Abstract
Introduction Coagulopathy plays an important role in sepsis. The aim of this study was to determine whether bone marrow stromal cell (BMSC) administration could attenuate coagulopathy in sepsis.
Materials and methods In vitro: endothelial cells were cultured with/without BMSCs for 6 h following LPS stimulation and were collected for thrombomodulin (TM) and endothelial protein C receptor (EPCR) measurements. In vivo: Thirty-six mice were randomized into sham, sepsis, and sepsis + BMSC groups (n = 12 each group). Sepsis was induced through cecal ligation and puncture (CLP). BMSC infusion was started at 6 h after CLP. Lung tissues and plasma samples were collected at 24 h after CLP for enzyme-linked immunosorbent assay (ELISA), quantitative real-time RT-PCR, western blot, and immunohistochemistry analysis.
Results In vitro: BMSCs attenuated the decrease in TM and EPCR mRNA and protein expression levels in LPS-stimulated endothelial cells. In vivo: BMSC treatment decreased lung injury and mesenteric perfusion impairment, and ameliorated coagulopathy, as suggested by the reduction in elevated TF, vWF, and TAT circulation levels. BMSC infusion decreased TF mRNA transcription and protein expression levels in lung tissues, and increased TM and EPCR mRNA transcription and expression levels.
Discussion BMSC administration attenuated coagulopathy, and decreased lung injury and mesenteric perfusion impairment in sepsis.
BMSCs increased the expression of TM and EPCR from endothelium cells exposed to LPS in vitro.
BMSC treatment attenuated lung injury and coagulopathy in the mice cecal ligation and puncture (CLP) model.
BMSC administration-attenuated coagulopathy is related to the reduced expression of TF and increased expression of TM and EPCR.
Key messages
Introduction
Sepsis remains a severe issue with its high morbidity and mortality (Citation1). More than 50% of sepsis patients develop coagulation abnormalities (Citation2). Coagulopathy has been shown to worsen sepsis outcomes through widespread thrombosis in microvasculature, resulting in multiple organ failure and death (Citation3–7). Anticoagulant therapies have been proven to reduce mortality rates associated with severe sepsis (Citation2,Citation8).
Sepsis-related coagulopathy is related to the following: first, upregulated pro-coagulation characterized as an increased expression of tissue factor (TF); second, downregulated anti-coagulation mainly through thrombomodulin (TM), endothelial protein C receptor (EPCR), protein S, and tissue factor pathway inhibitor (TFPI) levels (Citation9). The protein C (PC) system, as a crucial endogenous anticoagulant mechanism, has been impaired in sepsis due to the reduced expression of TM and EPCR on endothelial cells (Citation10). Furthermore, fibrinolysis was suppressed due to increased plasminogen activator inhibitor-1 (PAI-1) (Citation7). In addition, endothelial dysfunction contributes to organ failure and high mortality in sepsis, since the endothelium provides an interface between inflammation and coagulation (Citation11). Novel interventions directed at re-establishing endothelial barrier function revealed some promising results (Citation12).
Bone marrow stromal cells (BMSCs), as a connective tissue stem cell population, are multi-potent and self-renewing cells that can differentiate into muscle, bone, fat, fibroblasts, and cartilage (Citation13). The transplantation of BMSCs has been widely tested in clinics and achieved encouraging results in cardiovascular (Citation14,Citation15), neurological (Citation16,Citation17), and immunological diseases (Citation18,Citation19). BMSC transplantation has been shown to modulate innate and adaptive immune response (Citation20), promote the neovascularization of ischemic tissues (Citation17), and enhance the proliferation of epithelial cells (Citation21). BMSCs represent a novel cellular therapeutic treatment strategy for sepsis through its anti-inflammatory effects (Citation22–26). However, its effect on coagulation in sepsis remains unknown. We hypothesized that BMSCs could attenuate sepsis-related coagulopathy through modulating Von Willebrand factor (vWF), thrombin–antithrombin (TAT), TM, and EPCR levels.
Materials and methods
This study was approved by the local Ethics Committee of Wenzhou Medical University (China). Care and handling of animals were in accordance with the guidelines of the National Institutes of Health.
The experiment was divided into two parts: in vitro and in vivo parts.
In vitro
Cell identification
Bone marrow stromal cells (BMSCs; isolated from the bone marrow of C57BL/6 mice) were obtained from Cyagen Biosciences (Cyagen, Guangzhou, China). Cell identification was performed according to cell surface phenotypes and multipotency suggested by the supplier: shortly, cells were immune-fluorescently stained with fluorochrome-conjugated antibodies specific to cell surface antigens CD34, CD29, CD117, CD44, Sca-1, and CD31. The potential for adipogenic and osteogenic differentiation was determined by staining with oil red-O and alizarin red, respectively, following culture in adipogenic or osteogenic differentiation media (Cyagen Biosciences Inc., China) for 2–3 weeks.
Endothelial–bone marrow mesenchymal stem cell co-culture experiments
Endothelial cells and BMSCs were cultured in DMEM medium with 10% FBS, 1% penicillin–streptomycin, and 1% glutamine at 37 °C with 5% CO2. This co-culture experiment was divided into four groups: (I) phosphate-buffered saline (PBS) control group; (II) BMSCs control group; (III) lipopolysaccharide (LPS; Sigma, St. Louis, MO) group; and (IV) LPS + BMSCs-treated group. A concentration of 4 × 105 cells per 2 ml was plated in six-well transwell plates. Then, an equal number of BMSCs was added into the upper compartments of the transwell system (Polyster Membrane Transwell 0.4-μm pore-size polycarbonate membrane; Corning Incorporated, NY) in the BMSCs control group and LPS + BMSCs-treated group. An isopycnic medium (Cyagen, Guangzhou, China) was added to the other two groups. After overnight incubation, LPS was added to reach a final concentration of 1 μg/ml for the LPS group and LPS + BMSCs-treated group (the dose used was based on a pilot study, unpublished data). At the same time, an isopycnic medium was added to PBS for the other two groups. Six hours after LPS stimulation, the upper compartments of the transwell system was removed. Endothelial cells in the lower compartment were collected for PCR and western blot to determine TM and EPCR mRNA and protein expression levels, respectively. All experiments were repeated three times.
In vivo
Thirty-six 6–8 weeks old male C57BL/6 mice (18–25 g) were purchased from Shanghai SLAC Laboratory Animal Co., Ltd (Shanghai, China). Care for the animals was according to the local animal welfare policy. All animals had free access to water and chow before surgery.
Surgical preparation
The cecal ligation and puncture (CLP) procedure was performed as previously described (Citation27). Briefly, mice were anesthetized by intraperitoneal injection of 4% chloral hydrate (0.1 ml/10 g body weight; Solarbio, Beijing, China). After midline laparotomy, the cecum was exposed and ligated at the terminal part with a 4-0 silk suture (YuanKang, Yangzhou, China), and punctured twice with a 21G needle (Kindly, Shanghai, China). A small quantity of feces was squeezed into the abdominal cavity. The cecum was returned to the peritoneal cavity and the abdominal wall was closed in two layers with a 6-0 silk suture (YuanKang, Yangzhou, China). After surgery, all mice were fluid-resuscitated subcutaneously in the neck with 0.8 ml of pre-warmed normal sterile saline (Zhejiang Kangle Pharmaceutical Co. Ltd., Wenzhou, China). For sham-operated mice, all performed procedures were similar to CLP, except for ligation and puncture.
Administration of BMSCs
Mice were randomly assigned to the following groups (n = 12 each group): (I) sham group: i.v. administration of 0.3 ml of sterile PBS; (II) CLP control group: i.v. administration of 0.3 ml of PBS (III) CLP + BMSCs group: i.v. administration of 0.3 ml of PBS with one million BMSCs (Cyagen Biosciences, Guangzhou, China). The infusion of BMSCs/PBS was started at 6 h after CLP. Eight mice from each group were sacrificed 24 h after CLP. Plasma and lung tissue samples were collected for histological staining, enzyme-linked immunosorbent assay (ELISA), quantitative real-time RT-PCR, western blot, and immunohistochemistry measurements.
Histological staining
After autopsy, the whole lung was fixed with 4% paraformaldehyde, dehydrated, embedded, and cut into 4-μm sections. Following hematoxylin and eosin (H&E) staining, histological examination was performed to assess morphological damage. Lung injury scores were used to evaluate severity: 0 for no injury, 1 for mild injury, 2 for moderate injury, and 3 for severe injury (Citation28).
Mesenteric microcirculation
The abdomen of rest four mice in each group was re-opened after 24 h under sedation, and a mini-contact probe (Probe 404-1; Perimed Stockholm, Sweden) was placed on the superior mesenteric artery to observe microcirculation. Three 15-s videos were recorded for later analysis. Images were acquired with the PeriFlux system 5000 and analyzed by PeriSoft for Windows Examination Manager (PSW EXM; Perimed Stockholm, Sweden) software. Thereafter, the four mice were sacrificed.
Measurements of vWF, TAT, and TF plasma levels
Plasma vWF and thrombin levels were examined by ELISA specific for the vWF and TAT complex (eBioscience, San Diego, CA) following manufacturer’s instructions. Plasma TF levels were also measured using a mouse TF Elisa kit (eBioscience, San Diego, CA).
Quantitative real-time RT-PCR analysis
Total RNA was extracted from the frozen lung tissues (50–100 mg) of each mouse using Trizol reagent (Invitrogen, Carlsbad, CA) following manufacturer’s protocol. cDNA was synthesized with a cDNA Reverse Transcription Kit (Takara, Biotech, Japan), according to manufacturer’s instructions. Quantitative real-time PCR was performed by an ABI 7500 Sequence Detection System using specific primers (Invitrogen) and SYBR Green Real-Time PCR Master Mix (TaKara, Biotech, Japan). The final reaction volume was 20 μl with each primer. Sequences of primers used for this study were as follows: TF (NM_010171.3) forward: 5′-GCCACCATCTTTATCATCCTCC-3′, reverse: 5′-AGCCTTTCCTCTATGCCAAGC-3′; TM (NM_009378.3) forward: 5′-AGCGAGGGTAGGGAGGACTTGA-3′, reverse: 5′-CAGCAGCACTAGGAGGTGAGGT-3′; and EPCR (NM_011171.2) forward: 5′-TTGACGAAGTTTCTGCCGCTAC-3′, reverse: 5′-CCTGATGCCTCACATGATGGTT-3′ (Invitrogen, CA).
Western blot analysis
Lung tissues and endothelial cells (1 × 106 cells; Chi Scientific, Wuxi, China) were homogenized in lysis buffer (10 mM of Tris-HCl pH 7.5, 1% Triton X-100, 120 mM of NaCl, 1% sodium deoxycholate and 0.1% sodium dodecyl sulfate) containing a protease inhibitor cocktail (Roche Diagnostics). All lysed samples were kept on ice for 40 min, and centrifuged at 12,000 × g for 15 min at 4 °C (Thermo, MA). The supernatant was collected and stored at −20 °C until further analysis. Total lysates were fractionated on SDS-PAGE gels (8–12%) and transferred onto a polyvinylidene difluoride membrane (EDM Millipore, Billerica, MA). Then, membranes were blocked with 5% milk in Tris-buffered saline-Tween 20 (TBST) for 1 h. After washing three times with TBST, membranes were incubated overnight at 4 °C with primary antibodies. The following primary antibodies were used: anti-TF antibody (1:1000; Abcam, Cambridge, UK), anti-TM antibody (1:500; Abcam, Cambridge, UK) and anti-EPCR antibody (1:500; Abcam, Cambridge, UK). Goat anti-rabbit HRP-conjugated polyclonal antibody (1:5000; Abcam, Cambridge, UK) was used as the secondary antibody, which was incubated for 1 h at room temperature.
Immunohistochemistry
Immunohistochemistry was used to examine the expression of TF, TM, and EPCR in lungs using anti-TF antibody (1:250; Abcam, Cambridge, UK), anti-TM antibody (1:300; Santa Cruz Biotech, CA), and anti-EPCR antibody (1:100; Santa Cruz Biotech, CA). In brief, paraffin-embedded lung tissues were dewaxed and rehydrated in a graded concentration of ethanol. Tissues were subjected to antigen retrieval with citric acid buffer (0.01 M, pH 6) at 95 °C for 30 min. Thereafter, the sections were treated with 5% bovine serum albumin (BSA) for 30 min. Anti-TF, Anti-TM, or Anti-EPCR was applied and incubated overnight at 4 °C. After washing three times with Tris-buffered saline, horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary antibody (Zhongshan Golden Bridge Biotechnology, Beijing, China) was used to incubate for 30 min at room temperature; and a DAB kit (Sigma-Aldrich, St. Louis, MO) was used to detect immunohistochemical reactions. The slides were examined under a phase contrast light microscope (Nikon, Tokyo, Japan), and average integral optical density of each positively stained slide was measured using Image-Pro plus 6.0 true color image analysis system. Three areas were randomly chosen from each section for measurement (n = 4).
Statistical analysis
GraphPad Prism software was used to analyze data. Data were expressed as means ± standard error (SEM). The difference among groups was analyzed with one-way Analysis of Variance (ANOVA) and Student–Newman–Keuls test. Student’s t-test with Bonferroni correction was used for two-group analysis. p < 0.05 was considered statistically significant.
Results
In vitro
Cell identification
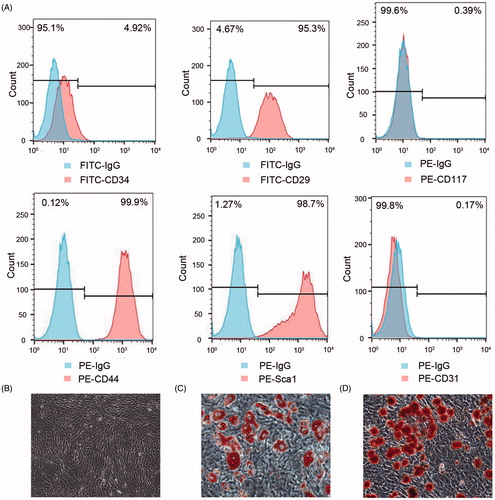
The expression of cell surface antigens CD29, CD44, and Sca-1 exhibited ≥70% positivity, while the expression of cell surface antigens CD34, CD31, and CD117 exhibited ≤5% positivity. Surface phenotypes were CD34−, CD44+, CD29+, CD117−, SCA-1+, and CD31− (). Tri-lineage differentiation of adipocytes and osteocytes were discovered when cultured in appropriate differentiation media ().
Figure 1. Identification of BMSCs isolated from the bone marrow of C57BL/6 mice. Cell surface markers including CD34, CD29, CD117, CD44, Sca-1, and CD31 were analyzed by flow cytometry (A). Morphology of BMSCs at the seventh passage (B, ×100) and multilineage differentiation capacities of BMSCs including adipogenic differentiation stained with oil red-O (C, ×200) and osteogenic differentiation stained with alizarin red (D, ×200) were observed with a microscope.
Endothelial–bone marrow mesenchymal stem cell co-culture experiments
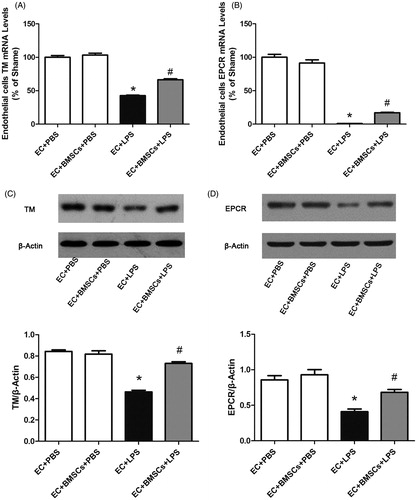
Lipopolysaccharide (LPS) stimulation significantly decreased TM and EPCR mRNA transcription and protein expression in the endothelium in the LPS group, compared to the PBS control group. However, this decrease was significantly ameliorated after co-culture with BMSCs ().
Figure 2. Endothelial cells in co-culture systems were collected 6 h after LPS stimulation. (A) RNA and (B) protein expression levels of TM in endothelial cells were determined by real-time PCR and western blot, respectively. RNA (C) and protein (D) expression levels of EPCR in endothelial cells were determined. TM and EPCR expression in each sample was normalized to the expression of β-actin. Each blot was quantified by densitometry analysis. Data were compared by Student’s t-test; *p < 0.05 versus the sham group; #p < 0.05 versus the CLP control group.
In vivo
Lung histology
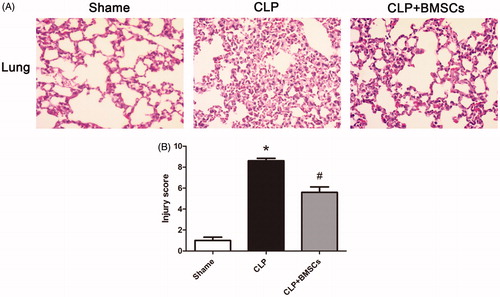
Typical histological images are shown in . Lung injury score was higher in the CLP control group compared to the sham group, and was significantly lower with BMSCs ().
Figure 3. (A) Representative photomicrographs of lung tissues stained with hematoxylin and eosin 24 h after the surgery. The sections are shown at ×400 magnification. CLP caused the infiltration of inflammatory cells into the interstitium and alveolar spaces of the lung, alveolar wall thickening, and intra-alveolar exudation. (B) Histological scoring of lung injury in different groups was quantified, as described in the “Materials and methods” section. Lung-injury score significantly decreased in the CLP + BMSCs group compared with the CLP control group. Data are expressed as mean ± SEM (n = 4, per group), and were compared by one-way ANOVA and SNK method; *p < 0.05 versus the sham group; #p < 0.05 versus the CLP control group. ANOVA: Analysis of Variance; CLP: cecal ligation and puncture; SEM: standard error of the mean; SNK: Student–Newman–Keuls.
Mesenteric perfusion area
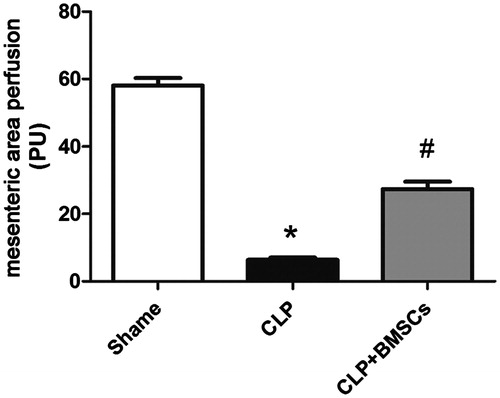
At 24 h after CLP, mesenteric perfusion was significantly impaired in the CLP control group compared with the sham group; while this was better maintained with the administration of BMSCs ().
Figure 4. Mice treated with BMSCs presented a significantly increased mesenteric perfusion area compared to CLP control mice (n = 4, per group). Data are expressed as mean ± SEM, and were compared by one-way ANOVA and SNK method; *p < 0.05 versus the sham group; #p < 0.05 versus the CLP control group. ANOVA: Analysis of Variance; CLP: cecal ligation and puncture; SEM: standard error of the mean; SNK: Student–Newman–Keuls.
Coagulation
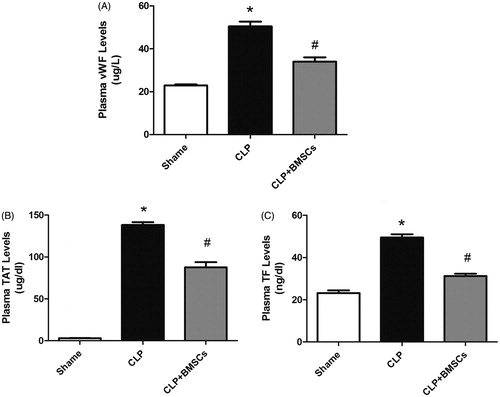
Plasma vWF, TF, and TAT complex levels increased at 24 h after CLP in all groups. The vWF, TF, and TAT levels markedly increased in the CLP control group vs. the sham group, but this was significantly tempered by the administration of BMSCs ().
Figure 5. Plasma levels of vWF, TAT, and TF in the three groups were measured by enzyme-linked immunosorbent assay (ELISA) (n = 4–8, per group): (A) vWF, (B) TAT, and (C) TF. Data are expressed as mean ± SEM, and were compared by one-way ANOVA and SNK method; *p < 0.05 versus the sham group; #p < 0.05 versus the CLP control group. ANOVA: Analysis of Variance; CLP: cecal ligation and puncture; SEM: standard error of the mean; SNK: Student–Newman–Keuls.
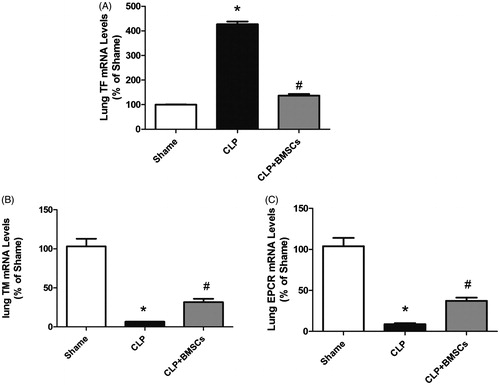
Lung TF, TM, and EPCR mRNA measurements
Tissue factor (TF) RNA expression in lung tissues was significantly upregulated in the CLP control group compared to the sham group, while this upregulated expression was significantly lower after treatment with BMSCs. However, the expression of TM and ECPR in lung tissues was significantly downregulated in the CLP control group compared to the sham group, and this downregulated expression was significantly attenuated after treatment with BMSCs ().
Figure 6. Effect of BMSCs on (A) TF, (B) TM, and (C) EPCR gene expression levels in lung tissues of CLP mice. RNA was isolated from lung tissues, and relative mRNA expression levels of TF, TM, and EPCR were measured by real-time RT-PCR. Results are reported as fold-change relative to CLP control (untreated) animals. Data are represented as mean ± SEM (n = 4 mice/group), and compared by one-way ANOVA and SNK method; *p < 0.05 versus sham group; #p < 0.05 versus the CLP control group. ANOVA: Analysis of Variance; CLP: cecal ligation and puncture; SEM: standard error of the mean; SNK: Student–Newman–Keuls.
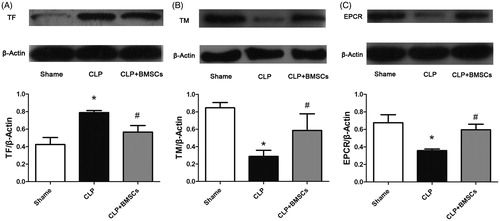
Western blotting
Western blotting revealed a similar pattern for TF, TM, and ECPR protein expression levels ().
Figure 7. (A) TF, (B) TM, and (C) EPCR expression levels in lung tissues in the three groups were determined by western blot. β-Actin was used as a total protein loading control. Each blot was quantified by densitometry analysis. Data are expressed as mean ± SEM (n = 4 mice/group), and were compared by one-way ANOVA and SNK method; *p < 0.05 versus sham group; #p < 0.05 versus the CLP control group.
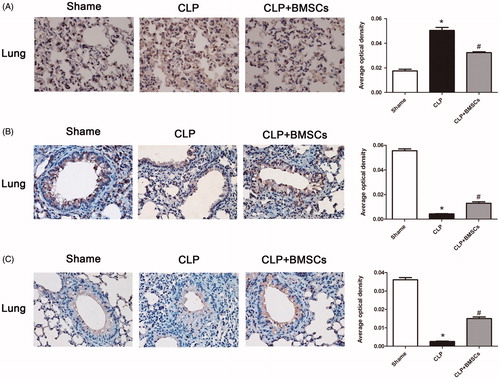
Immunohistochemistry
Immunohistochemistry results confirmed the results of western blotting (), in which the average integral optical density of positive staining revealed a consistent result.
Figure 8. Treatment with BMSCs inhibited (A) TF, but increased (B) TM, and (C) EPCR expression levels in lung tissues after CLP-induced sepsis in mice. After 24 h of CLP surgery, lung samples were processed for immunohistochemistry analysis. Graphical representation of immunostaining for TF, TM, and EPCR expression levels evaluated in lung tissues is shown. Mean intensity of TF, TM, and EPCR staining were determined from image analysis, and are represented as optical density. Each column represents the mean ± SEM of four mice per group and represents three independent experiments; *p < 0.05 versus sham group; #p < 0.05 versus the CLP control group.
Discussion
Although the co-culture with endothelium cells in vitro were stimulated by LPS and post-infusion after CLP in vivo, BMSC administration in our study has been shown to: (I) increase the expression of TM and EPCR from endothelium cells exposed to LPS in vitro; (II) attenuate sepsis-induced coagulopathy, as suggested by the tempered increase in vWF, TAT, and TF circulation levels; (III) ameliorate mesenteric perfusion area microcirculation impairment and decrease lung injury.
In sepsis, TF forms a complex with activated VII (FVIIa), which activates the coagulation system via the extrinsic pathway (Citation29–32). Increasing evidence has shown that TF is the principal initiator of thrombin generation in sepsis (Citation33). The TAT complex, as a marker of thrombin generation, has been proven as a good predictor of 28-day mortality; which has allowed the early identification of severe sepsis (Citation34). The vWF might serve as a marker to detect systemic endothelial activation upon systemic inflammation. High levels of vWF reflect endothelial damage and correlate with poor survival and fewer organ-failure days (Citation35). Excessive thrombin generation from the endothelium and subsequent fibrin deposition in sepsis provoke disseminated intravascular coagulation (DIC) development (Citation36). This study revealed that the administration of BMSCs markedly decreased vWF, TF, and TAT plasma levels following reduced thrombin generation, which may inhibit the extrinsic coagulation pathway and be responsible for ameliorated sepsis-related coagulopathy.
In sepsis, endothelial cell TM and EPCR expression levels were strongly downregulated and caused the impaired activation of PC, which is central in the modulation of coagulation activation and inflammatory processes (Citation37). The administration of soluble TM has been shown to improve the derangement of coagulation, ameliorate inflammatory responses, and possibly restore organ dysfunction (Citation38). Indeed, the widespread thrombosis in the microcirculation of different tissues causes hypoxic-ischemic tissue injury and contribute to poor sepsis outcome. The amelioration of DIC by various interventions improved organ failure (Citation39). In our study, we discovered that BMSCs attenuated the downregulation of TM in endothelial cells in vivo and in vitro; which may result in the preservation of endothelial cells, less inflammatory response, and improved lung injury and the impairment of microcirculation.
The limitations of this study are as follows. (I) Single dose BMSCs from the previous pilot experiment was used in the present study. Thus, whether dose-dependent effects exist or not remains unknown. (II) The experiment was performed for 24 h, and the long-term effects of BMSCs were not studied. Furthermore, the adverse effects of BMSCs, if any, could not be identified. (III) Young and healthy animals were used, which is clearly different from clinical patients with co-morbidities. (IV) Antibiotics, vasopressors, and fluids were not used in the resuscitated model, which might decrease the clinical relevance of the sepsis model. (V) BMSC infusion started at 6 h after CLP, which might be different from clinical reality. Nevertheless, this intervention remains as a post-treatment. (VI) Finally, rodent animals were not patients; thus, these results should be carefully extrapolated. Further studies on relevant clinical models are necessary to confirm our results.
Disclosure statement
The authors report no conflicts of interest.
Funding information
Jingye Pan is supported by the National Natural Science Foundation of China (81171787).
References
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–10.
- Wheeler AP, Bernard GR. Treating patients with severe sepsis. N Engl J Med. 1999;340:207–14.
- Semeraro N, Ammollo CT, Semeraro F, Colucci M. Sepsis, thrombosis and organ dysfunction. Thromb Res. 2012;129:290–5.
- Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–61.
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–35.
- Kinasewitz GT, Yan SB, Basson B, Comp P, Russell JA, Cariou A, et al. Universal changes in biomarkers of coagulation and inflammation occur in patients with severe sepsis, regardless of causative micro-organism [ISRCTN74215569]. Crit Care. 2004;8:R82–90.
- Semeraro N, Ammollo CT, Semeraro F, Colucci M. Coagulopathy of acute sepsis. Semin Thromb Hemost. 2015;41:650–8.
- Iba T, Nagaoka I, Boulat M. The anticoagulant therapy for sepsis-associated disseminated intravascular coagulation. Thromb Res. 2013;131:383–9.
- van der Poll T, Levi M. Crosstalk between inflammation and coagulation: the lessons of sepsis. Curr Vasc Pharmacol. 2012;10:632–8.
- Levi M, van der Poll T. Inflammation and coagulation. Crit Care Med. 2010;38:S26–34.
- Deutschman CS, Tracey KJ. Sepsis: current dogma and new perspectives. Immunity. 2014;40:463–75.
- Jones CA, Nishiya N, London NR, Zhu W, Sorensen LK, Chan AC, et al. Slit2-Robo4 signalling promotes vascular stability by blocking Arf6 activity. Nat Cell Biol. 2009;11:1325–31.
- Kim DH, Yoo KH, Choi KS, Choi J, Choi SY, Yang SE, et al. Gene expression profile of cytokine and growth factor during differentiation of bone marrow-derived mesenchymal stem cell. Cytokine. 2005;31:119–26.
- Ripa RS, Haack-Sorensen M, Wang Y, Jorgensen E, Mortensen S, Bindslev L, et al. Bone marrow derived mesenchymal cell mobilization by granulocyte-colony stimulating factor after acute myocardial infarction: results from the Stem Cells in Myocardial Infarction (STEMMI) trial. Circulation. 2007;116:I24–30.
- Chen SL, Fang WW, Ye F, Liu YH, Qian J, Shan SJ, et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004;94:92–5.
- Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874–82.
- Lee PH, Kim JW, Bang OY, Ahn YH, Joo IS, Huh K. Autologous mesenchymal stem cell therapy delays the progression of neurological deficits in patients with multiple system atrophy. Clin Pharmacol Ther. 2008;83:723–30.
- Ringden O, Uzunel M, Rasmusson I, Remberger M, Sundberg B, Lonnies H, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81:1390–7.
- Lazarus HM, Koc ON, Devine SM, Curtin P, Maziarz RT, Holland HK, et al. Cotransplantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biol Blood Marrow Transplant. 2005;11:389–98.
- Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–22.
- Herrera MB, Bussolati B, Bruno S, Fonsato V, Romanazzi GM, Camussi G. Mesenchymal stem cells contribute to the renal repair of acute tubular epithelial injury. Int J Mol Med. 2004;14:1035–41.
- Elman JS, Li M, Wang F, Gimble JM, Parekkadan B. A comparison of adipose and bone marrow-derived mesenchymal stromal cell secreted factors in the treatment of systemic inflammation. J Inflamm (Lond). 2014;11:1. doi: 10.1186/1476-9255-11-1.
- Kusadasi N, Groeneveld AB. A perspective on mesenchymal stromal cell transplantation in the treatment of sepsis. Shock. 2013;40:352–7.
- Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–9.
- Shin S, Kim Y, Jeong S, Hong S, Kim I, Lee W, et al. The therapeutic effect of human adult stem cells derived from adipose tissue in endotoxemic rat model. Int J Med Sci. 2013;10:8–18.
- Yagi H, Soto-Gutierrez A, Navarro-Alvarez N, Nahmias Y, Goldwasser Y, Kitagawa Y, et al. Reactive bone marrow stromal cells attenuate systemic inflammation via sTNFR1. Mol Ther. 2010;18:1857–64.
- Dejager L, Pinheiro I, Dejonckheere E, Libert C. Cecal ligation and puncture: the gold standard model for polymicrobial sepsis? Trends Microbiol. 2011;19:198–208.
- Giangola MD, Yang WL, Rajayer SR, Nicastro J, Coppa GF, Wang P. Growth arrest-specific protein 6 attenuates neutrophil migration and acute lung injury in sepsis. Shock. 2013;40:485–91.
- Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–50.
- Iyer JK, Khurana T, Langer M, West CM, Ballard JD, Metcalf JP, et al. Inflammatory cytokine response to Bacillus anthracis peptidoglycan requires phagocytosis and lysosomal trafficking. Infect Immun. 2010;78:2418–28.
- Keshari RS, Silasi-Mansat R, Zhu H, Popescu NI, Peer G, Chaaban H, et al. Acute lung injury and fibrosis in a baboon model of Escherichia coli sepsis. Am J Respir Cell Mol Biol. 2014;50:439–50.
- Taylor FB, Jr, Kinasewitz GT, Lupu F. Pathophysiology, staging and therapy of severe sepsis in baboon models. J Cell Mol Med. 2012;16:672–82.
- Pawlinski R, Mackman N. Cellular sources of tissue factor in endotoxemia and sepsis. Thromb Res. 2010;125:S70–3.
- Koyama K, Madoiwa S, Nunomiya S, Koinuma T, Wada M, Sakata A, et al. Combination of thrombin-antithrombin complex, plasminogen activator inhibitor-1, and protein C activity for early identification of severe coagulopathy in initial phase of sepsis: a prospective observational study. Crit Care. 2014;18:R13.
- Page AV, Liles WC. Biomarkers of endothelial activation/dysfunction in infectious diseases. Virulence. 2013;4:507–16.
- Uaylor FB, Jr, Toh CH, Hoots WK, Wada H, Levi M. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86:1327–30.
- Mohan Rao LV, Esmon CT, Pendurthi UR. Endothelial cell protein C receptor: a multiliganded and multifunctional receptor. Blood. 2014;124:1553–62.
- Levi M, van der Poll T. The role of natural anticoagulants in the pathogenesis and management of systemic activation of coagulation and inflammation in critically ill patients. Semin Thromb Hemost. 2008;34:459–68.
- Levi M, Schultz M, van der Poll T. Disseminated intravascular coagulation in infectious disease. Semin Thromb Hemost. 2010;36:367–77.
