Abstract
Background In this study, we investigated the independent and combined effects of sleep duration and afternoon napping on the risk of incident diabetes among a cohort of middle-aged and older Chinese adults.
Methods Information of sleep and napping was obtained by questionnaires during face-to-face interviews. We categorized sleep duration into <7 h, 7∼<8 h (reference), 8∼<9 h, 9∼<10 h, and ≥ 10 h. Afternoon napping was divided into no napping (0 min) (reference), 1–30 min, 31–60 min, 61–90 min, and > 90 min. Cox proportional hazard regression models were used.
Results Compared with referential sleeping group, subjects sleeping ≥10 h had a 42% higher risk of developing diabetes. The HR was 1.28 for napping > 90 min when compared with no napping. These associations were more pronounced in individuals without hypertension. Combined effects of long sleep duration and afternoon napping were further identified. Individuals with both sleep duration ≥ 10 h and napping > 60 min had a 72% higher risk of incident diabetes than those with sleeping 7∼<8 h and napping 0 min (all above p < 0.05).
Conclusions Both long sleep duration and afternoon napping were independently and jointly associated with higher risk of incident diabetes.
Sleep duration was associated with diabetes, but whether it is a real cause of incident diabetes especially in Chinese still remains to be elucidated.
The association of afternoon napping and diabetes was not consistent and definite, we clarified this association in a large prospective study.
Long sleep duration and afternoon napping were independently and jointly associated with higher risk of incident diabetes.
Key messages
Introduction
Diabetes remains a critical public health concern worldwide. The overall diabetes prevalence is estimated to be over 11.6% and affects more than 100 million Chinese adults (Citation1). Besides genetic factors and traditional lifestyle risk factors, other nontraditional behavioral and environmental risk elements also play an important role in diabetes epidemic (Citation2). Efforts are needed to further understand more determinants of this disease and to develop effective prevention strategies (Citation3).
Sleep is essential in many physical, cognitive, and psychological processes; an optimal sleeping condition appears to be a health imperative (Citation4). In the past few years, the number of studies concerning the relationship between sleep duration and diabetes has nearly doubled (Citation3,Citation5–12). Several studies have reported a U-shaped association between sleep duration and diabetes (Citation13,Citation14), but other studies did not find consistent relationship (Citation15,Citation16). A latest meta-analysis including 10 prospective studies reported that compared with 7 h sleep per day, an hour shorter and an hour longer sleep duration was associated with a 9% and 14% elevated risk of developing diabetes respectively (Citation17). However, most of these studies were conducted in the Europeans; in addition, the definitions of short and long sleep duration differed across studies, which made the interpretation of the results complicated. Therefore, it still remains to elucidate whether short or long sleep duration is a real cause of incident diabetes, especially in other populations including the Chinese population.
Afternoon napping is popular in China, especially among older adults and is regarded as a healthy habit. Appropriate napping can be beneficial for daytime functioning as well as mental health (Citation18,Citation19). However, the health consequences of napping are poorly investigated. Cross-sectional (Citation2,Citation20–22) and cohort (Citation12) studies have explored the relationship between afternoon napping and diabetes but findings were inconsistent. A prospective study in the National Institutes of Health (NIH)-AARP Diet and Health Study indicated that both short and long napping were associated with a higher risk of developing diabetes (Citation12). However, in the Chinese population the cross-sectional studies conducted in the Dongfeng-Tongji cohort (Citation2) and the Guangzhou Biobank (Citation21) suggested that this association existed merely among long nappers. To clarify this association, it is considerably important to conduct a large prospective study.
To further clarify the associations of sleep duration and afternoon napping with diabetes risk, in this study we investigated whether sleep duration and afternoon napping were independently and jointly associated with risk of incident diabetes among a cohort of middle-aged and older Chinese adults.
Materials and methods
Study population
The design, methods and other details of the Dongfeng-Tongji cohort have been described elsewhere (Citation23). Briefly, a total of 27,009 retired employees were recruited in the cohort and completed baseline questionnaires, medical examinations, and provided baseline blood samples between September 2008 and June 2010. Among 25,978 individuals (96.2%) who completed the follow-up until October 2013, we excluded individuals with diabetes (n = 4970), self-reported CHD (n = 3584), stroke (n = 843), and cancer (n = 1183) at baseline, as well as those with missing information related to sleep duration, afternoon napping, or other covariates (n = 222), resulting in a final study sample of 16,399 subjects (7083 males and 9316 females with a mean age of 62.5 years). The study has been approved by the Ethics and Human Subject Committee of the School of Public Health, Tongji Medical College, and Dongfeng General Hospital, the Dongfeng Motor Corporation (DMC). All study participants provided written informed consents.
Data collection
Determination of sleep duration and afternoon napping
Sleep duration data were obtained by trained interviewers during face-to-face interviews via asking the following question “What time did you usually go to sleep at night and wake up in the morning over the past six months?” We categorized sleep duration into five groups: < 7 h, 7∼< 8 h, 8∼< 9 h, 9∼< 10 h, and ≥ 10 h. Afternoon napping was assessed by asking “Did you have a habit of taking afternoon napping over the past six months?” Those who gave positive responses were further asked about the duration of their napping. Afternoon napping was thereby divided into no napping (0 min), 1–30 min, 31–60 min, 61–90 min, and > 90 min. Sleep quality was also interviewed by asking “How was your sleep quality?” and categorized into good, fair poor without hypnotics and very poor with hypnotics (combined into poor).
Ascertainment of baseline and incident diabetes
The diagnosis of diabetes was on the basis of American Diabetes Association (ADA) criteria (Citation24) as meeting any of the following criteria in follow-up interviews or laboratory examinations: (i) self-report of a physician’s diagnosis of diabetes, (ii) fasting blood glucose level of ≥ 7.0 mmol/L, 3) HbA1c level ≥ 6.5%, 4) 2-h 75-g oral glucose tolerance test (OGTT) value of ≥ 11.1 mmol/L, and 5) usage of diabetes medication (insulin or oral hypoglycemic agent). The incident diabetic cases were those occurred after baseline survey but before the end of October 2013. Because the OGTT test was not conducted in this study and HbA1c levels were only assayed during the follow-up in 2013, baseline and incident diabetic cases were thereby ascertained according to self-report data, fasting blood glucose levels and usage of diabetes medication. A total of 1123 incident diabetic cases were diagnosed during the follow-up period in the present study.
Assessment of covariates
Baseline data were collected by trained interviewers by semi-structured questionnaires during face-to-face interviews. Information on socio-demographic factors such as age, sex, education, marital status, medications, health status, and lifestyle including smoking status, alcohol consumption status, and physical activity were included in the questionnaires. Education was classified into five groups: primary or below, junior high school, high school, college or above. Participants were asked about their medical history diagnosed by physicians, including diabetes, CHD, stroke, hypertension, hyperlipidemia, and cancer. Hypertension was defined as individuals with self-reported physician diagnosis of hypertension, or blood pressure ≥140/90 mmHg, or current usage of antihypertensive medication. Hyperlipidemia was defined as total cholesterol >5.72 mmol/L or triglycerides >1.70 mmol/L at medical examination, current usage of lipid-lowing medication, or a previous physician diagnosis of hyperlipidemia.
The general health examination was performed at the same time. Standing height, body weight and waist circumference were measured with participants in light indoor clothing and without shoes. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared.
All subjects were examined in the morning after overnight fasting and 15 ml of fasting blood was drawn with three vacuum (ethylenediamine tetraacetic acid, EDTA) anticoagulation tubes for plasma; coagulation tube for serum. Blood glucose level was determined through Glucose Oxidase method by Abbott Aeroset analyzer. Triglyceride, total cholesterol, low-density lipoprotein (LDL) cholesterol, and high-density lipoprotein (HDL) cholesterol levels were measured in the hospital’s laboratory with ARCHITECT Ci8200 automatic analyzer (ABBOTT Laboratories. Abbott Park, IL, USA) using the Abbott Diagnostics reagents according to the instructions of the manufacturer.
Statistical analysis
All statistical analysis was performed using SPSS 13.0 software (SPSS Inc., Chicago, IL). Categorical variables were presented in percentages and compared by Chi-square analysis. Continuous variables were expressed in means (SD) and compared by Student’s t-test or analysis of variation (ANOVA) unless otherwise specified. Because in the present population the percentage of subjects night sleeping < 5 h, < 6 h, and 6∼< 7 h was only 0.3%, 1.0%, and 6.6%, respectively, we combined the three groups into < 7 h group. Sleep duration was divided into <7 h, 7∼<8 h, 8∼<10 h, and ≥ 10 h groups and afternoon napping was categorized into no napping, 1–30 min, 31–60 min, and > 60 min groups. The multivariable adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) were computed by the Cox proportional hazard regression models to separately evaluate the associations of sleep and napping duration categories with incident diabetes risk, taking sleep duration of 7∼< 8 h and no afternoon napping as the reference groups, based on most existing researches (Citation2,Citation3,Citation7,Citation12). Potential confounders were adjusted involving age, sex, BMI, education, smoking status, alcohol consumption status, physical activity, baseline comorbidities (hypertension and hyperlipidemia), and family history of diabetes. Sleep quality was also adjusted because some studies reported that poor sleep quality was associated with higher risk of developing diabetes (Citation6,Citation16,Citation25). We further adjusted for afternoon napping for the sleep-incident diabetes relationship and adjusted for sleep duration for the nap-incident diabetes relationship. The curvilinear relation was also tested by including linear and quadratic terms of the median sleep duration or afternoon napping within each sleep duration or afternoon napping category as continuous variables in the regression models. Stratification analysis was performed on the basis of baseline characteristics [including age (< 65, ≥ 65 years), sex, BMI (< 24, ≥ 24 kg/m2), current smoking (yes, no), alcohol consumption (yes, no), physical activity (yes, no), hypertension (yes, no), hyperlipidemia (yes, no), and family history of diabetes (yes, no)]. Moreover, we tested the potential interactions by adding interaction terms of these covariates with sleep duration or afternoon napping, respectively. Combined effects of sleep duration and afternoon napping on diabetes risk were further evaluated. Sensitivity analysis by exclusion of the incident diabetic cases during the first two years of follow-up was also performed. A two-side p value of < 0.05 was considered to be statistically significant.
Results
Baseline characteristics of the study population according to categories of sleep duration and napping are summarized in . Overall, 7.9%, 28.2%, 41.9%, 16.9%, and 5.1% of participants reported sleeping < 7 h, 7–< 8 h, 8–< 9 h, 9–< 10 h, and ≥ 10 h per day respectively; 33.2%, 17.1%, 29.1%, 12.0%, and 8.6% of participants reported no napping, napping 1–30 min, 31–60 min, 61–90 min, and > 90 min, respectively. Compared with the reference groups, individuals sleeping ≥ 10 h or napping > 90 min were more likely to be males, current smokers, current alcohol consumers, and have good sleep quality. Those sleeping ≥ 10 h were more likely to be physically inactive and had higher triglycerides levels and lower HDL-cholesterol levels at baseline. Meanwhile, subjects napping > 90 min were more likely to have higher levels of fasting blood glucose.
Table 1. Baseline characteristics of the study population according to sleep duration and afternoon napping.
As shown in , after multivariable adjustment for potential confounders, long sleep duration and afternoon napping were independently associated with increased incidence of diabetes. Compared with the reference groups, the full adjusted HR (95% CI) was 1.42 (95% CI: 1.08, 1.87) for sleeping ≥ 10 h/night (p = 0.012) (p for quadratic trend = 0.013). Similarly, individuals reported napping > 90 min (HR, 1.28; 95% CI: 1.03, 1.59; p = 0.027) were also at increased risk of incident diabetes with a significant quadratic trend (p for quadratic trend = 0.002) while HR (95% CI) for those reported napping 1–30 min, 31–60 min, and 61–90 min was 0.88 (0.73–1.07), 1.02 (0.87, 1.19), and 1.18 (0.97, 1.44), respectively.
Table 2. Hazard ratios (95% CI) for incident diabetes according to sleep duration and afternoon napping.
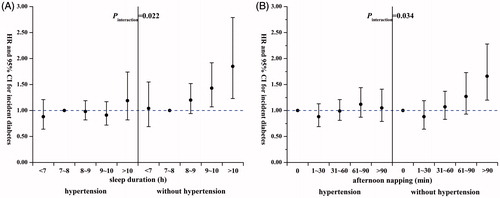
In stratification and interaction analysis, the above-mentioned associations were more prominent among individuals without hypertension. Compared with the reference groups, the HRs were 1.43 (95% CI: 1.07, 1.92) and 1.85 (95% CI: 1.23, 2.79) for sleep duration of 9∼< 10 h and ≥ 10 h, respectively (p for interaction = 0.022; and Supplementary Table 1); 1.66 (95% CI, 1.20–2.28) for afternoon napping > 90 min (p for interaction = 0.034; and Supplementary Table 2). In addition, these associations were both evident among individuals < 65 years of age. However, we did not find significant interactions of sleep duration or afternoon napping with these covariates (all p for interaction > 0.05).
Figure 1. Hazard ratios (95% CI) for incident diabetes in subjects with and without hypertension according to (A) sleep duration and (B) afternoon napping. The adjusted covariates included age, sex, BMI, education, smoking status, alcohol consumption status, physical activity, hyperlipidemia, family history of diabetes, sleep quality, sleep duration, and afternoon napping. Each group adjusted for the other covariates except for itself. The reference groups were 7–<8 h for sleep duration and 0 min for afternoon napping.
We then examined the combined effects of sleep duration and afternoon napping on the incident diabetes. The sleep duration was divided into < 7 h, 7–< 8 h, 8–< 10 h, and ≥ 10 h; afternoon napping was categorized into no napping, 1–30 min, 31–60 min, and > 60 min. Compared with participants with sleep duration 7–< 8 h and without afternoon napping, subjects with both long sleep duration (≥ 10 h) and long afternoon napping (> 60 min) (HR, 1.72; 95% CI: 1.03, 2.85; p = 0.037) as well as those with both sleep duration ≥ 10 h and no napping (HR, 1.63; 95% CI: 1.05, 2.53; p = 0.029) showed significantly increased incident diabetes risk ().
Table 3. Combined effects (hazard ratios with 95% CI) of sleep duration and afternoon napping duration on incident diabetes.
To reduce the possibility of reverse causation that pre-clinical disease status might cause longer sleep and napping duration, we performed sensitivity analysis by excluding the newly diagnosed incident diabetic cases during the first two years of follow-up (2009 and 2010, n = 122). As a result, the association of long sleep duration (HR, 1.67; 95% CI: 1.04, 2.68; p = 0.035), afternoon napping of > 90 min (HR, 1.47; 95% CI: 1.12, 1.85; p = 0.027) with incident diabetes risk did not alter materially (data not shown).
Discussion
In this large prospective study, we found that long sleep duration (≥ 10 h) and long afternoon napping (> 90 min) were independently associated with increased risk of incident diabetes after controlling for a variety of potential confounders. In addition, there were combined effects of long sleep duration and long afternoon napping on incident diabetes risk.
To our best knowledge, this is the first study simultaneously exploring the independent and combined effects of the sleep duration and afternoon napping on the incident diabetes in middle-aged and older Chinese population. Several prospective studies have investigated the relationship between sleep duration and incident diabetes and majority of them showed increased incidence of diabetes among short sleepers (< 7 h) (Citation3,Citation5,Citation16,Citation17). However, because of the limited incident diabetic cases (n = 82) among individuals sleeping < 7 h, we did not further categorize the short sleepers (< 7 h) group in the present study. The association of increased incident diabetes risk with long sleep duration is largely consistent with earlier studies (Citation3,Citation5,Citation25). Our analysis further discovered that long afternoon napping was also an independent predictor of incident diabetes, which was in accordance with preceding studies indicating a positive association between long afternoon napping and elevated diabetes incidence and mortality (Citation2,Citation12,Citation21).
Notably, the aforementioned associations were more evident in participants without hypertension. Although the underlying pathophysiological mechanisms are blurring, it is possible that the presence of potential diabetes risk factors or lifestyle improvement may cover up the influence of long sleep duration and afternoon napping on diabetes among high-risk individuals and leave the detrimental effects robust in relatively healthy adults.
Preceding studies indicated that disease status might influence sleep patterns during short follow-up periods (Citation7), we performed sensitivity analysis by exclusion of the incident diabetic cases diagnosed during the first two years of follow-up and the results did not alter materially. Additionally, in the present study we observed significant combined effects of sleep duration and afternoon napping on the risk of incident diabetes, which emphasized the deleterious health consequences of long sleep duration and afternoon napping as well as their independent and joint prediction of diabetes development. These findings need verification from further researches.
Potential mechanisms mediating the relationship of long sleep duration and afternoon napping with increased incidence of diabetes are still under investigation, however, several mechanisms might involve in these associations. First, compared with normal sleepers and nappers, long sleepers and nappers may have increased sleep fragmentation and more frequent awakenings, leading to changes in inflammatory markers such as elevated levels of blood interleukin-6, C-reactive protein, fibrinogen and decreased albumin levels (Citation26), which can increase the incident diabetes risk by damaging the body’s glucose stability and β-cell function (Citation27). Second, obstructive sleep apnoea, which usually includes heavy snoring, is more common among individuals with long sleep and napping duration. Snoring or obstructive sleep apnoea could induce oxygen desaturation, which elevates catecholamine and cortisol levels, contributing to glucose intolerance and insulin resistance (Citation28). Third, long sleep duration and napping may be partly due to less exercise and result in reciprocal changes in circulating levels of leptin and ghrelin, which might increase appetite and caloric intake, reduce energy expenditure and facilitate obesity development and impaired glycemic control (Citation29). Finally, longer napping duration might be associated with nonrapid eye movement sleep and disrupts circadian rhythms in the nap takers (Citation2); glucose tolerance fluctuates in circadian rhythm and recent studies have described that disturbance in such rhythm might be a predisposing environmental risk factor for diabetes development (Citation30).
The current study has several strengths. Firstly, the prospective design could assist us to infer causative and temporal relationships; the large sample size provided relatively strong evidence. Secondly, the diabetes information in the Dongfeng-Tongji cohort was fairly complete; the baseline and incident diabetes was diagnosed in terms of rigorous standards and the false positive could be reduced to a large extent. Thirdly, besides diabetic subjects we also excluded individuals with CHD, stroke and cancer at baseline. Comorbidities of baseline hypertension and hyperlipidemia, as well as a large number of potential confounders were further controlled; thus the bias of potential confounders could be minimized. Fourthly, as far as we know, this is the first study to examine the individual effects of sleep duration, afternoon napping, and their joint effects on incident diabetes risk simultaneously in a middle-aged and older Chinese population. The findings may provide some new insight into diabetes prediction and prevention.
However, some limitations should also be taken into consideration. Firstly, the sleep duration and afternoon napping were obtained from self-report data using a single question, which might under- or over-estimate sleep duration and napping and measurement error and misclassification might exist. Secondly, the information of sleep duration and napping habits was collected once at baseline and this habit might change during the long follow-up period. Thirdly, the obstructive sleep apnea is known to increase the risk of diabetes (Citation31,Citation32) and is associated with napping and longer sleep duration. However, in the present study we did not collect the information about obstructive sleep apnea and this might slightly bias our findings. Furthermore, the lack of other potential confounders such as depressive symptoms, Alzheimer’s disease and other neuropsychiatric disorders might also limit further exploration into the relationship. Fourthly, participants included in the present study were all middle-aged and older Chinese free of diabetes, CHD, stroke and cancer; therefore the findings may not be generalized to populations of younger age, different health conditions, and other ethnicities.
In this study, we found long sleep duration and afternoon napping independently and jointly associated with higher risk of incident diabetes in a middle-aged and older Chinese population. Our findings suggested the particular importance of regular sleep duration and afternoon napping for diabetes prevention.
Funding information
This work was supported by the grant from the National Natural Science Foundation (grant NSFC-81473051, 81522040, and 81230069); the 111 Project (No. B12004); the Program for Changjiang Scholars; Innovative Research Team in University of Ministry of Education of China (No. IRT1246); China Medical Board (No. 12-113) and the Program for the New Century Excellent Talents in University (NCET-11-0169) for Meian He.
Acknowledgements
The authors would like to appreciate all study subjects for participating in the present Dongfeng-Tongji Cohort study as well as all volunteers for assisting in collecting the sample and questionnaire data. We also acknowledge all the staff for collecting the clinic data.
Disclosure statement
The authors declare no conflict of interest.
References
- Xu Y, Wang LM, He J, Bi Y, Li M, Wang T, et al. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310:948–58.
- Fang W, Li Z, Wu L, Cao Z, Liang Y, Yang H, et al. Longer habitual afternoon napping is associated with a higher risk for impaired fasting plasma glucose and diabetes mellitus in older adults: results from the Dongfeng-Tongji cohort of retired workers. Sleep Med. 2013;14:950–4.
- Yaggi HK, Araujo AB, McKinlay JB. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care. 2006;29:657–61.
- Dolgin E. Deprivation: a wake-up call. Nature. 2013;497:S6–7.
- Ayas NT, White DP, Al-Delaimy WK, Manson JE, Stampfer MJ, Speizer FE, et al. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care. 2003;26:380–4.
- Boyko EJ, Seelig AD, Jacobson IG, Hooper TI, Smith B, Smith TC, et al. Sleep characteristics, mental health, and diabetes risk: a prospective study of U.S. military service members in the Millennium Cohort Study. Diabetes Care. 2013;36:3154–61.
- Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Opler MG, et al. Sleep duration associated with mortality in elderly, but not middle-aged, adults in a large US sample. Sleep. 2008;31:1087–96.
- Hayashino Y, Fukuhara S, Suzukamo Y, Okamura T, Tanaka T, Ueshima H, et al. Relation between sleep quality and quantity, quality of life, and risk of developing diabetes in healthy workers in Japan: the High-risk and Population Strategy for Occupational Health Promotion (HIPOP-OHP) Study. BMC Pub Health. 2007;7:129.
- Kita T, Yoshioka E, Satoh H, Saijo Y, Kawaharada M, Okada E, et al. Short sleep duration and poor sleep quality increase the risk of diabetes in Japanese workers with no family history of diabetes. Diabetes Care. 2012;35:313–18.
- Tuomilehto H, Peltonen M, Partinen M, Lavigne G, Eriksson JG, Herder C, et al. Sleep duration, lifestyle intervention, and incidence of type 2 diabetes in impaired glucose tolerance: The Finnish Diabetes Prevention Study. Diabetes Care. 2009;32:1965–71.
- von Ruesten A, Weikert C, Fietze I, Boeing H. Association of sleep duration with chronic diseases in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. PLoS One. 2012;7:e30972.
- Xu Q, Song YQ, Hollenbeck A, Blair A, Schatzkin A, Chen HL. Day napping and short night sleeping are associated with higher risk of diabetes in older adults. Diabetes Care. 2010;33:78–83.
- Gottlieb DJ, Punjabi NM, Newman AB, Resnick HE, Redline S, Baldwin CM, et al. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med. 2005;165:863–8.
- Jackson CL, Redline S, Kawachi I, Hu FB. Association between sleep duration and diabetes in black and white adults. Diabetes Care. 2013;36:3557–65.
- Beihl DA, Liese AD, Haffner SM. Sleep duration as a risk factor for incident type 2 diabetes in a multiethnic cohort. Ann Epidemiol. 2009;19:351–7.
- Mallon L, Broman JE, Hetta J. High incidence of diabetes in men with sleep complaints or short sleep duration: a 12-year follow-up study of a middle-aged population. Diabetes Care. 2005;28:2762–7.
- Shan ZL, Ma HF, Xie ML, Yan PP, Guo YJ, Bao W, et al. Sleep duration and risk of type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care. 2015;38:529–37.
- Dhand R, Sohal H. Good sleep, bad sleep! The role of daytime naps in healthy adults. Curr Opin Pulm Med. 2006;12:379–82.
- Milner CE, Cote KA. Benefits of napping in healthy adults: impact of nap length, time of day, age, and experience with napping. J Sleep Res. 2009;18:272–81.
- Goldman SE, Hall M, Boudreau R, Matthews KA, Cauley JA, Ancoli-Israel S, et al. Association between nighttime sleep and napping in older adults. Sleep. 2008;31:733–40.
- Lam KBH, Jiang CQ, Thomas GN, Arora T, Sen Zhang W, Taheri S, et al. Napping is associated with increased risk of type 2 diabetes: the Guangzhou Biobank cohort study. Sleep. 2010;33:402–7.
- Picarsic JL, Glynn N, Taylor CA, Katula JA, Goldman SE, Studenski SA, et al. Self-reported napping and duration and quality of sleep in the lifestyle interventions and independence for elders pilot study. J Am Geriatr Soc. 2008;56:1674–80.
- Wang F, Zhu J, Yao P, Li XL, He MA, Liu YW, et al. Cohort profile: the Dongfeng-Tongji cohort study of retired workers. Int J Epidemiol. 2013;42:731–40.
- Assoc AD. Standards of medical care in diabetes–2010. Diabetes Care. 2010;33:S11–61.
- Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33:414–20.
- Dowd JB, Goldman N, Weinstein M. Sleep duration, sleep quality, and biomarkers of inflammation in a Taiwanese population. Ann Epidemiol. 2011;21:799–806.
- Wisse BE. The inflammatory syndrome: the role of adipose tissue cytokines in metabolic disorders linked to obesity. J Am Soc Nephrol. 2004;15:2792–800.
- Al-Delaimy WK, Manson JE, Willett WC, Stampfer MJ, Hu FB. Snoring as a risk factor for type II diabetes mellitus: a prospective study. Am J Epidemiol. 2002;155:387–93.
- Lucassen EA, Rother KI, Cizza G. Interacting epidemics? Sleep curtailment, insulin resistance, and obesity. Ann N Y Acad Sci. 2012;1264:110–34.
- Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–31.
- Tasali E, Leproult R, Spiegel K. Reduced sleep duration or quality: relationships with insulin resistance and type 2 diabetes. Prog Cardiovasc Dis. 2009;51:381–91.
- Tasali E, Mokhlesi B, Van Cauter E. Obstructive sleep apnea and type 2 diabetes: interacting epidemics. Chest. 2008;133:496–506.
