Abstract
Purpose To evaluate the risk of acute pancreatitis hospitalization with sitagliptin use in patients with type 2 diabetes mellitus (T2DM).
Methods This retrospective cohort analysis included newly diagnosed T2DM with onset age ≥25 years between 1999 and 2010 from the National Health Insurance database. Ever users (n = 89,800) and never users (n = 449,000) of sitagliptin were followed until end of 2011. A time-dependent approach was used to calculate event incidence and estimate hazard ratios adjusted for propensity score.
Results During follow-up, 261 ever users and 5,840 never users were hospitalized for acute pancreatitis (respective incidence, 224.0 and 168.4 per 100,000 person-years), with adjusted hazard ratio of 1.59 (95% CI 1.40–1.81). The respective hazard ratio for the first, second, and third tertile of time since starting sitagliptin <9.5, 9.5–21.0, and >21.0 months was 8.10 (6.80–9.65), 1.70 (1.38–2.11), and 0.41 (0.30–0.56); 3.26 (2.67–3.98), 1.86 (1.52–2.27), and 0.76 (0.59–0.98) for cumulative duration <3.7, 3.7–10.3, and >10.3 months; and 3.21 (2.65–3.90), 1.89 (1.54–2.32), and 0.73 (0.57–0.95) for cumulative dose <9,000, 9,000–28,000, and >28,000 mg.
Conclusions Sitagliptin is associated with a higher risk of acute pancreatitis within the first 2 years of its initiation. The risk diminishes thereafter, probably due to the depletion of susceptible patients.
Sitagliptin is significantly associated with an increased risk of acute pancreatitis within the first 2 years of its initiation, but the risk diminishes thereafter, probably due to the depletion of susceptible patients.
The overall hazard ratio after adjustment for propensity score for ever users versus never users was 1.59 (95% CI 1.40–1.81).
For users with cumulative dose >28,000 mg, the hazard ratio was 0.73 (0.57–0.95).
Key Messages
Introduction
Incretin-based therapy, either with a dipeptidyl peptidase-4 (DPP4) inhibitor or a glucagon-like peptide-1 (GLP-1) agonist, has become a main therapy for patients with type 2 diabetes mellitus (T2DM). However, acute pancreatitis associated with incretin-based therapy has been reported since 2006 (Citation1) and has become a major concern for their use.
Administrative database analyses from the adverse event reporting system of the US Food and Drug Administration (FDA) (Citation2,Citation3), the Italian Spontaneous Adverse Drug Reactions Reporting Database (Citation4), and the French Pharmacovigilance Database (Citation5) all suggested a disproportionally higher reporting rate (up to 30-fold) of acute pancreatitis for incretin-based therapy. However, studies using the adverse event reporting systems may suffer from a potential Weber effect (notoriety bias) (Citation6).
Although secondary analyses of randomized controlled trials did not find a significant link (Citation7–10), these trials were not designed primarily for evaluating acute pancreatitis, and therefore the incident cases of acute pancreatitis were generally too small (mostly <30 cases in each comparing group) for sufficient statistical power. Some observational studies supported such a significant association (Citation7,Citation11), but this could not be similarly demonstrated by others (Citation7,Citation12–18).
Sitagliptin was the first DPP4 inhibitor approved for clinical use in Taiwan (on 13 July 2007). The other DPP4 inhibitors (i.e. saxagliptin, vildagliptin, and linagliptin) and GLP-1 agonists (i.e. exenatide and liraglutide) were not approved until after 2009. Using the reimbursement database of the National Health Insurance (NHI), the present study aimed at evaluating whether sitagliptin use in Taiwanese patients with T2DM would affect the risk of acute pancreatitis. Other incretins were not evaluated because they were not used commonly during the study period. A new-user design and a time-dependent approach for sitagliptin use were applied in order to minimize the potential ‘prevalent user bias’ (Citation19) and ‘immortal time bias’ (Citation20,Citation21).
Materials and methods
This retrospective cohort analysis was approved by the National Health Research Institutes (NHRI) with approval number 99274. Written informed consent was not required according to local regulations because the identification information was scrambled prior to analysis for privacy protection.
Since March 1995 a compulsory and universal system of NHI was implemented. The NHI covers more than 99% of the Taiwanese population and has contracts with over 98% of the hospitals nationwide. Detailed records including outpatient visits, emergency department visits, and hospital admission are kept in the database. The International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) has been used during the study period, and diabetes was coded 250.XX. Information of hospitalization for acute pancreatitis (ICD-9-CM 577.0) was linked from the hospitalization database.
shows the flow chart for the procedures followed in creating the cohort of newly diagnosed T2DM patients with onset age ≥25 years for the study. The NHRI created a cohort of 120,000 newly diagnosed diabetic patients in each calendar year for a 12-year period from 1999 to 2010 from the whole nation. The longitudinal reimbursement records of these patients from 1996 to 2011 can be provided for academic research after approval. A patient should not have a diagnosis of diabetes in the previous years when he/she was randomly selected into the cohort for each specific year. The definition of diabetes was based on one of the following two criteria: 1) diagnosis of diabetes during an admission to the hospital or having been prescribed with antidiabetic drugs during hospitalization; or 2) in an outpatient setting within 1 year, a patient has been diagnosed twice or more as having diabetes, or diagnosed once as having diabetes plus prescribed with antidiabetic drugs once. As a result, a total of 1,440,000 patients with newly diagnosed diabetes were available within these 12 years.
Figure 1. Flow chart showing the procedures followed in creating the cohort of patients with newly diagnosed type 2 diabetes mellitus and aged ≥25 years at diabetes onset during 1999–2010 for the study. NHI = National Health Insurance; NHRI = National Health Research Institutes.
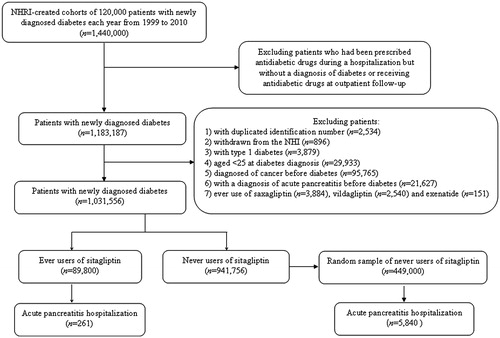
In consideration that some patients might have been given insulin, oral antidiabetic drugs, or GLP-1 agonists during an admission for some medical conditions but they might not be real cases of diabetes, patients who were recruited based on the criterion of having been prescribed with antidiabetic drugs during hospitalization but had not been followed at the outpatient clinics with a diagnosis of diabetes or had not received antidiabetic drugs at outpatient follow-up were first excluded. This resulted in a sample size of 1,183,187 patients. After further exclusion of patients with duplicated identification number (n = 2,534), withdrawn from the NHI (n = 896), with a diagnosis of type 1 diabetes (n = 3,879), aged <25 at diabetes diagnosis (n = 29,933), with a diagnosis of any cancer before diabetes diagnosis (n = 95,765), with a diagnosis of acute pancreatitis before diabetes diagnosis (n = 21,627), and ever use of saxagliptin (n = 3,884), vildagliptin (n = 2,540), and exenatide (n = 151), a total of 1,031,556 patients were available (linagliptin and liraglutide were not available in Taiwan during the study period). Among them, 89,800 patients had been prescribed with sitagliptin (ever users), and 941,756 patients had not been treated with sitagliptin (never users). A subsample of the never users was randomly selected with a predetermined sample size of 5-fold the ever users (i.e. n = 449,000) as a comparison group for analyses.
Age was defined at the time of diabetes diagnosis, and a number of comorbidities and covariates were determined as a status/diagnosis at the time of censor. The ICD-9-CM codes for the comorbidities were (Citation22–27): hypertension 401–405; chronic obstructive pulmonary disease (a surrogate for smoking) 490–496; stroke 430–438; nephropathy 580–589; ischemic heart disease 410–414; peripheral arterial disease 250.7, 785.4, 443.81, and 440–448; eye disease 250.5, 362.0, 369, 366.41, and 365.44; obesity 278; dyslipidemia 272.0–272.4; alcohol-related diagnosis 291, 303, 535.3, 571.0–571.3, 980.0; and gallstone 574.00, 574.01, 574.10, 574.11, 574.20, 574.21, and A348. Medications included sulfonylurea, meglitinide, acarbose, pioglitazone, rosiglitazone, insulin, statin, fibrate, angiotensin-converting enzyme inhibitor and/or angiotensin receptor blocker, calcium channel blocker, aspirin, ticlopidine, clopidogrel, dipyridamole, and non-steroidal anti-inflammatory drugs (excluding aspirin).
The characteristics of sitagliptin never users and ever users were compared by Student’s t test for age and by chi-square test for others. The crude incidence density of acute pancreatitis was calculated for sitagliptin ever users and never users and for the tertiles of the following exposure parameters calculated from the database: time since starting sitagliptin (months), cumulative duration (months), and cumulative dose (mg). Follow-up started on the first day of diabetes diagnosis and ended on 31 December 2011, at the time of a new diagnosis of acute pancreatitis during hospitalization, or at the date of the last reimbursement record. Lacking information on the mortality or migration status of the patients, the last reimbursement record may serve as a surrogate because these patients should be withdrawn from the NHI in Taiwan. Exposure to sitagliptin was treated as a time-dependent variable. Therefore, the sitagliptin ever users contributed person-years to the non-sitagliptin group until they started using sitagliptin, and after starting sitagliptin, to the sitagliptin group.
Time-dependent Cox regression was performed to estimate the hazard ratios for acute pancreatitis among sitagliptin ever users versus never users, and for the various tertiles of the exposure parameters. The hazard ratios were adjusted for propensity score (PS) derived from the above-mentioned characteristics because biased estimates may result from systematic differences between treatment groups in non-randomized studies, and adjustment for PS may reduce this bias (Citation28). In consideration that ever users and never users of sitagliptin might be recruited during different periods of time, which might have introduced some unknown bias, sensitivity analyses for the above Cox regression models were also created by including calendar year of recruitment in the calculation of PS.
The following sensitivity analyses were conducted to estimate the PS-adjusted hazard ratios for ever users versus never users: 1) in subgroups of men, women, age <50 years, and age ≥50 years, respectively; 2) excluding insulin users, because insulin is always used as the last resort for glycemic control in Taiwan and therefore its use can serve as a surrogate for poor glycemic control; 3) excluding users of fibrate, because fibrate is mainly used for the treatment of hypertriglyceridemia, a well-recognized risk factor for acute pancreatitis; and 4) excluding patients with a diagnosis of alcohol-related diagnosis and/or gallstone, because these are considered as the most important risk factors for acute pancreatitis (Citation29).
Analyses were conducted using SAS statistical software, version 9.3 (SAS Institute, Cary, NC, USA). P < 0.05 was considered statistically significant.
Results
compares the characteristics between ever users and never users of sitagliptin. All variables differed significantly. Ever users were characterized by a younger age, slightly higher proportion of male sex, and a higher proportion of dyslipidemia but lower proportions of alcohol-related diagnoses and gallstone. Except for dipyridamole, ever users were more likely to use other medications. Hypertension, ischemic heart disease, peripheral arterial disease, eye disease, and obesity were more prevalent among ever users, while chronic obstructive pulmonary disease, stroke, and nephropathy were less prevalent.
Table I. Characteristics of sitagliptin never users and ever users.
shows the incidences of acute pancreatitis hospitalization in different exposure groups and the hazard ratios comparing exposed to unexposed. In models that did not consider calendar year of recruitment, the overall PS-adjusted hazard ratio was 1.59 (95% CI 1.40–1.81). For all three exposure parameters, significantly increased risk was observed for the first and second tertiles, but the risk diminished in the third tertiles. The results were not remarkably influenced when models also included calendar year in PS calculation.
shows the overall PS-adjusted hazard ratios in the sensitivity analyses. The risk of acute pancreatitis associated with sitagliptin use was consistently increased disregarding age and sex. The results were not affected by residual confounding from poor glycemic control (insulin use as a surrogate), hypertriglyceridemia (fibrate use as a surrogate), and alcohol-related diagnosis and/or gallstone.
Table II. Sitagliptin and incidence of acute pancreatitis hospitalization and hazard ratios comparing exposed to unexposed.
Table III. Sensitivity analyses for sitagliptin and acute pancreatitis.
Discussion
The study supported that sitagliptin use might increase the risk of acute pancreatitis. The increased risk was mainly observed within 21 months after sitagliptin initiation, within a cumulative duration of approximately 10 months or within a cumulative dose of 28,000 mg (). After 21 months of its initiation, after a longer duration of its use, or after a higher cumulative dose, the observed risk diminished ().
The higher risk of acute pancreatitis within 2 years of its initiation was consistent with most of the case reports. For example, Sue et al. reported a Japanese man who developed acute pancreatitis after 8 months of sitagliptin use, and summarized four additional cases of acute pancreatitis developed within 8 weeks of sitagliptin use (two women) or during change of sitagliptin to vildagliptin (one man and one woman) (Citation30). Scheen reported acute pancreatitis in diabetic patients treated with either sitagliptin (one man and two women) or vildagliptin (two men and one woman) within a few weeks to a maximum of 28 weeks after the initiation of the drugs (Citation7). Other case reports of acute pancreatitis associated with incretin-based therapy included a 49-year-old Indian man who used vildagliptin for 3 weeks (Citation31), a 67-year-old Italian man who used liraglutide for 5 months (Citation32), and a 63-year-old French man who used liraglutide for 11 months (Citation33).
The diminished risk associated with sitagliptin after a longer duration or a larger dose of its exposure could be explained as follows. When some patients developed acute pancreatitis after the use of sitagliptin, the physicians would surely discontinue its use, leaving patients with a low susceptibility to keep on using sitagliptin. Because it is extremely rare for drugs to increase risk in one period and decrease in another, the significantly lower risk of acute pancreatitis observed in the third tertiles of the dose-response parameters () could be a spurious finding resulting from the depletion of susceptible cases in the cohort.
The discrepant risk association with regard to exposure duration and dose is an interesting finding not reported previously. This may explain the discrepant conclusions made in different observational studies. Therefore, studies that include mostly patients within the first 2 years of sitagliptin use may give an overall significant risk estimate, but studies including more patients with longer duration of exposure would surely conclude with a neutral or even protective effect. These observations may also explain why the estimated relative risk for acute pancreatitis among current users of exenatide (1.3, 95% CI 1.0–1.7) was not as remarkable as among past users (1.6, 95% CI 1.2–2.1) in an analysis that pooled two US cohort studies using separate commercial health insurance databases (Citation18).
Although two recent meta-analyses still did not conclude with a significantly higher risk associated with incretin-based therapy (Citation34,Citation35), they included randomized clinical trials and observational studies with varying sample sizes and follow-up durations, and did not specify the effect of sitagliptin. It is worth pointing out that in the just published randomized placebo-controlled trial investigating the effect of sitagliptin on cardiovascular outcomes in patients with T2DM (TECOS: Trial Evaluating Cardiovascular Outcomes with Sitagliptin; median follow-up, 3.0 years), even though not statistically significant, cases of incident acute pancreatitis in patients randomized to sitagliptin outnumbered cases in those who used placebo (23 versus 12 cases), with an estimated hazard ratio of 1.93 (95% CI 0.96–3.88, P = 0.07) (Citation36).
From animal studies, the potential mechanisms for acute pancreatitis associated with incretin-based therapy may involve an increased replication of pancreatic duct and induction of acinar to ductal metaplasia, resulting in outflow obstruction (Citation37). A recent autopsy study supported such animal findings (Citation38). Butler et al. compared the pathological changes in the pancreas in brain-dead organ donors in three groups of patients: non-diabetes (n = 14), T2DM without incretin-based therapy (n = 12), and T2DM with incretin-based therapy (n = 8; 7 sitagliptin users and 1 user of exenatide) (Citation38). The findings suggested that patients on incretin-based therapy had marked expansion of both exocrine and endocrine pancreas (Citation38). However, other investigators pointed out some potential limitations in this study, including the small numbers of cases, the imbalance in age and pancreatic weight, and the lack of control of potential confounders (Citation39).
The association between incretin-based therapy and acute pancreatitis has long been a great concern. It is critical that this potential adverse effect should be fully characterized because the use of incretins has been increasing dramatically for the treatment of T2DM in recent years. Clinical trials are unlikely to provide a definite answer in a short observation time because acute pancreatitis occurs at a very low frequency. Despite some limitations, epidemiological analyses of existing large databases as conducted in the present study may offer a promising approach for understanding the relationship at the present time. However, analyses of similar large databases by using different approaches may give different conclusions. For example, a recently published paper that also used the NHI databases for analyses came to a different conclusion. The investigators found that, while compared to new users of sulfonylurea, new users of DPP4 inhibitors (not specified and included sitagliptin, saxagliptin, and vildagliptin) had a significantly lower risk of acute pancreatitis with adjusted hazard ratio of 0.36 (95% CI 0.17–0.75). However, the hazard ratio comparing new users of DPP4 inhibitors to new users of metformin was not significant (Citation40). Scrutiny of this paper suggests that several inherent flaws might have led to such a conclusion. First, they included a small sample size of users of DPP4 inhibitors (n = 4,113) and did not specify the DPP4 inhibitors in the study. Second, the observation time was only approximately 60 days across all three drug groups (i.e. DPP4 inhibitors, metformin, and sulfonylurea), which would be too short to make any definite conclusion. Third, they did not exclude patients with a history of pancreatitis at baseline, and the difference in the distribution of baseline pancreatitis in users of DPP4 inhibitors, metformin, and sulfonylurea was statistically significant (P ≤ 0.01): 0.19%, 0.18%, and 0.28%, respectively (Citation40). Because pancreatitis always recurs in the same patients, it was surprisingly coincident that the estimated hazard ratio of 0.36 comparing users of DPP4 inhibitors to users of sulfonylurea simply reflects the different distribution in baseline pancreatitis. When the difference in the prevalence of baseline pancreatitis in users of sulfonylurea and DPP4 inhibitors (0.28% minus 0.19% = 0.09%) was divided by the prevalence of baseline pancreatitis in users of sulfonylurea (0.28%), the resulting value of 0.32 from this calculation was very close to the estimated hazard ratio of 0.36. There are several additional strengths in the present study by using similar NHI databases. First, we made a special request to include all longitudinal data of the patients to cover the whole period since the availability of the database in 1996. Second, the study has the merits of a large sample size and representativeness for the whole nation. Third, the observation time could be more than 2 years in a large sample of sitagliptin users and patients with pancreatitis at baseline had been excluded.
There are some limitations that deserve discussion. First, the choice of an optimal comparator group is a difficult problem that cannot be easily or fully resolved. Inclusion of all never users of sitagliptin as the comparator group in the present study might not be the best choice. Users of sitagliptin (a second- or third-line drug) might not be fully matched in risk distribution to never users that mixed patients with a variety of anti-diabetic medications including first-, second-, and third-line treatments. Furthermore, the selection of never users in the present study indicated that they could be patients followed before the marketing period of sitagliptin in Taiwan, but ever users included the follow-up starting after the marketing date of sitagliptin. To avoid potential bias introduced by recruiting ever users and never users of sitagliptin during different periods of time, additional analyses were performed by including calendar year of recruitment in the calculation of PS. The similar results () strengthened the conclusion of the study. Such a consistent finding could partly be due to a very steady trend of incidence of acute pancreatitis during a 10-year period from 2000 to 2009 in Taiwan, in contrary to an increasing trend observed in Western countries (Citation41). Additionally, because the diagnosis of diabetes during a hospital admission could be sufficient to enter the cohort, never users of sitagliptin could also include patients who were never pharmacologically treated. However, when examining the use of anti-diabetic medications in the cohort, only a small proportion of the recruited patients (n = 2,800, or 0.52%) were never pharmacologically treated. Even when these patients were excluded in secondary analyses, the results in did not change remarkably (data not shown).
Second, age, poor glycemic control, hypertriglyceridemia, alcoholism, and gallstone are well-recognized risk factors for acute pancreatitis. Models adjusted for PS without considering disease severity (e.g. glycemic control and diabetes duration) and important risk factors (e.g. triglyceride level) might not be able to exclude the residual confounding of these risk factors. However, we did try to include important risk factors of acute pancreatitis (e.g. age, obesity, dyslipidemia, alcohol-related diagnosis, and gallstone) in the calculation of PS used for adjustment. Because of the lack of biochemical data, use of insulin and use of fibrate could only be used as surrogates for poor glycemic control and hypertriglyceridemia, respectively. The consistency in the models adjusted for these surrogates () and after excluding these surrogates () indicated a true risk association between sitagliptin and acute pancreatitis. Even though users of sitagliptin were more prone to possess risk factors of obesity and dyslipidemia, they were less likely to have other risk factors such as older age, alcohol-related diagnosis, and gallstone (). Because excessive alcohol use and gallstone are the most important risk factors for acute pancreatitis (Citation29), the lower proportions of these risk factors together with a younger age in the group of ever users of sitagliptin () did not favor a bias toward a higher risk of acute pancreatitis among this group.
Third, the diagnosis of acute pancreatitis was made through hospital discharge records. Although the use of medical records may reduce the bias related to self-reporting, medical history and laboratory data to support such a diagnosis and to evaluate the severity of acute pancreatitis were not available in the reimbursement database. Since acute pancreatitis can be a serious, sometimes deadly, event characterized by abdominal pain, hyperamylasemia, and ultrasound findings, whether acute pancreatitis in the present study might have been overdiagnosed from the hospital discharge records based simply on hyperamylasemia is not known. In secondary analyses when cases of acute pancreatitis labeled as ‘secondary discharge diagnosis’ (n = 2,186 or 35.8%, presumably indicating patients suffering from minor pancreatic injury with clinical manifestation of only hyperamylasemia) were excluded in the analyses, the estimated hazard ratio for ever users versus never users of sitagliptin was 1.638 (95% CI 1.393–1.926), and the hazard ratios for the tertile analyses were all very close to those shown in (data not shown) without any change in the conclusions. The much higher incidence of acute pancreatitis in ever users (224.0 per 100,000 person-years) and never users of sitagliptin (168.4 per 100,000 person-years) in the present study () than the reported incidence of acute pancreatitis of 30.1 per 100,000 person-years in the general population and 54.0 per 100,000 person-years in patients with T2DM in a UK study conducted during 1996 and 2006 (Citation42) might suggest such a potential overdiagnosis of acute pancreatitis. However, an even higher incidence of acute pancreatitis was reported in another US study conducted during 1999 and 2005, which estimated an incidence of 149.3 per 100,000 person-years in the non-diabetic cohort and 421.9 per 100,000 person-years in patients with T2DM (Citation43). Therefore, the incidence rates of acute pancreatitis reported in the present study, which fell between the rates reported from the UK and the US, might also be reasonable.
Another limitation of the present study was its inability to evaluate the effects of other DPP4 inhibitors and GLP-1 agonists because of the small sample sizes of users of these drugs.
In summary, the present study suggests a positive association between sitagliptin use and acute pancreatitis within the first 2 years of its use. The risk diminishes thereafter, probably due to the depletion of susceptible patients among users of sitagliptin from the cohort. Because of the unavoidable inherent limitations associated with observational studies using existent big databases, the findings of the present study should be better confirmed in the future by ongoing randomized clinical trials.
Funding
The study was supported by the Ministry of Science and Technology (MOST 103-2314-B-002-187-MY3) of Taiwan. The guarantor of the manuscript is C.-H. Tseng.
Declaration of interest
The author reports no conflicts of interest.
References
- Denker PS, Dimarco PE. Exenatide (exendin-4)-induced pancreatitis: a case report. Diabetes Care. 2006;29:471.
- Elashoff M, Matveyenko AV, Gier B, Elashoff R, Butler PC. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology. 2011;141:150–6.
- Butler PC, Elashoff M, Elashoff R, Gale EA. A critical analysis of the clinical use of incretin-based therapies: are the GLP-1 therapies safe? Diabetes Care. 2013;36:2118–25.
- Delfino M, Motola D, Benini A, Franzè GP, Barotto M, Campi A, et al. Incretin-mimetics associated pancreatitis: evidence from the spontaneous adverse drug reactions reporting in Italy. Expert Opin Drug Saf. 2014;13:151–6.
- Faillie JL, Babai S, Crépin S, Bres V, Laroche ML, Le Louet H, et al.; French Pharmacovigilance Centers Network. Pancreatitis associated with the use of GLP-1 analogs and DPP-4 inhibitors: a case/non-case study from the French Pharmacovigilance Database. Acta Diabetol. 2014;51:491–7.
- Giorda CB, Nada E, Tartaglino B, Marafetti L, Gnavi R. A systematic review of acute pancreatitis as an adverse event of type 2 diabetes drugs: from hard facts to a balanced position. Diabetes Obes Metab. 2014;16:1041–7.
- Scheen A. Gliptins (dipeptidyl peptidase-4 inhibitors) and risk of acute pancreatitis. Expert Opin Drug Saf. 2013;12:545–57.
- White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, et al.; EXAMINE Investigators. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–35.
- Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, et al.; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–26.
- Meier JJ, Nauck MA. Risk of pancreatitis in patients treated with incretin-based therapies. Diabetologia. 2014;57:1320–4.
- Singh S, Chang HY, Richards TM, Weiner JP, Clark JM, Segal JB. Glucagonlike peptide 1-based therapies and risk of hospitalization for acute pancreatitis in type 2 diabetes mellitus: a population-based matched case-control study. JAMA Intern Med. 2013;173:534–9.
- Garg R, Chen W, Pendergrass M. Acute pancreatitis in type 2 diabetes treated with exenatide or sitagliptin: a retrospective observational pharmacy claims analysis. Diabetes Care. 2010;33:2349–54.
- Romley JA, Goldman DP, Solomon M, McFadden D, Peters AL. Exenatide therapy and the risk of pancreatitis and pancreatic cancer in a privately insured population. Diabetes Technol Ther. 2012;14:904–11.
- Funch D, Gydesen H, Tornøe K, Major-Pedersen A, Chan KA. A prospective, claims-based assessment of the risk of pancreatitis and pancreatic cancer with liraglutide compared to other antidiabetic drugs. Diabetes Obes Metab. 2014;16:273–5.
- Faillie JL, Azoulay L, Patenaude V, Hillaire-Buys D, Suissa S. Incretin based drugs and risk of acute pancreatitis in patients with type 2 diabetes: cohort study. BMJ. 2014;348:g2780.
- Giorda CB, Picariello R, Nada E, Tartaglino B, Marafetti L, Costa G, et al. Incretin therapies and risk of hospital admission for acute pancreatitis in an unselected population of European patients with type 2 diabetes: a case-control study. Lancet Diabetes Endocrinol. 2014;2:111–15.
- Chou HC, Chen WW, Hsiao FY. Acute pancreatitis in patients with type 2 diabetes mellitus treated with dipeptidyl peptidase-4 inhibitors: a population-based nested case-control study. Drug Saf. 2014;37:521–8.
- Dore DD, Hussein M, Hoffman C, Pelletier EM, Smith DB, Seeger JD. A pooled analysis of exenatide use and risk of acute pancreatitis. Curr Med Res Opin. 2013;29:1577–86.
- Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158:915–20.
- Stricker BH, Stijnen T. Analysis of individual drug use as a time-varying determinant of exposure in prospective population-based cohort studies. Eur J Epidemiol. 2010;25:245–51.
- Suissa S. Immortal time bias in pharmaco-epidemiology. Am J Epidemiol. 2008;167:492–9.
- Tseng CH. Pioglitazone and bladder cancer: a population-based study of Taiwanese. Diabetes Care. 2012;35:278–80.
- Tseng CH. Diabetes is not an independent risk factor for hepatocellular carcinoma. Diabetes Metab Res Rev. 2013;29:515–24.
- Tseng CH. Metformin may reduce bladder cancer risk in Taiwanese patients with type 2 diabetes. Acta Diabetol. 2014;51:295–303.
- Tseng CH. Diabetes but not insulin increases the risk of lung cancer: a Taiwanese population-based study. PLoS One. 2014;9:e101553.
- Tseng CH. Pioglitazone does not affect the risk of kidney cancer in patients with type 2 diabetes. Metabolism. 2014;63:1049–55.
- Tseng CH. Treatment with human insulin does not increase thyroid cancer risk in patients with type 2 diabetes. Eur J Clin Invest. 2014;44:736–42.
- D’Agostino RB Jr. Propensity scores in cardiovascular research. Circulation. 2007;115:2340–3.
- Whitcomb DC. Clinical practice. Acute pancreatitis. N Engl J Med. 2006;354:2142–50.
- Sue M, Yoshihara A, Kuboki K, Hiroi N, Yoshino G. A case of severe acute necrotizing pancreatitis after administration of sitagliptin. Clin Med Insights Case Rep. 2013;6:23–7.
- Kunjathaya P, Ramaswami PK, Krishnamurthy AN, Bhat N. Acute necrotizing pancreatitis associated with vildagliptin. JOP. 2013;14:81–4.
- Famularo G, Gasbarrone L, Minisola G. Pancreatitis during treatment with liraglutide. JOP. 2012;13:540–1.
- Bourezane H, Kastler B, Kantelip JP. Late and severe acute necrotizing pancreatitis in a patient with liraglutide. Therapie. 2012;67:539–43.
- Li L, Shen J, Bala MM, Busse JW, Ebrahim S, Vandvik PO, et al. Incretin treatment and risk of pancreatitis in patients with type 2 diabetes mellitus: systematic review and meta-analysis of randomised and non-randomised studies. BMJ. 2014;348:g2366.
- Monami M, Dicembrini I, Mannucci E. Dipeptidyl peptidase-4 inhibitors and pancreatitis risk: a meta-analysis of randomized clinical trials. Diabetes Obes Metab. 2014;16:48–56.
- Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, et al.; TECOS Study Group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232–42.
- Butler PC, Dry S, Elashoff R. GLP-1-based therapy for diabetes: what you do not know can hurt you. Diabetes Care. 2010;33:453–5.
- Butler AE, Campbell-Thompson M, Gurlo T, Dawson DW, Atkinson M, Butler PC. Marked expansion of exocrine and endocrine pancreas with incretin therapy in humans with increased exocrine pancreas dysplasia and the potential for glucagon-producing neuroendocrine tumors. Diabetes. 2013;62:2595–604.
- Bonner-Weir S, In’t Veld PA, Weir GC. Reanalysis of study of pancreatic effects of incretin therapy: methodological deficiencies. Diabetes Obes Metab. 2014;16:661–6.
- Chang HY, Hsieh CF, Singh S, Tang W, Chiang YT, Huang WF. Anti-diabetic therapies and the risk of acute pancreatitis: a nationwide retrospective cohort study from Taiwan. Pharmacoepidemiol Drug Saf. 2015;24:567–75.
- Shen HN, Lu CL, Li CY. Epidemiology of first-attack acute pancreatitis in Taiwan from 2000 through 2009: a nationwide population-based study. Pancreas. 2012;41:696–702.
- Gonzalez-Perez A, Schlienger RG, Rodríguez LA. Acute pancreatitis in association with type 2 diabetes and antidiabetic drugs: a population-based cohort study. Diabetes Care. 2010;33:2580–5.
- Noel RA, Braun DK, Patterson RE, Bloomgren GL. Increased risk of acute pancreatitis and biliary disease observed in patients with type 2 diabetes: a retrospective cohort study. Diabetes Care. 2009;32:834–8.
