculitis]. Z Rheumatol. 2003;62:402–5.
- Weinstein RS. Glucocorticoid-induced bone disease. N Engl J Med. 2011;365:62–70.
- Weinstein RS. Glucocorticoid-induced osteoporosis and osteonecrosis. Endocrinol Metab Clin North Am. 2012;41:595–611.
- Steinbuch M, Youket TE, Cohen S. Oral glucocorticoid use is associated with an increased risk of fracture. Osteoporos Int. 2004;15:323–8.
- De Vries F, Cooper AL, Cockle SM, van Staa T-P, Cooper C. Fracture risk in patients receiving acid-suppressant medication alone and in combination with bisphosphonates. Osteoporos Int. 2009;20:1989–98.
