ABSTRACT
Introduction Circadian rhythm disturbance increases cardiovascular risk but the effects of daylight saving time (DST) transitions on the risk of myocardial infarction (MI) are unclear.
Methods We studied association of DST transitions in 2001–2009 with incidence and in-hospital mortality of MI admissions nationwide in Finland. Incidence rations (IR) of observed incidences on seven days following DST transition were compared to expected incidences.
Results Incidence of MI increased on Wednesday (IR 1.16; CI 1.01–1.34) after spring transition (6298 patients’ cohort). After autumn transition (8161 patients’ cohort), MI incidence decreased on Monday (IR 0.85; CI 0.74–0.97) but increased on Thursday (IR 1.15; CI 1.02–1.30). The overall incidence of MI during the week after each DST transition did not differ from control weeks. Patient age or gender, type of MI or in-hospital mortality were not associated with transitions. Renal insufficiency was more common among MI patients after spring transition (OR 1.81; CI 1.06–3.09; p < 0.05). Diabetes was less common after spring transition (OR 0.71; CI 0.55–0.91; p = 0.007), but more common after autumn transition (OR 1.21; 1.00–1.46; p < 0.05).
Conclusions DST transitions are followed by changes in the temporal pattern but not the overall rate of MI incidence. Comorbidities may modulate the effects DST transitions.
Both spring and autumn daylight saving time transitions changed the temporal occurrence pattern but not the overall incidence of myocardial infarction occurrence on the week following the clock shift.
The age or gender distribution of patients, ratio of different types of myocardial infarctions or in-hospital mortality were not affected by clock shifts.
The effect of daylight saving time transitions on MI incidence may be modified by the presence of diabetes.
KEY MESSAGES
Introduction
Sleep is essential for well-being and its disturbances have been associated with disruption of numerous physiological processes and changes in cardiovascular risk factors (Citation1,Citation2). Sleep disordered breathing has been associated with risk of coronary heart disease (Citation3,Citation4) and sleep impairment with prognosis of myocardial infarction (MI) (Citation5). Moreover, fragmentation and instability of circadian rhythms have been associated with increase in overall mortality (Citation6).
Daylight saving time (DST) is used in many countries including the United States and the members of the European Union for prolonging of sun-light proportion of day. Clock shifts however alter and disrupt chronobiological rhythms and impair sleep (Citation7,Citation8) providing a “natural experiment” for studying the effects of rhythm and sleep disruptions on the incidence of vascular events. Although chronobiological factors have been shown to affect the incidence of MI (Citation9,Citation10), studies on the association of DST and the incidence of MI have been partly conflicting. With one exception (Citation11), all studies show changes in the temporal distribution of MI in the week following DST transitions but the patterns of change differ (Citation12–15) and there is no agreement about the impact of these changes on the overall incidence of MI (Citation11–16).
MI is classified into two categories based on electrocardiogram (ECG) presentation: ST-segment elevation MI (STEMI) represents total abrupt occlusion of coronary artery requiring immediate reperfusion therapy, while non-ST-segment elevation MI (NSTEMI) represents more transient myocardial ischemia. STEMI and NSTEMI differ in pathophysiology and treatment guidelines but share the same risk factors (Citation17). Previous research indicates that DST transition may affect STEMI and NSTEMI differently (Citation14), but this has not been evaluated in all studies. Potential effects of DST transitions on the type of MI may yield further clues to evaluating the effects of circadian disruption on cardiac health and the mechanisms behind it.
We studied whether the transitions to and from DST affected the incidence, type and in-hospital mortality of MI in Finland.
Methods
Study patients
Patients aged ≥18 years admitted to participating hospitals with MI (ICD-10 codes I21x) (Citation18) as primary discharge diagnosis in 2001–2009 two weeks prior and three weeks after DST transition were included. Type of MI was classified as STEMI (ICD-10 codes I21.0x-21.3x) and NSTEMI (ICD-10 codes I21.4x-21.9x) (Citation18). Data were collected from all 22 Finnish hospitals with coronary catheterization laboratory that treat emergency cardiac patients by using the Finnish Care Register for Health Care (CRHC), a nationwide, obligatory and automatically collected hospital discharge database. Information of co-diagnoses (based on ICD-10 codes) was identified from the same registry. The study was approved by the National Institute for Health and Welfare (permission THL/1576/5.05.00/2010).
Analysis
Association of DST and incidence of MI was measured by calculating incidence rations (IR) of observed incidences on seven days following DST transition (study group) to expected incidences (control group). Expected incidences were calculated as the mean of two control weeks prior to DST and two weeks following the week after DST transition. DST starts in Finland on the last Sunday of March and ends on the last Sunday of October. Because the Monday following Easter Sunday is a bank holiday in Finland, years with DST spring transition on Easter Sunday were excluded from the analysis (2002 and 2005) to increase international comparability and avoid confounding (Citation12). In addition, when Easter Sunday was celebrated within 2 weeks after DST transition, post-DST control weeks after Easter were selected. Number of events on shorter Sunday after transition into DST was adjusted with multiplying by 24/23 and lengthening of Sunday after transition out of DST with multiplying by 24/25 (Citation12). Confidence intervals (95% CI) of incidence were calculated by CINV function, a method based on the relation between chi-square and Poisson distributions (Citation14,Citation16,Citation19). Population-based incidence of MI admissions to participating hospitals during spring and autumn periods were calculated using corresponding population data of mainland Finland obtained from Statistics Finland and standardized to European standard population 2013 by using the direct method.
Characteristics and co-diagnoses of patients during a week following DST transition and control weeks were compared by using logistic regression modelling with MI during the week following DST transition as the outcome variable and patient characteristic/co-morbidity as an independent variable. Model was adjusted for study year. In-hospital mortality was analyzed using Cox’s proportional hazards regression stratified for study year. Variable selection to Cox’s proportional hazards regression model was performed by using augmented backward elimination procedure with significance threshold α = 0.2 and a change-in-estimate threshold τ = 0.05 (Citation20). Age, gender and occurrence of MI during seven days after DST transition were forced into the Coxs model. SAS for windows v. 9.4. was used for analyses (SAS Institute, Cary, NC).
Results
Spring transition
Spring cohort included 6298 patients (60.9% male, mean age 71.2, SD 12.6 years). Of these, 1269 (20.2%) were admitted during 7 days following transition into DST and 5029 (79.9%) during control weeks. Standardized incidence of MI admissions in participating hospitals during spring study period was 259/100,000 person-years. Incidence of MI admissions was similar to control weeks for Sunday–Tuesday after DST transition (). However, on fourth day after transition (Wednesday), there was a significant increase in MI incidence compared to control weeks (IR 1.16; CI 1.01–1.34). Incidence of MI during the whole study week after DST transition was similar to control weeks (IR 1.01; CI 0.96–1.07). Overall MI incidence was similar also during three weeks after DST transition when compared to two weeks prior to transition (IR 0.98; CI 0.94–1.02).
Figure 1. Hospital admissions for acute myocardial infarction (MI) in the weeks following spring transition into daylight saving time (DST) compared to control weeks. Numbers show MI admissions on post-DST transition week per admissions on control weeks. Error bars represent 95% confidence intervals.
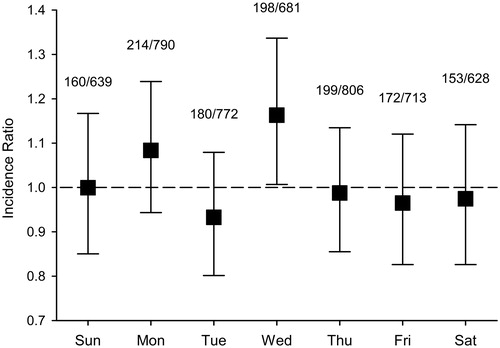
Patient characteristics between study group and control group in spring did not differ with the exceptions of co-diagnoses of diabetes mellitus, ventricular arrhythmias and renal failure (). Patients admitted during the week after DST transition were less likely to have diagnosed diabetes or ventricular arrhythmias compared to patients admitted during control weeks, but had diagnosed renal failure more often. Transfer into DST did not affect in-hospital mortality during the week following the transition (mortality 9.7%) when compared to control weeks (8.5%) (HR 1.06; CI 0.87–1.29, p = 0.577). Variables included in multivariate regression and connected HRs are given in Appendix 1 of Supplementary material. Transfer into DST did not affect the duration of admissions (). Proportion of STEMI patients was also similar between study week and control weeks. Infarction was located anteriorly in similar proportion of STEMI patients in study and control weeks ().
Table 1. Patient characteristics and co-diagnoses during the spring weeks after shift into DST (study group) and control weeks (control group) or represent association of a patient feature with occurrence of MI during a week after DST transition (study group) versus Control weeks (control group) (see Methods for details).
Autumn transition
In autumn period, 8161 patients (60.1% male, mean age 71.0, SD 12.9 years) were admitted because of MI. Of these patients, 20.0% (n = 1628) were admitted during the first 7 days after transition out of DST and 80.1% (n = 6533) during control weeks. During the autumn period, the incidence of MI admissions was 263/100,000 person-years. Number of MI admissions was decreased on the first working day after DST transition (IR 0.85; CI 0.74–0.97) (). Incidence remained similar to control weeks during Tuesday and Wednesday, but there was a significant increase on Thursday after transition out of DST (IR 1.15; CI 1.02–1.30). There were no differences in total incidence of MI admissions during the week after transition out of DST when compared to control weeks (IR 0.99; CI 0.94–1.04), or during three weeks after compared to two weeks before transition (IR 0.99; CI 0.96–1.02).
Figure 2. Hospital admissions for acute myocardial infarction (MI) in the weeks following autumn transition out of daylight saving time (DST) compared to control weeks. Numbers show MI admissions on post-DST transition week per admissions on control weeks. Error bars represent 95% confidence intervals.
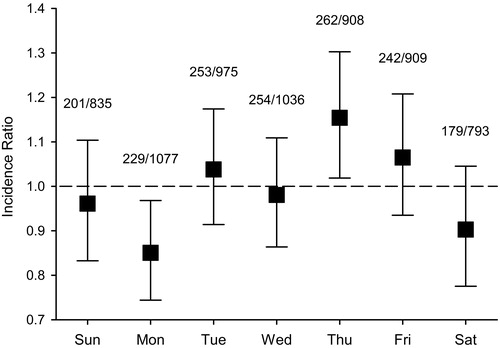
Gender distribution or age of patients did not differ between study week and control weeks in the autumn (). Diagnosed diabetes was more common in patients admitted during the weeks after transition out of DST. Otherwise, there were no differences in patient characteristics (). Proportions of STEMI patients and anterior STEMI patients were also similar between study and control weeks (). Transfer out of DST had no effect on in-hospital mortality (8.3% versus 8.5% in control weeks; HR 0.96; CI 0.79–1.16, p = 0.640). Variables included in multivariate regression and connected HRs are given in Appendix 2 of Supplementary material. Duration of MI admission was not affected by transition out of DST ().
Table 2. Patient characteristics and co-diagnoses during the weeks after autumn shift out of DST (study group) and control weeks (control group) or represent association of a patient feature with occurrence of MI during a week after DST transition (study group) versus Control weeks (control group) (see Methods for details).
Discussion
DST transitions offer a unique possibility to study the effects of disruptions of chronobiological rhythms and sleep on the occurrence of hard vascular endpoints. This national study investigated associations of transition to and from DST on MI incidence and mortality. Our results show that temporal distribution of MI is altered after DST transition. Time shifts did not have overall effect on in-hospital mortality of MI.
Previous studies have reported both no change (Citation14,Citation15) and an increase (Citation13,Citation16) in the incidence of MI following transition into DST. We found no difference in the overall MI incidence during the week following the transition, but changes in temporal distribution of MI occurrence were identified. Previous studies have found no change in MI incidence (Citation11) or an increase shortly after transition into DST, namely on Sundays (Citation14), Mondays (Citation15) or from Mondays to Wednesdays (Citation12) following the shift. In our data, the increase in MI incidence presented on Wednesday after the transition. This suggests that either effects of circadian cycle distraction to MI triggers appeared later, or possibly that stress caused by shift into DST accumulated further during the first working days after transition. This discrepancy may also be associated with different distribution of MI subtypes. Change into DST has previously been associated especially with increase in NSTEMI (Citation14) whereas our results show no change in the incidence ratio of STEMI versus NSTEMI. Furthermore, the location of STEMI was not affected by DST transitions in our study. Unlike in the USA (Citation21) and other countries (Citation22,Citation23) where the proportion of NSTEMI has been increasing in recent years, the opposite has, for unknown reason, occurred in Finland where the proportion of STEMI has increased (Citation18). There are also considerable differences in the proportion of STEMI versus NSTEMI between different countries (Citation24). Overall, although sleep deprivation and disruption of circadian rhythms have extensive physiological effects (Citation2), it seems possible that population-specific features result in divergence of clinical outcomes to some extent. This effect may be conveyed by factors such as chronotype which follows latitudinal variation (Citation25) and previous research has shown that chronotypes show a different distribution in Finland compared to many other countries (Citation26). Mechanisms of DST effects on MI remain, however, to be further studied.
We found autumn transition out of DST to be followed by an immediate reduction of incident MIs on Monday followed by an apparent rebound-effect of increased MI incidence on Thursday. This result is in line with a previous report which showed an immediate reduction on Sunday and a rebound-effect on the following Saturday (Citation14). Separate studies of MI incidence have also reported a slightly delayed reduction on Monday (Citation12) or Tuesday (Citation15) as well as a slightly delayed increase on Tuesday and Thursday (Citation13) following the autumn transition. One study has reported an increased risk of MI in the first 4 working days following the autumn transition with OR 1.44 (Citation13). Considering studies with more recent data (Citation12,Citation14,Citation15), it seems that the autumn transition is closely followed by a temporary reduction in MI incidence, although a recent study including also out-of-hospital cardiac deaths showed no change in the temporal distribution (Citation11). The rebound-effect suggests that transfer out of DST does not prevent MI, but merely delays the occurrence. Indeed, most studies, including ours, agree in that the overall incidence of MI, measured during one week following the transition, is not affected by the transition out of DST (Citation11,Citation12,Citation14,Citation15,Citation16). Furthermore, we found no increase in overall MI incidence during three weeks following DST transitions, although more delayed effect cannot be excluded (Citation27). The change in occurrence patterns of MI did not affect the severity of MI as detected by in-hospital mortality or treatment duration in our study.
There were some differences in frequencies of comorbidities in MI patients between study and control periods. Following the spring transition, patients with MI had renal insufficiency more often than patients admitted in the control period. Even moderate renal failure raises the risk of MI (Citation28–30) and these patients may have increased susceptibility to the adverse consequences of disruption of circadian blood pressure control maintained by the kidneys (Citation31). Contrary to previous reports (Citation11,Citation13,Citation14,Citation16), we found significant variation in prevalence of diabetes among MI patients in both spring and autumn DST transition suggesting that diabetic patients are affected differently by DST shifts. Curiously, the proportion of diabetic patients was smaller during the week after transition into DST and higher after transition out of DST. Both circadian rhythm disturbances (Citation32) and diabetes (Citation33) are known to disturb the immune system which, in turn, has been associated with MI risk (Citation34).The combined effect of these factors could be amplified by the months-long circadian unadjustment following transition into DST (Citation27) with the transition out of DST providing the final disruption needed to trigger MI in these patients. Sleep duration and circadian disruption have been shown to adversely affect the glycaemic status in both diabetic and non-diabetic persons (Citation35–37). However, the consequences of a very small shift in circadian rhythm and slight sleep deprivation such that occur with DST transitions have not been studied in diabetics. Further research is warranted, as the effects of circadian rhythm and its disruption affect glucose tolerance independently (Citation38) and the relationship between sleep duration and cardiovascular risk factors in diabetes is not linear (Citation39). Possible modulatory effects of medications (Citation11,Citation16) should also be taken into account in future studies.
This was a retrospective study based on registry information of hospitalized patients. Diagnoses were made by treating physicians, and we had no access to clinical data. Possible limitations of misdiagnosis and erroneous or missing coding are thus present. CRCH, however, has been reported to be reliable (Citation40) and we further limited our observation to hospitals with high level of diagnostic and treatment capabilities. Possibilities of random errors are limited by the number of study patients. However, DST transitions in themselves may have affected the rate of diagnostic errors, something we could not control for. We were unable to control for out-of-hospital MI deaths after DST transition. A recent study on subject (Citation11) indicates that this limitation is however unlikely to significantly bias our results. The exact timing of MI onset was not available so hospital admission time was used as a proxy, as has been done in most other studies on the subject. Although we included all MI patients admitted to 22 hospitals after DST transitions of 9 years, the study power to detect subtle changes caused by one hour DST transitions may still be limited. There was no information on the chronotype of patients available and further research is needed on their effects on vascular outcomes of DST transitions.
In conclusion, DST transitions are associated with temporal distribution of MI, while diabetes and renal insufficiency may modulate this association.
Declaration of interest
Jussi O.T. Sipilä has received travel grants (Orion Corporation, Merck Serono, Lundbeck, Sanquin) and holds shares (Orion Corporation). Päivi Rautava and Ville Kytö report no conflicts of interest. This study was funded by the governmental VTR-funding of the hospital district of Southwestern Finland and grant funding of the Finnish Cardiac Society.
References
- Maury E, Ramsey KMK, Bass J. Circadian rhythms and metabolic syndrome: from experimental genetics to human disease. Circ Res. 2010;106:447–62.
- McEwen BS, Karatsoreos IN. Sleep deprivation and circadian disruption: stress, allostasis and allostatic load. Sleep Med Clin. 2015;10:1–10.
- Cepeda-Valery B, Acharjee S, Romero-Corral A, Pressman GS, Gami AS. Obstructive sleep apnea and acute coronary syndromes: etiology, risk, and management. Curr Cardiol Rep. 2014;16:535.
- Wang X, Ouyang Y, Wang Z, Zhao G, Liu L, Bi Y. Obstructive sleep apnea and risk of cardiovascular disease and all-cause mortality: a meta-analysis of prospective cohort studies. Int J Cardiol. 2013;169:207–14.
- Clark A, Lange T, Hallqvist J, Jennum P, Rod NH. Sleep impairment and prognosis of acute myocardial infarction: a prospective cohort study. Sleep. 2014;37:851–8.
- Zuurbier LA, Luik AI, Hofman A, Franco OH, Van Someren EJ, Tiemeier H. Fragmentation and stability of circadian activity rhythms predict mortality: the Rotterdam study. Am J Epidemiol. 2015;181:54–63.
- Hadlow NC, Brown S, Wardrop R, Henley D. The effects of season, daylight saving and time of sunrise on serum cortisol in a large population. Chronobiol Int. 2014;31:243–51.
- Lahti TA, Leppämäki S, Lönnqvist J, Partonen T. Transition to daylight saving time reduces sleep duration plus sleep efficiency of the deprived sleep. Neurosci Lett. 2006;406:174–7.
- Spielberg C, Falkenhahn D, Willich SN, Wegscheider K, Völler H. Circadian, day-of-week, and seasonal variability in myocardial infarction: comparison between working and retired patients. Am Heart J. 1996;132:579–85.
- Culić V. Acute risk factors for myocardial infarction. Int J Cardiol. 2007;117:260–9.
- Kirchberger I, Wolf K, Heier M, Kuch B, von Scheidt W, Peters A, et al. Are daylight saving time transitions associated with changes in myocardial infarction incidence? Results from the German MONICA/KORA Myocardial Infarction Registry. BMC Public Health. 2015;15:778.
- Janszky I, Ljung R. Shifts to and from daylight saving time and incidence of myocardial infarction. N Engl J Med. 2008;359:1966–8.
- Culić V. Daylight saving time transitions and acute myocardial infarction. Chronobiol Int. 2013;30:662–8.
- Jiddou MR, Pica M, Boura J, Qu L, Franklin BA. Incidence of myocardial infarction with shifts to and from daylight savings time. Am J Cardiol. 2013;111:631–5.
- Sandhu A, Seth M, Gurm HS. Daylight savings time and myocardial infarction. Open Heart. 2014;1:e000019.
- Janszky I, Ahnve S, Ljung R, Mukamal KJ, Gautam S, Wallentin L, et al. Daylight saving time shifts and incidence of acute myocardial infarction – Swedish Register of Information and Knowledge About Swedish Heart Intensive Care Admissions (RIKS-HIA). Sleep Med. 2012;13:237–42.
- Santos-Gallego CG, Picatoste B, Badimón JJ. Pathophysiology of acute coronary syndrome. Curr Atheroscler Rep. 2014;16:401.
- Kytö V, Sipilä J, Rautava P. Likelihood and predictors of ST-elevation in patients hospitalized for myocardial infarction. PLoS One. 2014;9:e108440.
- Sun J, Ono Y, Takeuchi Y. A simple method for calculation the exact confidence interval of the standardized mortality ratio with an SAS function. J Occup Health. 1996;38:196–7.
- Dunkler D, Plischke M, Leffondré K, Heinze G. Augmented backward elimination: a pragmatic and purposeful way to develop statistical models. PLoS One. 2014;21:e113677.
- Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362:2155–216.
- Jennings SM, Bennett K, Lonergan M, Shelley E. Trends in hospitalisation for acute myocardial infarction in Ireland, 1997-2008. Heart. 2012;98:1285–9.
- Ortolani P, Marino M, Melandri G, Guastaroba P, Corzani A, Berti E, et al. Recent temporal trends for first-time hospitalization for acute myocardial infarction. Treatment patterns and clinical outcome in a large cohort study. Am Heart J. 2013;166:846–54.
- André R, Bongard V, Elosua R, Kirchberger I, Farmakis D, Häkkinen U, et al. International differences in acute coronary syndrome patients’ baseline characteristics, clinical management and outcomes in Western Europe: the EURHOBOP study. Heart. 2014;100:1201–7.
- Miguel M, Oliveira VC, Pereira D, Pedrazzoli M. Detecting chronotype differences associated to latitude: a comparison between Horne-Östberg and Munich Chronotype questionnaires. Ann Hum Biol. 2014;41:105–8.
- Merikanto I, Kronholm E, Peltonen M, Laatikainen T, Lahti T, Partonen T. Relation of chronotype to sleep complaints in the general Finnish population. Chronobiol Int. 2012;29:311–17.
- Kantermann T, Juda M, Roenneberg T. The human circadian clock’s seasonal adjustment is disrupted by daylight savings time. Curr Biol. 2007;22:1196–2000.
- Beddhu S, Allen-Brady K, Cheung AK, Horne BD, Bair T, Muhlestein JB, et al. Impact of renal failure on the risk of myocardial infarction and death. Kidney Int. 2002;62:1776–83.
- Brugts JJ, Knetsch AM, Mattace-Raso FUS, Hofman A, Witteman JCM. Renal function and risk of myocardial infarction in an elderly population: the Rotterdam Study. Arch Intern Med. 2005;165:2659–65.
- Meisinger C, Döring A, Löwel H, KORA Study Group. Chronic kidney disease and risk of incident myocardial infarction and all-cause and cardiovascular disease mortality in middle-aged men and women from the general population. Eur Heart J. 2006;27:1245–50.
- Bonny O, Firsov D. Circadian regulation of renal function and potential role in hypertension. Curr Opin Nephrol Hypertens. 2013;22:439–44.
- Cermakian N, Lange T, Golombek D, Sarkar D, Nakao A, Shibata S, et al. Crosstalk between the circadian clock circuitry and the immune system. Chronobiol Int. 2013;30:870–88.
- Frostegård J. Immune mechanisms in atherosclerosis especially in diabetes type 2. Front Endocrinol (Lausanne). 2013;29:162.
- Mann DL, Kirchenbaum L. The emerging role of innate immunity in the heart and vascular system: for whom the cell tolls. Circ Res. 2011;108:1133–45.
- Depner CM, Stothard ER, Wright KP. Jr. Metabolic consequences of sleep and circadian disorders. Curr Diab Rep. 2014;14:507.
- Donga E, van Dijk M, van Dijk JG, Biermasz NR, Lammers GJ, van Kralingen K, et al. Partial sleep restriction decreases insulin sensitivity in type 1 diabetes. Diabetes Care. 2010;33:1573–7.
- Knutson KL, Van Cauter E, Zee P, Liu K, Lauderdale DS. Cross-sectional associations between measures of sleep and markers of glucose metabolism among subjects with and without diabetes: the Coronary Artery Risk Development in Young Adults (CARDIA) Sleep Study. Diabetes Care. 2011;34:1171–6.
- Morris CJ, Yang JN, Garcia JI, Myers S, Bozzi I, Wang W, et al. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc Natl Acad Sci USA. 2015;112:E2225–34.
- Cooper AJM, Westgate K, Brage S, Prevost AT, Griffin SJ, Simmons RK. Sleep duration and cardiometabolic risk factors among individuals with type 2 diabetes. Sleep Med. 2015;16:119–25.
- Sund R. Quality of the Finnish Hospital Discharge Register: a systematic review. Scand J Public Health. 2012;40:505–15.
