Abstract
Introduction: Maternal obesity is associated with several adverse long-term health outcomes in the offspring. In this study, we examined the association between maternal body mass index (BMI) and offspring physical and psychosocial functioning in late adulthood. Methods: The study included 1759 men and women born during 1934–1944 and belonging to the Helsinki Birth Cohort Study. Data on maternal weight and height in late pregnancy and on offspring birth weight were retrieved from hospital birth records. Physical and psychosocial functioning was assessed using the Short Form 36 scale. Results: Maternal BMI was positively associated with poorer physical and psychosocial functioning among men, but not among women. This association was not mediated by birth weight. Discussion: The present study emphasizes the importance of preventing overweight and obesity among women of childbearing age.
Maternal BMI is known to be associated with adverse health outcomes among adult offspring.
We found that higher maternal BMI was associated with poorer physical and psychosocial functioning among male offspring in late adulthood.
The association between maternal BMI and offspring physical and psychosocial functioning was not mediated by birth weight.
Key messages
Introduction
Obesity has become a considerable health issue among pregnant women globally. This is also the case within the European Union, where about one-third of women of childbearing age are overweight and every fifth is obese (Citation1,Citation2). It is well documented that a high maternal body mass index (BMI) is associated with a number of pregnancy complications, including miscarriages and congenital defects (Citation3,Citation4). However, increasing evidence suggest that maternal BMI also alters the long-term health outcomes of the offspring.
Several studies have shown that maternal obesity is associated with obesity in the offspring (Citation5,Citation6). This association is stronger for maternal than it is for paternal obesity, indicating that it is not only due to a common genetic background (Citation7). Moreover, maternal obesity increases the risk of cardiovascular events and premature mortality among the adult offspring (Citation8). We have previously shown in the Helsinki Birth Cohort Study (HBCS) among 13,345 men and women born during 1934–1944, that higher maternal BMI was associated with an increased risk of type 2 diabetes, cancer and cardiovascular disease as well as all-cause and cancer mortality among the offspring (Citation9).
The underlying mechanisms that potentially mediate the long-term association between high maternal BMI and offspring health outcomes in later life can include genetic factors, adapting the mother’s lifestyle when growing up, and in utero programming (Citation10–16). Programming is defined as a consequence of a stimulus or insult during a critical time period inducing permanent changes in the structure and function of the developing tissues and organs. Epidemiological studies on this phenomenon have mostly used birth weight as a marker of intrauterine conditions (Citation17). Birth weight is influenced by intrauterine conditions, however, it does not capture all factors active during fetal development that influence offspring later health, such as maternal characteristics (Citation18,Citation19).
In the HBCS, low birth weight and slow growth during childhood have previously been shown to associate with poorer physical functioning, and early life stress, with both poorer physical and psychosocial functioning (Citation20,Citation21). Furthermore, findings from The British 1946 Birth Cohort indicate that poor maternal health increase the risk of poorer offspring physical functioning (Citation22). In the present study, we investigate the possible association between maternal BMI and physical and psychosocial functioning in late adulthood in men and women belonging to the HBCS. Moreover, we study whether this association could be mediated by offspring birth weight or adulthood BMI.
Materials and methods
HBCS includes 13,345 men and women who were born in the two largest maternity hospitals in Helsinki between 1934 and 1944, who attended child welfare clinics in the city and who lived in Finland in 1971, when all Finnish residents were assigned a unique personal identification number. In the year 2000, a questionnaire was sent to all members of the study population living in Finland at the time. Out of the 6874 individuals who responded, 2901 were randomly chosen and invited to take part in a clinical study. Altogether 2003 participated in the clinical examinations, which were conducted between the years 2001 and 2004. Those 1759 individuals (810 men and 949 women), who had complete data available on maternal weight and height before delivery and who answered a questionnaire regarding physical and psychosocial functioning at the time of the clinical examination, were included in the study. Maternal height and weight before delivery were retrieved from hospital birth records. Using these data we calculated maternal BMI as weight (kg)/(height (m2)) and divided it into tertiles. The lowest BMI group included 579 participants with a mean value of 23.7 kg/m2 (range 15.6–25.1 kg/m2), the middle group included 590 participants with a mean BMI of 26.3 kg/m2 (range 25.2–27.4 kg/m2) and the highest group included 585 participants with a mean BMI of 29.7 kg/m2 (range 27.4–43.4 kg/m2). Data on the participants’ birth weight and height were extracted from hospital birth records and the participants were divided into three pre-specified groups consistent with previous publications; birth weight below 2500 grams (n = 33), birth weight between 2500 and 3000 grams (n = 248) and birth weight over 3000 grams (n = 1362). Childhood socioeconomic class was defined as fathers’ highest occupational class using data from birth, child welfare and school healthcare records. The participants’ smoking status was assessed at the clinical examinations and categorized as current smoker, ex-smoker and never smoked.
The participants’ physical and psychosocial functioning was assessed at the clinical examination using the Finnish validated version of the RAND 36-Item Health Survey 1.0 (Short Form 36 (SF-36)) (Citation23–26). The SF-36 has been found to accurately assess health related quality of life in the Finnish population. The physical functioning scale of the SF-36 has been widely used to measure physical functioning in the older population (Citation27,Citation28). Physical functioning was assessed with the physical component summary of SF-36, which included four subscales; physical functioning, role limitations due to physical problems, pain and general health. Each subscale included 2–10 items and every item was scored as 0 = a lot of problems or unable to perform, 50 = some problems and 100 = no problems. The physical functioning component summary score was calculated by summarizing the scores of each item and dividing the sum by the total amount of items (Citation20). The score ranged from 0 to 100, median score being 85 for men and 81 for women. The physical functioning component summary score was divided into thirds and those in the bottom third (less than 78 for men and 70 for women) were considered to have poor physical functioning.
Four subscales from the SF-36, role limitations due to emotional problems, energy, emotional well-being and social functioning, were used to assess psychosocial functioning. Each subscale included 2–10 items, which were scored as 0 = a lot of problems or unable to perform, 50 = some problems and 100 = no problems. The psychosocial functioning summary component was calculated by summarizing the scores for each item and dividing the sum with the number of items. For men, a median score was 90, for women it was 85. The summary score was divided into thirds and those in the bottom third (less than 84 for men and 78 for women) were considered to have poor psychosocial functioning.
Statistical methods
Descriptive results are expressed as means and standard deviations for continuous variables and as percentages for categorical variables, and were calculated separately for men and women. The relation between maternal BMI and physical and psychosocial functioning was assessed with logistic regression analyses, using the lowest maternal BMI group as a reference. Separate analyses were done for physical and psychosocial functioning, and the analyses were performed both for men and women together and separately for both sexes. These analyses were adjusted first for age at clinical examination, and then further for parity, gestational age, childhood social class and smoking. Finally, birth weight, BMI at clinical examination and maternal age were separately added to the analyses, in order to test whether these factors mediated the association between maternal BMI and functioning in older age. The associations between birth weight and physical and psychosocial functioning were also assessed with logistic regression analyses, using the group with a birth weight above 3000 grams as a reference. These analyses were adjusted for parity, gestational age, childhood social class, smoking, age at clinical examination and sex, and performed first, for both sexes combined and secondly, for men and women separately. All analyses were carried out with IBM SPSS version 22. The study complies with the guidelines of the Declaration of Helsinki and it was approved by the Ethics Committee of Epidemiology and Public Health of the Hospital District of Helsinki and Uusimaa and that of the National Public Health Institute, Helsinki. All participants gave a written informed consent.
Results
Maternal characteristics of the participating men and women are shown in . Odds ratios (OR) and 95% confidence intervals (CI) for physical and psychosocial functioning according to tertiles of maternal BMI are illustrated in for both sexes combined, in for men and in for women, respectively. Higher maternal BMI was weakly associated with poorer physical functioning in older age among the offspring, OR for the group with the highest maternal BMI was 1.20 (95% CI 0.92, 1.55) in the analysis pooled by gender and adjusted for age, parity, gestational age, childhood social class and smoking (model 2). Further adjustment for birth weight slightly strengthened the association (OR 1.23; 95% CI 0.94, 1.60). The association between maternal BMI and birth weight was 0.29 (p < 0.001) for both sexes combined. Including adjustment for adult BMI in model 2 attenuated the association (OR 1.07; 95% CI 0.85, 1.40). In the analyses done separately for men, the association between higher maternal BMI and poorer physical functioning was stronger and statistically significant, OR being 1.60 (95% CI 1.08, 2.37) in model 2. Additional adjustment for birth weight further strengthened the association (OR 1.69; 95% CI 1.13, 2.52), while it was not significantly altered by further adjustment for adult BMI. There was no significant association between maternal BMI and physical functioning among women. Including maternal age as a variable in the analyses assessing the association between maternal BMI and offspring physical functioning did not alter the main results. OR and 95% CI for physical and psychosocial functioning according to thirds of offspring birth weight are illustrated in . Lower birth weight was associated with poorer physical functioning regardless of sex. In the lowest birth weight group OR was 3.09 (95% CI 1.50, 6.39) in the pooled analysis.
Figure 1. (a) Odds ratio (OR) and 95% confidence intervals (CI) for poor physical functioning (dots) and psychosocial functioning (squares) according to tertiles of maternal body mass index, both sexes combined. Model 1 is adjusted for age and sex; Model 2, in addition to age, is also adjusted for parity, gestational age, childhood social class and smoking; Model 3, in addition to all previous adjustments, is adjusted for birth weight. (b) Odds ratio (OR) and 95% confidence intervals (CI) for poor physical functioning (dots) and psychosocial functioning (squares) according to tertiles of maternal body mass index for men. Model 1 is adjusted for age; Model 2, in addition to age, is also adjusted for parity, gestational age, childhood social class and smoking; Model 3, in addition to all previous adjustments, is adjusted for birth weight. (c) Odds ratio (OR) and 95% confidence intervals (CI) for poor physical functioning (dots) and psychosocial functioning (squares) according to tertiles of maternal body mass index for women. Model 1 is adjusted for age; Model 2, in addition to age, is also adjusted for parity, gestational age, childhood social class and smoking; Model 3, in addition to all previous adjustments, is adjusted for birth weight.
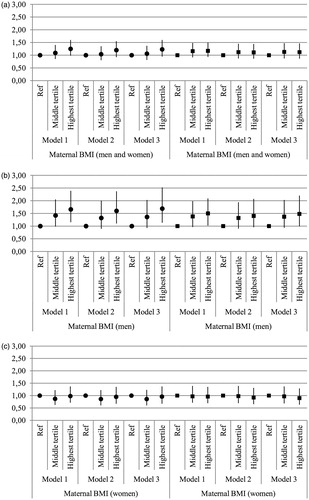
Table 1. Maternal characteristics of men and women belonging to the Helsinki Birth Cohort Study.
Table 2. Odds ratio (OR) and 95% confidence intervals (95% CI) for poorer physical and psychosocial functioning according to thirds of offspring birth weight.
Higher maternal BMI was weakly associated with poor psychosocial functioning in the analyses pooled by gender. Adjustment for birth weight slightly strengthened the association, while adjustment for adult BMI slightly attenuated it. Among men this association was statistically significant, age-adjusted OR in the highest maternal BMI group being 1.46 (95% CI 1.02, 2.09), however, further adjustment for parity, gestational age, socioeconomic status in childhood and smoking in adulthood attenuated the association. The association was not affected by additional adjustment for birth weight (model 3), BMI in adulthood further attenuated it. Including adjustment for maternal age in model 3, however, strengthened the association (OR 1.59; 95% CI 1.10, 2.39). Maternal BMI was not associated with psychosocial functioning among women. Lower birth weight was associated with poor psychosocial functioning in the pooled analysis (OR 2.14; 95% CI 1.05, 4.36) and in the analysis performed separately for men, but only weakly associated in the analysis performed for women.
Discussion
Using data from the HBCS we have studied the associations between maternal BMI during late pregnancy and offspring physical and psychosocial functioning in later life. This is, to our knowledge, the first study on this topic. Our main finding is that a higher maternal BMI was associated with poorer physical and psychosocial functioning in men in later life. The association between maternal BMI and physical functioning in men was independent of parity, gestational age, childhood social class, current age, smoking history, birth weight, adulthood BMI and maternal age. Similar associations were, however, not observed in women.
The associations between maternal BMI and functioning were further strengthened by additional adjustment for birth weight, thus concluding that birth weight was not a mediating factor in the association. As we have reported previously (Citation20), birth weight was independently associated with self-reported physical functioning. We found a similar association between body size at birth and later psychosocial functioning, an association that has been little studied so far (Citation29).
As stated earlier, high maternal BMI is a well-known risk factor for obesity in the offspring (Citation5,Citation6). In the HBCS, we have previously shown that adiposity in adulthood is independently associated with poor physical functioning in older age (Citation30). Nevertheless, in this study, additional adjustment for adulthood BMI only slightly attenuated the main associations; maternal BMI was significantly associated with poorer physical functioning among men regardless of it. Thus, obesity in adulthood, which could be explained by genetic factors and by adapting maternal lifestyle when growing up, does not solely explain our findings.
While the underlining mechanisms behind our findings remains unclear, one mediator of the associations is most likely a non-optimal prenatal environment, caused by maternal overweight or obesity. According to the overnutrition hypothesis, maternal obesity results in high maternal glucose, insulin and free fatty acid levels, which in turn lead to adverse in utero programming, potentially permanently altering the development of the fetus (Citation11,Citation12,Citation15). In the HBCS, we have previously shown that high maternal BMI is associated with a number of adverse health outcomes in the offspring, including cardiovascular events and type 2 diabetes. In utero programming is often characterized by gender differences, and gender differences were reported both in previous studies and in the current study. Type 2 diabetes and stroke are found to be more strongly associated with maternal BMI in women than in men, while coronary heart disease is more strongly associated with maternal BMI in men (Citation9,Citation31). These chronic diseases are also well-known risk factors for poor physical functioning (Citation32,Citation33).
Our study has some limitations. The data in the HBCS are restricted to people who were both born in Helsinki between 1934 and 1944, and who attended child welfare clinics in Helsinki. Most families attended these clinics, since they were free of charge. Attendance was, however, voluntary, and therefore the people in our study may not be representative of all people in Helsinki, for example, we lack information about the people who gave birth at private clinics. Speculatively, our study can thus have a different distribution of socioeconomic groups than the entire population of Helsinki at the time. However, adjusting for socioeconomic status did not alter the results. We calculated maternal BMI by using maternal weight just before delivery, which means that the weight was influenced by both the mothers’ weight before and weight gain during pregnancy. Nonetheless, maternal obesity before pregnancy and excessive gestational weight gain are associated with the same adverse pregnancy and neonatal outcomes (Citation16). Additionally, most of the mothers in our study were not obese by today’s standards; the group with the highest maternal BMI had a mean of 29.7 kg/m2. Therefore, we did not focus on the obese group but rather on the trend with BMI. We also do not have information about gestational diabetes among the mothers, which was rarely diagnosed at that time, and could thus not assess the effect the disease might have had on offspring physical and psychosocial functioning. Some of the children were born during World War II, which might have had an impact on the health of the child and mother.
The strengths of our study include reliable, hospital birth record based, information on maternal and prenatal characteristics. Moreover, the study cohort has been followed up for a long period, from birth until mean age of 62 years. Few studies have had the possibility to simultaneously assessing the association between maternal weight and body size at birth on later physical and psychosocial functioning.
In conclusion, the present study suggests that a higher maternal BMI is associated with poorer physical and psychosocial functioning in older age among men, but not among women. The association is, however, not mediated by birth weight. Our findings stress the importance of maternal influence during pregnancy on offspring later health. Awareness of the risks of overweight and obesity during pregnancy should be raised among women of childbearing age.
Disclosure statement
The authors report no conflicts of interest.
Funding information
HBCS has been supported by grants from Finska Läkarsällskapet, the Finnish Special Governmental Subsidy for Health Sciences, Academy of Finland, Samfundet Folkhälsan, Liv och Hälsa, the Signe and Ane Gyllenberg Foundation, and EU FP7 (DORIAN) project number 278603. The Academy of Finland supported MBvB (grant no. 257239); J.G.E. (grant no. 129369, 129907, 135072, 129255 and 126775) and E.K. (127437, 129306, 130326, 134791, and 263924). E.K. has received funding from the Foundation for Pediatric Research, Juho Vainio Foundation, Novo Nordisk Foundation, Signe and Ane Gyllenberg Foundation, Sigrid Juselius Foundation.
References
- World Obesity Federation: Numbers of females of reproductive age either overweight or obese by region. Available at: http://www.worldobesity.org/resources/obesity-data-repository/resources/charts/21/ (accessed 20 November 2015).
- Eurostat: Population on 1 January by age and sex. Available at: http://ec.europa.eu/eurostat/en/web/products-datasets/-/DEMO_PJAN (accessed 20 November 2015).
- Owens LA, O’Sullivan EP, Kirwan B, Avalos G, Gaffney G, Dunne F, ATLANTIC DIP Collaborators. ATLANTIC DIP: the impact of obesity on pregnancy outcome in glucose-tolerant women. Diabetes Care. 2010;33:577–9.
- Roman AS, Rebarber A, Fox NS, Klauser CK, Istwan N, Rhea D, Saltzman D. The effect of maternal obesity on pregnancy outcomes in women with gestational diabetes. J Matern Fetal Neonatal Med. 2011;24:723–7.
- Drake AJ, Reynolds RM. Impact of maternal obesity on offspring obesity and cardiometabolic disease risk. Reproduction. 2010;140:387–98.
- Reynolds RM, Osmond C, Phillips DI, Godfrey KM. Maternal BMI, parity, and pregnancy weight gain: influences on offspring adiposity in young adulthood. J Clin Endocrinol Metab. 2010;95:5365–9.
- Pirkola J, Pouta A, Bloigu A, Hartikainen AL, Laitinen J, Jarvelin MR, Vaarasmaki M. Risks of overweight and abdominal obesity at age 16 years associated with prenatal exposures to maternal prepregnancy overweight and gestational diabetes mellitus. Diabetes Care. 2010;33:1115–21.
- Reynolds RM, Allan KM, Raja EA, Bhattacharya S, McNeill G, Hannaford PC, et al. Maternal obesity during pregnancy and premature mortality from cardiovascular event in adult offspring: follow-up of 1 323 275 person years. BMJ. 2013;347:f4539.
- Eriksson JG, Sandboge S, Salonen MK, Kajantie E, Osmond C. Long-term consequences of maternal overweight in pregnancy on offspring later health: findings from the Helsinki Birth Cohort Study. Ann Med. 2014;46:434–8.
- Godfrey KM, Gluckman PD, Hanson MA. Developmental origins of metabolic disease: life course and intergenerational perspectives. Trends Endocrinol Metab. 2010;21:199–205.
- Ramsay JE, Ferrell WR, Crawford L, Wallace AM, Greer IA, Sattar N. Maternal obesity is associated with dysregulation of metabolic, vascular, and inflammatory pathways. J Clin Endocrinol Metab. 2002;87:4231–7.
- Catalano PM, Ehrenberg HM. The short- and long-term implications of maternal obesity on the mother and her offspring. BJOG. 2006;113:1126–33.
- Catalano PM, Presley L, Minium J, Hauguel-de Mouzon S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care. 2009;32:1076–80.
- Sewell MF, Huston-Presley L, Super DM, Catalano P. Increased neonatal fat mass, not lean body mass, is associated with maternal obesity. Am J Obstet Gynecol. 2006;195:1100–3.
- Gluckman PD, Hanson MA. Developmental and epigenetic pathways to obesity: an evolutionary-developmental perspective. Int J Obes. 2008;32:S62–71.
- Li N, Liu E, Guo J, Pan L, Li B, Wang P, et al. Maternal prepregnancy body mass index and gestational weight gain on pregnancy outcomes. PLoS One. 2013;8:e82310.
- Bateson P, Barker D, Clutton-Brock T, Deb D, D'Udine B, Foley RA, et al. Developmental plasticity and human health. Nature 2004;430:419–21.
- Barker DJ, Osmond C, Kajantie E, Eriksson JG. Growth and chronic disease: findings in the Helsinki birth cohort. Ann Hum Biol. 2009;36:445–58.
- Roseboom TJ, van der Meulen JH, Ravelli AC, Osmond C, Barker DJ, Bleker OP. Effects of prenatal exposure to the Dutch famine on adult disease in later life: an overview. Twin Res. 2001;4:293–8.
- von Bonsdorff MB, Rantanen T, Sipila S, Salonen MK, Kajantie E, Osmond C, et al. Birth size and childhood growth as determinants of physical functioning in older age: the Helsinki Birth Cohort Study. Am J Epidemiol. 2011;174:1336–44.
- Alastalo H, von Bonsdorff MB, Raikkonen K, Pesonen AK, Osmond C, Barker DJ, et al. Early life stress and physical and psychosocial functioning in late adulthood. PLoS One. 2013;8:e69011.
- Mishra GD, Black S, Stafford M, Cooper R, Kuh D, National Survey of Health and Development scientific and data collection team. National Survey of Health and Development scientific and data collection team. Childhood and maternal effects on physical health related quality of life five decades later: the British 1946 birth cohort. PLoS One. 2014;9:e88524.
- Hays RD, Sherbourne CD, Mazel RM. The RAND 36-item health survey 1.0. Health Econ. 1993;2:217–27.
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. conceptual framework and item selection. Med Care. 1992;30:473–83.
- Aalto AM, Aro S, Aro AR, Mähönen M. RAND 36-Item Health Survey 1.0. Finnish version on the Health-related Quality of Life Questionnaire. National Research and Development Centre for Welfare and Health (STAKES) report 2/1995. 1995.
- Aalto AM, Aro AR, Teperi J. Rand-36 as a Measure of Health-related Quality of Life, Reliability, Construct Validity and Reference Values in the Finnish General Population. National Research and Development Centre for Welfare and Health (STAKES). 1999.
- Bohannon RW, DePasquale L. Physical functioning scale of the short-form (SF) 36: internal consistency and validity with older adults. J Geriatr Phys Ther. 2010;33:16–8.
- Brazier Je, Harper R, Jones Nm, O'Cathain A, Thomas KJ, Usherwood T, Westlake L. Validating the SF-36 Health Survey Questionnaire: new outcome measure for primary care. BMJ. 1992;305:160–4.
- Stafford M, Gale CR, Mishra G, Richards M, Black S, Kuh DL. Childhood environment and mental wellbeing at age 60-64 years: prospective evidence from the MRC national survey of health and development. PLoS One. 2015;10:e0126683.
- Eriksson JG, Osmond C, Perala MM, Salonen MK, Simonen M, Pohjolainen P, et al. Prenatal and childhood growth and physical performance in old age-findings from the Helsinki birth cohort study 1934–1944. Age (Dordr). 2015;37:108.
- Eriksson JG, Forsen T, Tuomilehto J, Osmond C, Barker DJ. Early growth and coronary heart disease in later life: longitudinal study. BMJ. 2001;322:949–53.
- Gregg EW, Mangione CM, Cauley JA, Thompson TJ, Schwartz AV, Ensrud KE, et al. Diabetes and incidence of functional disability in older women. Diabetes Care. 2002;25:61–7.
- Pinsky JL, Jette AM, Branch LG, Kannel WB, Feinleib M. The framingham disability study: relationship of various coronary heart disease manifestations to disability in older persons living in the community. Am J Public Health. 1990;80:1363–7.
