Abstract
Introduction. Both sedentary behaviour and fatty liver are associated with increased risk of obesity and non-communicable diseases, but their relationship remains unknown. We investigated the relationship of television (TV) viewing time with serum gamma-glutamyltransferase (GGT) and Fatty Liver Index (FLI), and ultrasonographically assessed liver fat.
Methods. A total of 1,367 adults of the population-based Cardiovascular Risk in Young Finns study (748 women, 619 men, aged 34–49 years) had fasting serum GGT, triglycerides, weight, height, and waist circumference, and self-reported TV time data from 2001, 2007, and 2011. Changes in GGT and FLI, and liver ultrasound images in 2011 were studied in groups with constantly low (≤ 1 h/d), moderate (1–3 h/d), or high (≥ 3 h/d) daily TV time, and in groups with ≥ 1 hour increase/decrease in daily TV time between 2001 and 2011.
Results. Constantly high TV time was associated with higher GGT and FLI (P < 0.02 in both), and 2.3-fold (95% CI 1.2–4.5) increased risk of fatty liver regardless of age, sex, leisure-time and occupational physical activity, energy intake, diet composition, alcohol use, sleep duration, socioeconomic status, and smoking. Adjustment for BMI partly attenuated the associations.
Conclusions. High TV viewing increases fatty liver risk. It may be one mechanism linking sedentary behaviour with increased cardiometabolic disease risks.
Sedentary lifestyle is increasing, and both sedentary behaviour and non-alcoholic fatty liver disease (NAFLD) are associated with increased cardiometabolic risks.
TV viewing, as the most common leisure-time sedentary behaviour, is independently associated with increased risk of fatty liver.
This is most probably mediated, at least partly, by increased body weight.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a disorder with increased hepatic triglyceride accumulation (> 5% of hepatocytes) unrelated to excess alcohol intake, viral infection, or other specific liver disease (Citation1). NAFLD is estimated to affect 20% of adults in the general population and the majority of obese individuals (Citation2,Citation3). In our study population the prevalence of fatty liver has been reported earlier as 19% (Citation4). The prevalence increases in parallel with the obesity pandemic (Citation5–8), and fatty liver is associated with increased risk of obesity (Citation9,Citation10), metabolic syndrome (Citation11), insulin resistance (Citation12), type 2 diabetes (Citation13), and cardiovascular disease (Citation14). It may result in liver inflammation, fibrosis, fat cell necrosis, and eventually cirrhosis and liver cancer (Citation3,Citation12).
Regular physical activity (PA) reduces the risk of NAFLD (Citation15–17), increases whole-body fatty acid oxidation (Citation18), enhances insulin sensitivity, reduces the risk of type 2 diabetes, and favourably modifies serum lipids (Citation19,Citation20). The benefit of PA in obese individuals, without weight loss, is similar to that observed following weight loss alone (Citation21).
Sedentary behaviour, defined as an immobile state of the body (e.g. sitting) resulting in energy expenditure close to the resting metabolic rate, is associated with increased risk of obesity (Citation22,Citation23), metabolic syndrome (Citation24), type 2 diabetes, cardiovascular disease, and mortality (Citation25–27). An increased risk of liver cancer in sedentary individuals has also been reported (Citation28). TV viewing time (TV time) is a non-occupational sedentary behaviour most frequently associated with adverse health outcomes (Citation22), and high TV time is causally antecedent to weight increase (Citation23). Derangement of hepatic function may be involved, but the relationship between sedentary behaviour and fatty liver has not been studied, and it is unknown whether such association is independent of PA and other risks of fatty liver.
Various methods can be applied to assess NAFLD. Serum gamma-glutamyltransferase (GGT) is a well-established measure of liver function. Its concentration increases very early in response to liver fat accumulation (Citation1,Citation29,Citation30). Fatty Liver Index (FLI) is a marker calculated from serum triglycerides, GGT, BMI, and waist circumference used to predict hepatic steatosis (Citation31). Non-invasive liver ultrasound is the first-line method in large population studies used to demonstrate visually the presence of liver fat accumulation (Citation32–34).
To test the hypothesis that sedentary behaviour is related to NAFLD, we evaluated the longitudinal, 10-year association of TV time with GGT and FLI, in a cross-sectional setting with ultrasonographically diagnosed fatty liver.
Material and methods
Participants
The Cardiovascular Risk in Young Finns Study is an ongoing, multicentre follow-up study of atherosclerosis risk factors (Citation35). The first cross-sectional survey was conducted in 1980, when 3,596 individuals randomly selected from the national registry (aged 3–18 years) participated in clinical examinations. The latest follow-up study was conducted in 2011.
The sample drawn for the longitudinal part of this study comprised 1,367 participants (748 women and 619 men) aged 34–49 who had data on daily TV time and FLI in 2001, 2007, and 2011. For cross-sectional analyses 2,029 subjects having a liver ultrasound scan in 2011 were evaluated.
The participants gave written informed consent, and the study has been approved by the Ethics Committee of the Hospital District of Southwest Finland.
TV viewing time (sedentary behaviour)
Self-reported TV time was assessed by standardized questionnaires at each follow-up and was used as a measure of sedentary behaviour. The participants were enquired how much time on average they spent watching TV daily.
The study population was divided in five TV time groups, i.e. constantly low (≤ 1 h/day, n = 196), moderate (1–3 h, n = 233), or high (≥ 3 h, n = 84) TV time in 2001, 2007, and 2011, and groups with at least one hour increase (n = 218) or decrease (n = 213) in daily TV time between 2001 and 2011. Of the participants, 423 did not fulfil the TV time group criteria. They were evaluated further in an attrition analysis.
GGT, triglycerides, BMI, and waist circumference
Venous blood was drawn after an overnight fast and serum separated for GGT and triglyceride analyses. Body weight was measured with a digital scale in light clothing without shoes (accuracy of 0.1 kg), and height with a wall-mounted stadiometer (0.1 cm accuracy). BMI was calculated as weight (kg)/[height (m)]2. Waist circumference was measured with a measuring tape at the end of expiration at the mid-axillary line between the iliac crest and the lowest rib (accuracy of 0.1 cm).
Fatty Liver Index (FLI)
The FLI comprises triglycerides, GGT, body mass index (BMI), and waist circumference. It is calculated as:
FLI = (e0.953 × loge (TRIGLY) + 0.718 × loge (GGT) + 0.139 × BMI + 0.053 × WAIST − 15.745)/ (1 + e0.953 × loge (TRIGLY) + 0.718 × loge (GGT) + 0.139 × BMI + 0.053 × WAIST − 15.745) × 100 (Citation31). It has an accuracy (area under curve, AUC) of 0.84 (95% CI 0.81–0.87) in detecting fatty liver (Citation31). In our study the AUC for FLI versus overall visual evaluation of liver ultrasound images was 0.86 (0.84–0.88), and versus ultrasonographic FLI (see below) 0.83 (0.81–0.85). The respective AUCs for GGT were 0.81 (0.79–0.83) and 0.78 (0.75–0.80).
Liver ultrasound imaging
Ultrasonographic liver imaging was performed in 2011 for 2,040 study participants using a validated protocol (Citation36). Sequoia 512 ultrasound mainframes (Acuson, Mountain View, CA, USA) and 4.0 MHz adult abdominal transducers were used. The diagnosis of fatty liver was primarily based on an overall visual evaluation of the images by a trained ultrasonographer, i.e. 1) normal; or 2) fatty (mildly fatty or clearly fatty). Additionally, fatty liver diagnosis was evaluated by combining various ultrasonographic parameters to create a continuous ultrasonographic Fatty Liver Index (uFLI, range 4–12) (Citation4). The criteria for uFLI were: A) liver-to-kidney contrast (1, normal; or 2, abnormal); B) hepatic parenchymal brightness or echogenicity (1, normal; 2, mildly increased; 3, moderately increased; 4, severely increased); C) ultrasound deep beam attenuation between the posterior liver and the diaphragm (1, no attenuation, diaphragm zone clearly visible; 2, mild attenuation; 3, clear attenuation, diaphragm not visible); and D) visibility or brightness of the walls of the small intrahepatic vessels (1, normally visible; 2, partly visible; 3, not visible) (Citation37). In overall visual evaluation at least mild fatty deposition and in uFLI values > 6 were defined as indicative of fatty liver (18.3% and 18.8% of subjects, respectively).
Physical activity
A self-administered questionnaire was used to collect data on PA, including leisure-time physical exercise, active commuting, and occupational PA.
Leisure-time physical exercise frequency was categorized as: 1) not at all; 2) once a month; 3) once a week; 4) 2–3 times a week; 5) 4–6 times a week; 6) daily. The average duration of a single bout of exercise was defined as: 1) < 20 minutes; 2) 20–40 minutes; 3) 40–60 minutes; 4) > 60 minutes. For exercise intensity the categories were: 1) not getting out of breath nor sweating; 2) getting out of breath and sweating slightly; 3) getting out of breath and sweating heavily (Citation38). The metabolic equivalent (MET) h/week for active commuting to work was calculated based on travel mode (cycling or walking) and length of the commuting distance. A MET index (range 0–163 MET h/week) was calculated by multiplying leisure-time physical exercise intensity, frequency, and duration, and adding the MET h/week for active commuting. The index has been validated against data collected with accelerometers and pedometers (Citation37).
The level of occupational PA was classified as: 1) light sedentary work; 2) other sedentary work; 3) physically light work, performed mainly standing, or including light activity; 4) work including moderate PA; 5) physically strenuous work; or 6) physically very strenuous work. The test–retest reliability of this question has been reported to be reasonably good (Citation38), and the associations with physical fitness have been demonstrated (Citation39).
Diet, alcohol consumption, smoking, sleep duration, and socioeconomic status
Food consumption data were collected using a 131-item food frequency questionnaire (FFQ), developed and validated by the Finnish National Institute for Health and Welfare (Citation40). The subjects were asked to report the daily frequency and serving size of selected foods and dishes during the previous 12 months. The questionnaire included additional open questions to enable reporting of foods not listed in the FFQ. The daily specific food or food group consumption and nutrient intake were calculated using the latest version of the National Food Composition Database Fineli (Citation41).
A food-based diet score originally constructed to describe diet associated with lower risk of diabetes (Citation42) was used as an indicator of a healthy diet. With this score, each individual's adherence to the Nutrition Recommendations set by the National Nutrition Council (Citation43), the American Heart Association food guidelines (Citation44), and dietary guidelines for Americans (Citation45) was assessed. Data on alcohol consumption, smoking, sleeping time, and socioeconomic status were collected with a self-administered questionnaire. Alcohol consumption was calculated in standard drinks (12 g pure ethanol) per day, i.e. total number of doses (0.33 L of beer or cider, 0.12 L of wine, and 0.04 L of hard liquor) consumed per week divided by 7. Smoking was defined as daily cigarette smoking. The subjects reported how many hours they usually slept per night (range from ≤ 5 hours to ≥ 10 hours, scaling every 30 min). Socioeconomic status was determined based on the reported occupation: 1) manual; 2) lower, non-manual; or 3) upper, non-manual.
Statistical analysis
The mean GGTs and FLIs and differences between all TV time groups in a longitudinal setting were evaluated and compared with linear regression, multiple comparison corrected (Tukey–Kramer) test. To complement this data, the magnitude of the mean GGT and FLI changes in all TV time groups during the 10-year follow-up were evaluated, and t test was used to compare the differences between the extreme groups (constantly high versus constantly low TV time). Generalized linear modelling was used to calculate the relative risk (RR) of ultrasonograpically detected fatty liver, and secondarily of high uFLI, according to time spent watching TV during the 10 years of follow-up. Specificity and sensitivity of the FLI and GGT were estimated by calculating their AUCs in comparison to the direct liver ultrasound scan evaluation and uFLI.
All analyses were adjusted for age and sex, leisure-time and occupational PA, energy intake, diet composition, alcohol use, sleep duration, socioeconomic status, and smoking in 2007, and in longitudinal analyses for GGT, and FLI at baseline. In addition, adjustment for BMI was made, except for the FLI analyses, as BMI (and waist) were per definition included in the FLI.
Data were analysed with all subjects together, but due to sex-by-TV interaction for GGT at each follow-up, for FLI at 2001, and for uFLI in 2011 (P < 0.002 in all), also sex-stratified analyses were performed. Grouping of subjects according to the TV time criteria excluded 423 participants. Therefore, an attrition analysis comparing the excluded and included subjects was performed using Wilcoxon's test. There were no differences in age, BMI, waist circumference, or TV time in 2001, but the excluded subjects were more often women (data not shown). All statistical analyses were done with the SAS version 9.4, and statistical significance was inferred at a 2-tailed probability value < 0.05.
Results
In longitudinal analyses mean GGTs and FLIs in all TV time groups at each follow-up between 2001 and 2011 were evaluated. The mean GGTs and FLIs were similar in all TV time groups at baseline in 2001 (P > 0.05) (, and ).
Figure 1. Gamma-glutamyltransferase (GGT) concentration change in the TV time groups during 10 years of follow-up. (A) sexes combined. (B) women separately. (C) men separately. Analyses adjusted for age and sex, baseline GGT, physical activity, occupational physical strain, energy intake, diet composition, alcohol use, sleep duration, socioeconomic status, and smoking. Adjustment with BMI diluted the association to borderline significant (P = 0.053, data not shown). L = FLI different between the constantly high and constantly low TV time group; M = FLI different between the constantly high and constantly moderate TV time group; D = FLI different between the constantly high and decreased TV time group.
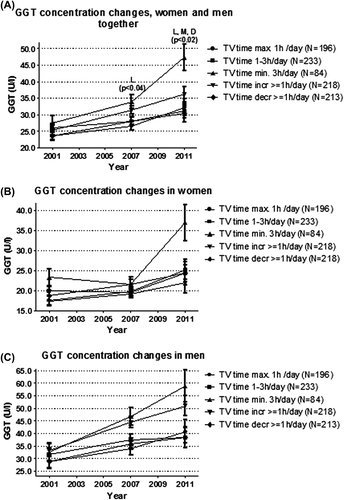
Figure 2. Fatty Liver Index (FLI) change in the TV time groups during 10 years of follow-up. (A) sexes combined. (B) women separately. (C) men separately. Analyses adjusted for age and sex, baseline FLI, physical activity, occupational physical strain, energy intake, diet composition, alcohol use, sleep duration, socioeconomic status, and smoking. BMI is included in the FLI as per definition. L = FLI different between the constantly high and constantly low TV time group; M = FLI different between the constantly high and constantly moderate TV time group; I = FLI different between the constantly high and increased TV time group; D = FLI different between the constantly high and decreased TV time group.
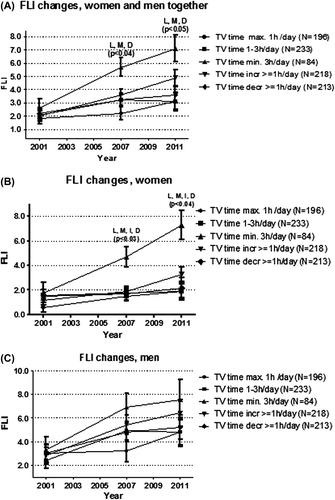
Table I. Mean increase in GGT and FLI during the 10-year follow-up in all TV time groups and by sex. Comparison of the magnitude of change between the constantly high and constantly low TV time groups is presented separately. Analyses adjusted for age and sex, baseline FLI, physical activity, occupational physical strain, energy intake, diet composition, alcohol use, sleep duration, socioeconomic status, and smoking. BMI is included in the FLI as per definition.
GGT concentration
In 2011 the mean GGT concentration was higher in the constantly high TV time group than in the constantly low and moderate and in the decreased TV time groups (P < 0.02 in all) (). A statistically significant difference was seen already between constantly high and low TV time groups in 2007. Adjustment for BMI diluted the association between high and low TV time groups to borderline significant (P = 0.053, data not shown).
The FLI
The mean FLIs in 2007 and 2011 were higher in the constantly high TV time group than in the constantly low and moderate, and the increased/decreased TV time groups (P < 0.04 in all) (). Adjustment for covariates did not change the results.
Changes of GGT and FLI
To complement the analysis of the mean GGT and FLI values at each follow-up in each TV group, the magnitude of increases in each TV group were evaluated. In addition, the GGT and FLI increases during the 10-year follow-up between the extreme groups (constantly high and constantly low TV time) were compared. The increase in GGT concentration and the FLI in the constantly high TV time group were about three times higher than in the constantly low TV time group (P < 0.0001 in all) ().
Hepatic ultrasound
In addition, a cross-sectional analysis was done to evaluate the risk of fatty liver in all TV time groups based on liver ultrasound findings performed in 2011. The risk of fatty liver increased 2.34-fold (95% CI 1.22–4.48) in the constantly high when compared to the increase in the constantly low TV time group (). The findings were similar for the uFLI (data not shown). Adjustment with BMI attenuated the associations only partially.
Table II. Risk ratios (RRs) and 95% confidence intervals (95% CI) for ultrasonographical diagnosis of fatty liver in all TV time groups. Generalized linear modelling adjusted for age and sex, leisure-time and occupational physical activity, energy intake, diet composition, alcohol use, sleep duration, socioeconomic status, and smoking, with and without BMI.
Discussion
We observed a steeper increase in GGT and FLI through 10 years of follow-up, and fatty liver risk increased in adults spending constantly more time viewing TV. Fatty liver was less prevalent in subjects reducing their TV time at least with one hour during the follow-up. The relationship between TV time and fatty liver was independent of various covariates, but BMI partly attenuated the association.
Pre-existing data
As data on sedentary behaviour and NAFLD do not exist, data from physical inactivity studies were evaluated. Decreased levels of daily PA (≤ 1 day/week versus ≥ 3 days/week) are associated with increased incidence of NAFLD, and women with lower PA have elevated liver enzymes (Citation46). Enforced physical inactivity in healthy, normal-weight women increases hepatic markers of NAFLD (alanine/aspartate transaminase, cytokeratin, angiopoietin-like protein 3) with hypertriglyceridaemia, suggesting alteration in hepatic metabolism independent of fat mass (Citation47). In hyperphagic OLETF rats daily PA prevents NAFLD by increasing hepatic mitochondrial content and function and by suppressing hepatic de novo lipogenesis (Citation46). Physical inactivity together with obesity may be the cause of disruption in hepatic insulin signalling and subsequent NAFLD. In obese mouse models of NAFLD, impaired hepatic insulin signalling under sedentary conditions markedly improves after an acute swimming bout (Citation46). Thus, current knowledge suggests that physical inactivity increases the risk of NAFLD, together with or independently of obesity, but the exact mechanism remains unconfirmed. Our results showing an independent association between constantly high TV viewing time and increased fatty liver risk using various markers are in line with these data.
Prolonged sitting may lead to low energy consumption, positive energy balance, and weight gain, even without increased energy intake, as described by Levine (Citation48); the non-exercise activity thermogenesis theory emphasizes the role of energy expenditure in all PA other than volitional sporting-like exercise. This is in line with our findings, since high TV viewing time was associated with increased risk of fatty liver regardless of PA and diet. Some studies have suggested that snacking while watching TV, especially in children, may be important for weight gain (Citation49), but the time of eating could not be evaluated in our study.
Based on our earlier study, sitting is causally antecedent to weight increase, and not the other way around (Citation23). The association between TV viewing and fatty liver was diluted after adjustment for BMI suggesting that the effect of high TV time is mediated, at least partly, by weight increase. In our previous studies (Citation22,Citation23) TV viewing was associated with weight increase regardless of energy intake and diet, but most probably also unhealthy diet may increase fatty liver risk.
Limitations and strengths
Due to significant sex-by-TV interaction, separate analyses also for women and men were performed. Not all analyses stratified by sex showed significant TV time group differences in our study ( and , ). This may be due to a lack of statistical power more than a true sex difference or non-significance of the results, as sex-stratified analyses showed a comparable trend with analyses combining men and women, i.e. alienation of curves and results during the 10-year follow-up ( and , and ).
Limitations of our study include subjective reporting of TV viewing time. However, TV viewing can be self-reported with adequate accuracy (Citation50). On the other hand, TV viewing makes up over 50% of the daily non-occupational time, and it is the type sedentary behaviour that has most frequently been associated with adverse health outcomes (Citation22), which makes it a good indicator of sedentary time.
It is important to note that TV programmes are increasingly followed using other devices than TV, especially by younger generations. An easier and high-speed wireless access to TV may change where and how we watch TV. This needs to be considered in future studies for data collection and evaluation purposes. Devices used for TV viewing may include computers and mobile devices, e.g. tablets or cell phones. This adds a more mobile aspect to TV viewing, i.e. especially shorter programmes may also be watched while being on the move. In contrast, watching movies and other longer TV programmes will most probably still result in prolonged sedentary time. On the other hand, mobile devices facilitate TV viewing regardless of location, which may result in an increase in overall TV viewing and other sedentary time spent in front of mobile devices. Nevertheless, as mentioned earlier, TV viewing time is the type of sedentary behaviour that has most often been associated with negative health outcomes, as seen also in our earlier study (Citation22). Even when screen time combining both TV and computer time was evaluated, TV viewing time seemed to be the driving force for the unfavourable associations. Similar findings have been seen in children and adolescents (Citation51). We also saw a stronger correlation with high TV viewing time and e.g. unfavourable diet habits than with high computer use (Citation22), which may contribute to the unfavourable associations seen with TV. In future studies, objectively collected PA data together with documentation of the device used for TV viewing may be needed to be able to evaluate the specific role of TV, computer, or mobile technology.
Most covariates used in the analyses were available only at 2007 follow-up, but the values reported around the middle of the follow-up period are likely representative of behaviour during the 10-year follow-up.
The duration of individual sitting bouts or breaks (Citation52,Citation53), or aerobic fitness (Citation54), and their association with fatty liver could not be evaluated in this study.
The strength of our study is the longitudinal setting in a relatively large and representative study population. In addition, important other risk factors for fatty liver could be taken into account. The attrition analyses performed for the current investigation, and for the whole study (Citation35), confirm that the direction of bias, if any, would only slightly dilute the results.
To conclude, based on this novel study, individuals spending constantly more time in front of the TV have a higher risk of fatty liver, regardless of several potential confounders. As domestic and working lives have become physically less active, and at the same time literally more sedentary (Citation55), we may need new ways to tackle the sedentary lifestyle. Reduction of TV time may offer potential to reduce the risk and cardiovascular and metabolic diseases associated with it. Such a hypothesis needs to be tested with interventional studies.
Acknowledgements
Statistical support during the preparation of this manuscript was given by Irina Lisinen and Ville Aalto.
Funding: The Cardiovascular Risk in Young Finns Study has been financially supported by the Academy of Finland (grants 126925, 121584, 124282, 129378, 117797, and 41071), the Social Insurance Institution of Finland, Kuopio, Tampere and Turku University Hospital Medical Funds, the Juho Vainio Foundation, the Paavo Nurmi Foundation, the Yrjö Jahnsson Foundation (T.L.), the Finnish Foundation of Cardiovascular Research and the Finnish Cultural Foundation, the Sigrid Juselius Foundation, the Tampere Tuberculosis Foundation and the Emil Aaltonen Foundation.
Declaration of interest: The authors have no competing interests.
References
- Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202–19.
- Ryan MC, Wilson AM, Slavin J, Best JD, Jenkins AJ, Desmond PV. Associations between liver histology and severity of the metabolic syndrome in subjects with nonalcoholic fatty liver disease. Diabetes Care. 2005;28:1222–24.
- Sattar N, Forrest E, Preiss D. Non-alcoholic fatty liver disease. BMJ. 2014;349:g4596.
- Suomela E, Oikonen M, Virtanen J, Parkkola R, Jokinen E, Laitinen T, et al. Prevalence and determinants of fatty liver in normal-weight and overweight young adults. The Cardiovascular Risk in Young Finns Study. Ann Med. 2015;47:40–6.
- Dixon JB, Bhathal PS, O’Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121:91–100.
- Sumida Y, Yoneda M, Hyogo H, Yamaguchi K, Ono M, Fujii H, et al. A simple clinical scoring system using ferritin, fasting insulin, and type IV collagen 7S for predicting steatohepatitis in nonalcoholic fatty liver disease. J Gastroenterol. 2011;46:257–68.
- Palekar NA, Naus R, Larson SP, Ward J, Harrison SA. Clinical model for distinguishing nonalcoholic steatohepatitis from simple steatosis in patients with nonalcoholic fatty liver disease. Liver Int. 2006;26:151–6.
- Poynard T, Ratziu V, Charlotte F, Messous D, Munteanu M, Imbert-Bismut F, et al. Diagnostic value of biochemical markers (NashTest) for the prediction of non alcoholic steatohepatitis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol. 2006;6:34.
- Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology. 2002;122:1649–57.
- Wiechowska A, McCullough AJ, Feldstein AE. Noninvasive diagnosis and monitoring of nonalcoholic steatohepatitis: Present and future. Hepatology. 2007;46:582–9.
- Stefan N, Kantartzis K, Haring HU. Causes and metabolic consequences of fatty liver. Endocr Rev. 2008;29:939–60.
- Adams LA, Lindor KD. Nonalcoholic fatty liver disease. Ann Epidemiol. 2007;17:863–9.
- Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346: 1221–31.
- Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363:1341–50.
- Hickman IJ, Jonsson JR, Prins JB, Ash S, Purdie DM, Clouston AD, et al. Modest weight loss and physical activity in overweight patients with chronic liver disease results in sustained improvement in alanine aminotransferase, fasting insulin, and quality of life. Gut. 2004;53:413–19.
- St George A, Bauman A, Johnston A, Farrell G, Chey T, George J. Independent effects of physical activity in patients with nonalcoholic fatty liver disease. Hepatology. 2009;50:68–76.
- Harrison SA, Day CP. Benefits of lifestyle modification in NAFLD. Gut. 2007;56:1760–9.
- Johnson NA, Stannard SR, Thompson MW. Muscle triglyceride and glycogen in endurance exercise: implications for performance. Sports Med. 2004;34:151–64.
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346: 393–403.
- Laaksonen DE, Lindstrom J, Lakka TA, Eriksson JG, Niskanen L, Wikstrom K, et al. Physical activity in the prevention of type 2 diabetes: the Finnish diabetes prevention study. Diabetes. 2005;54: 158–65.
- Solomon TPJ, Haus JM, Marchetti CM, Stanley WC, Kirwan JP. Effects of exercise training and diet on lipid kinetics during free fatty acid-induced insulin resistance in older obese humans with impaired glucose tolerance. Am J Physiol Endocr Metab. 2009; 297:E552–9.
- Heinonen I, Helajärvi H, Pahkala K, Heinonen OJ, Hirvensalo M, Pälve K, et al. Sedentary behaviours and obesity in adults: the Cardiovascular Risk in Young Finns Study. BMJ Open. 2013;3:002901.
- Helajärvi H, Rosenström T, Pahkala K, Kähönen M, Lehtimäki T, Heinonen OJ, et al. Exploring causality between TV viewing and weight change in young and middle-aged adults. The Cardiovascular Risk in Young Finns Study. PLoS One. 2014;9(7):e101860.
- Edwardson CL, Gorely T, Davies MJ, Gray LJ, Khunti K, Wilmot EG, et al. Association of sedentary behaviour with metabolic syndrome: a meta-analysis. PLoS One. 2012;7(4):e34916.
- Dunstan DW, Thorp AA, Healy GN. Prolonged sitting: is it a distinct coronary heart disease risk factor? Curr Opin Cardiol. 2011;26: 412–19.
- Grontved A, Hu FB. Television viewing and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality. JAMA. 2011;305: 2448–55.
- Wilmot EG, Edwardson CL, Achana FA, Davies MJ, Gorely T, Gray LJ, et al. Sedentary time in adults and the association with diabetes, cardiovascular disease and death: systematic review and meta-analysis. Diabetologia. 2012;55:2895–905.
- Ukawa S, Tamakoshi A, Wakai K, Kurozawa Y. Associations of daily walking and television viewing time with liver cancer mortality: findings from the Japan Collaborative Cohort Study. Cancer Causes Control. 2014 Jul;25:787–93.
- Tahan V, Canbakan B, Balci H, Dane F, Akin H, Can G, et al. Serum gamma-glutamyltranspeptidase distinguishes non-alcoholic fatty liver disease at high risk. Hepatogastroenterology. 2008;55:1433–8.
- Loguercio C, De Simone T, D’Auria MV, de Sio I, Federico A, Tuccillo C, et al.; Italian AISF Clinical Group. Non-alcoholic fatty liver disease: a multicentre clinical study by the Italian Association for the Study of the Liver. Dig Liver Dis. 2004;36:398–405.
- Bedogni G , Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33.
- Dasarathy S, Dasarathy J, Khiyami A, Joseph R, Lopez R, McCullough AJ. Validity of real time ultrasound in the diagnosis of hepatic steatosis: a prospective study. J Hepatol. 2009;51:1061–7.
- Loria P, Adinolfi LE, Bellentani S, Bugianesi E, Grieco A, Fargion S, et al. Practice guidelines for the diagnosis and management of nonalcoholic fatty liver disease. A decalogue from the Italian Association for the Study of the Liver (AISF) Expert Committee . Digest Liver Dis. 2010;42:272–82.
- Saverymuttu SH, Joseph AE, Maxwell JD. Ultrasound scanning in the detection of hepatic fibrosis and steatosis. Br Med J (Clin Res Ed). 1986;292:13–15.
- Raitakari OT, Juonala M, Rönnemaa T, Keltikangas-Järvinen L, Räsänen L, Pietikäinen M, et al. Cohort profile: the cardiovascular risk in Young Finns Study. Int J Epidemiol. 2008;37:1220–6.
- Edens MA, van Ooijen PM, Post WJ, Haagmans MJ, Kristanto W, Sijens PE, et al. Ultrasonography to quantify hepatic fat content: validation by 1H magnetic resonance spectroscopy. Obesity (Silver Spring). 2009;17:2239–44.
- Mansikkaniemi K, Juonala M, Taimela S, Hirvensalo M, Telama R, Huupponen R, et al. Cross-sectional associations between physical activity and selected coronary heart disease risk factors in young adults. The Cardiovascular Risk in Young Finns Study. Ann Med. 2011;44:733–44.
- Mälkiä E, Impivaara O, Maatela J, Aromaa A, Heliövaara M, Knekt P. Physical activity of Finnish adults. Report no. ML8. Turku: Publications of the Social Insurance Institution, Finland; 1988. p. 14–15.
- Tammelin T, Näyhä S, Rintamäki H, Zitting P. Occupational physical activity is related to physical fitness in young workers. Med Sci Sports Exerc. 2002;34:158–66.
- Paalanen L, Männisto S, Virtanen MJ, Knekt P, Räsänen L, Montonen J, et al. Validity of a food frequency questionnaire varied by age and body mass index. J Clin Epidemiol. 2006;59:994–1001.
- Fineli. Finnish Food Composition Database. 7. 2007. Helsinki, Finland: The National Public Health Institute, Nutrition Unit; 2007.
- Nettleton JA, Hivert M-F, Rozenn N, Lemaitre RN, McKeown NM, Mozaffarian D, et al. Meta-analysis investigating associations between healthy diet and fasting glucose and insulin levels and modification by loci associated with glucose homeostasis in data from 15 cohorts. Am J Epidemiol. 2013;177:103–15.
- National Nutrition Council. Finnish nutrition recommendations. Helsinki: Oy Edita; 2005.
- Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96.
- U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary guidelines for Americans, 2010. 7th ed. Washington, DC: U.S. Government Printing Office; 2011.
- Rector RS, Thyfault JP. Does physical inactivity cause nonalcoholic fatty liver disease? J Appl Physiol. 2011;111:1828–35.
- Rudwill F, Bergouignan A, Gastebois C, Gauquelin-Koch G, Lefai E, Blanc S, et al. Effect of enforced physical inactivity induced by 60-day of bed rest on hepatic markers of NAFLD in healthy normal-weight women. Liver Int. 2015;35:1700–6.
- Levine JA. Nonexercise activity thermogenesis—liberating the life-force. J Intern Med. 2007;262:273–87.
- Pearson N, Biddle SJ. Sedentary behaviour and dietary intake in children, adolescents, and adults a systematic review. Am J Prev Med. 2011;41:178–88.
- Atkin AJ, Gorely T, Clemes SA, Yates T, Edwardson C, Brage S, et al. Methods of measurement in epidemiology: sedentary behavior. Int J Epidemiol. 2012;41:1460–71.
- Coombs NA, Stamatakis E. Associations between objectively assessed and questionnaire-based sedentary behaviour with BMI-defined obesity among general population children and adolescents living in England. BMJ Open. 2015;5:e007172.
- Latouche C, Jowett JBM, Carey AL, Bertovic DA, Owen N, Dunstan DW, et al. Effects of breaking up prolonged sitting on skeletal muscle gene expression. J Appl Physiol. 2012;114:453–60.
- Healy GN, Dunstan DW, Salmon J, Cerin E, Shaw JE, Zimmet PZ, et al. Breaks in sedentary time: beneficial associations with metabolic risk. Diab Care. 2008;31:661–6.
- Johnson NA, George J. Fitness versus fatness: moving beyond weight loss in nonalcoholic fatty liver disease. Hepatology. 2010;52: 370–81.
- Church TS, Thomas DM, Tudor-Locke C, Katzmarzyk PT, Earnest CP, Rodarte RQ, et al. Trends over 5 decades in U.S. occupation-related physical activity and their associations with obesity. PLoS One. 2011;6:e19657.
