Abstract
Background. Rheumatoid arthritis (RA) is characterized by an uncontrolled spread of destructive joint inflammation resembling malignancy. Epidemiological studies have established strong correlation between inflammation and predisposition for cancer. Here we assess the predictive role of the circulating proto-oncogene survivin for clinical and radiological outcome of early RA.
Patients and methods. Serum survivin was measured by sandwich ELISA in 651 patients with early RA (mean duration 6 months). X-rays of hands and feet were prospectively obtained at base-line and after 1, 2, and 5 years and evaluated for the presence of bone destruction by a modified Sharp method. The predictive value of survivin for radiological destruction was calculated using multivariate regression models including antibodies against cyclic citrullinated peptides (aCCP) and rheumatoid factor (RF). Remission was assessed by the EULAR (European League Against Rheumatism) criteria and by criteria proposed by Mäkinen.
Results. At base-line, 391 patients (60%) had high levels of survivin. Radiological progression at 5 years was significantly more frequent (P= 0.001) among survivin-positive patients than among survivin-negative. Survivin positivity predicted radiological progression independently of aCCP and RF. The positive predictive value of survivin was proved both in the group of patients with and in the group without erosions at base-line. The combination of positive tests for both survivin and aCCP had the highest prediction for radiological progression (positive predictive value 0.75). Additionally, a positive test for survivin was an independent predictor of not being in remission.
Conclusion. Detection of survivin in early RA predicted joint destruction and failure of achieving remission after 5 years in patients with early RA.
Key messages
High circulating levels of proto-oncogene survivin are detected in a substantial proportion of patients with early rheumatoid arthritis at their first visit to the rheumatologist.
In this prospective cohort study we show that circulating survivin is an early and strong predictor of joint destruction within the following 5 years. Survivin predicted joint destruction independently of antibodies to citrullinated peptides and rheumatoid factor.
Detection of survivin in combination with antibodies to citrullinated peptides may improve the precision of pharmacological management of patients with early rheumatoid arthritis.
| Abbreviations | ||
| aCCP | = | antibodies against cyclic citrullinated peptides |
| BARFOT project | = | the Better AntiRheumatic FarmacOTherapy |
| CI | = | confidence interval |
| CRP | = | C-reactive protein |
| DAS28 | = | disease activity score based on evaluation of 28 joints |
| DMARDs | = | disease-modifying anti-rheumatic drugs |
| ELISA | = | enzyme-linked immunosorbent assay |
| ESR | = | erythrocyte sedimentation rate |
| EULAR | = | the European League Against Rheumatism |
| HAQ | = | health assessment questionnaire |
| OR | = | odds ratio |
| PPV | = | positive predictive value |
| PTEN | = | phosphatase and tensin homologue deleted on chromosome ten |
| RA | = | rheumatoid arthritis |
| RF | = | rheumatoid factor |
| SD | = | standard deviation |
| vdH-Sharp | = | the Sharp method as modified by van der Heijde |
Introduction
Rheumatoid arthritis (RA) is the most common inflammatory joint disease with a frequency of 0.5%–1% in the general population. The disease leads frequently to disability affecting the quality of life and causes major economical consequences for society (Citation1,Citation2). The course of RA may vary considerably (Citation3,Citation4). A growing number of new anti-rheumatic drugs may efficiently improve the outcome of RA. However, these drugs are associated with increased risks of serious complications and are associated with high costs. The requirement to start treatment of RA as early as possible (to prevent joint destruction) and the selection of those patients who have high probability of developing destructive disease urge the search for clinical, radiological, laboratory, and genetic predictive factors helping to establish an accurate disease prognosis already at the first visit to a physician.
The advent of more efficient treatment options has made it even more urgent than before to find reliable predictors of outcome to be used in early disease. Shared epitopes have been extensively studied as a potential predictor of erosive disease, but the results are contradictory (Citation5). Rheumatoid factor (RF) has long been appreciated as a major predictor of radiological damage. However, several reports have found that antibodies against cyclic citrullinated peptides (aCCP) have greater predictive value (Citation6–8). A recent meta-analysis of available studies (Citation9) concludes that aCCP may better predict erosive disease than RF. Thus, aCCP is regarded today as the best available single predictor of joint damage. However, the predictive value of aCCP is not optimal. Therefore, additional predictors are indeed needed. In this study we present a new parameter, circulating survivin, fulfilling these requirements.
Survivin is a multifunctional protein transcribed as a wild-type 16.5-kDa protein consisting of 142 amino acids and present in cytoplasm, nucleus, and in mitochondria (Citation10). In the cytoplasm survivin serves as an efficient inhibitor of apoptosis controlling both caspase-dependent and mitochondrial apoptosis cascades. Intranuclear survivin regulates cell cycle progression and mitosis by acting as a sub-unit in chromosomal passenger complex. A third pool of survivin has been recently described in mitochondria. However, most knowledge about survivin is accumulated with respect to oncogenesis. Survivin is abundantly expressed in most solid tumours and in haematological malignancies (Citation11). The growing evidence suggests that survivin expression in the tumour tissues is associated with clinical and morphological characteristics of the malignancy being frequently used to predict efficiency of treatment and overall survival (Citation12). It has been shown that modulation of survivin expression in the tumour cells in vitro has a regulatory effect on tumour growth and spreading.
During the course of RA, synovial tissue undergoes hyperplasia, growing invasively into the cartilage and subchondral bone. There are similarities between rheumatoid synovial tissue and tumour growth, not least regarding spreading tendency and expression of proto-oncogenes such as c-Myc, c-Ras, c-Jun, and low activity of tumour suppressor genes p53 and PTEN (Citation13,Citation14). Oncogenes c-Ras and c-Myc have even been suggested as promising targets for anti-rheumatic treatment (Citation15,Citation16); however, the prognostic value of oncogenes for the development of destructive joint disease has not been evaluated. We have recently reported the presence of extracellular survivin in circulation and in the joint fluid in a significant portion of patients with RA (Citation17). In the present study we assessed the predictive role of circulating survivin for clinical and radiological outcome of patients with early RA during a follow-up period of 5 years. Importantly, the predictive value of survivin was tested against the established predictive variables aCCP and RF.
Methods
A total of 698 patients with early RA, having disease duration of 1 year or less, were consecutively included into a multicentre observational study (the BARFOT project (Better AntiRheumatic FarmacO-Therapy)). All the patients fulfilled the classification criteria for RA established by the American College of Rheumatology (Citation18). No patient had received prior treatment for their RA with disease-modifying drugs (DMARDs) or glucocorticoids. At base-line, the treating rheumatologist made a decision regarding the need for DMARD treatment and was free to select any DMARD. Most patients received methotrexate or sulfasalazine. Biologics were seldom used in this patient cohort. Clinical, laboratory, and radiological assessments of the patients were performed at inclusion into the study (base-line), and thereafter at 1, 2, and 5 years of follow-up. All patients gave their informed consent, and the ethics committees approved the study. Disease activity was measured by the disease activity score based on the evaluation of 28 joints (DAS28) (Citation19). Remission was defined 1) as DAS28 <2.6 (EULAR criterion (European League Against Rheumatism)) (Citation20), and 2) as no swollen joints, no tender joints, and normal erythrocyte sedimentation rate (ESR) (women <30 and men <20 mm) (Mäkinen criteria) (Citation21). Point remission was assessed after 2 and 5 years of follow-up, and patients in remission at both these time points were considered to be in sustained remission. Functional disability was assessed using a Swedish version of the Stanford Health Assessment Questionnaire (HAQ) (Citation22).
Laboratory
Serum samples were collected at inclusion into the study, and samples were stored at −70°C until further analyses. Survivin was measured in 651 patients by a sandwich enzyme-linked immunosorbent assay (ELISA) using a pair of matched antibodies (R&D Systems, Abingdon, UK). Values of circulating survivin above 300 pg/mL, corresponding to 3 SD of a healthy control group, were defined as positive (Citation17). Rheumatoid factor (RF) was analysed using the Serodia agglutination test (Fujirebio, Tokyo, Japan). Antibodies to cyclic
citrullinated peptides (aCCP) were detected using the ELISA CCP2 test (Euro-Diagnostica, Malmö, Sweden).
Radiographic measurements
Joint destruction was assessed by X-ray changes. Posterior-anterior radiographs of the hands and feet were obtained at study entry in 597 patients, at 1 year in 572, at 2 years in 601, and at 5 years in 490 patients. Patients not having any X-rays did not differ significantly from patients with X-rays in base-line characteristics like age, disease duration, gender, aCCP, RF, survivin, DAS28, and HAQ. Radiographic damage was assessed according to the Sharp method as modified by van der Heijde (vdH-Sharp) (Citation23), which allows for separate analysis of a total score (TS) (range 0–448), an erosion score, and a joint space narrowing score. Since this study was performed in clinical practice, it was not feasible to read all films by two readers. Instead, the films were read by one of two experienced readers (KA and KF). Double readings of a fraction of films showed good agreement between the two readers. On 49 such double readings of base-line and 5 year films the smallest detectable change (SDC) could be calculated admitting radiological progression to be defined as a change in the total vdH- Sharp score over 5 years by >5.4.
Statistical analysis
Statistical analyses were performed using SPSS V.15.0 statistical software. To test the differences between groups, the Mann-Whitney U test or the independent t test was used for continuous variables, and the chi-square test for proportions. Pearson's or Spearman's correlation tests were used to assess the relations between two continuous variables. All significance tests were two-tailed and conducted at the 0.05 level of significance. Univariate analyses of the association of survivin and other base-line clinical and demographic variables with radiological and clinical outcome were performed. To determine whether survivin was an independent predictor of outcome, multiple logistic regression analyses with backwards elimination were performed, adjusting for those demographic and base-line clinical and radiological variables that were significantly (P < 0.1) associated with the outcomes in the univariate analyses. The criterion for elimination of a variable from the model was set at P < 0.05. The predictive performance of survivin, aCCP, and RF was analysed by 2 ×2 tables, and odds ratios (OR), sensitivity, specificity, and positive and negative predictive values were calculated.
Results
At base-line, the levels of survivin corresponding to above the mean + 3 SD of healthy blood donors (300 pg/mL) were found in 391 of 651 patients (60%). For the statistical evaluation the patients were stratified as survivin-positive (>300 pg/mL) or survivin-negative (<300 pg/mL). Clinical and demographic characteristics of survivin-positive and survivin-negative patients are shown in . The levels of survivin show sharply uneven distribution between the survivin-negative and survivin-positive groups. The table shows that survivin-positive patients had evidence of a severe disease course already at base-line. Thus, median C-reactive protein (CRP) and total vdH-Sharp score were significantly higher in these patients, and they had higher frequency of aCCP and RF. At base-line, all patients were DMARD and glucocorticoid naive. Treatment modalities during the follow-up period are shown in . The table shows that survivin-positive patients were more often started on DMARDs (P = 0.013) compared with survivin-negative patients. DMARDs as monotherapy and in combinations were used significantly more often by survivin-positive patients at 1, 2, and 5 years follow-up. In contrast, DMARD-free patients were significantly more often survivin-negative.
Table I. Clinical and demographic base-line characteristics of the survivin-positive and survivin-negative patients.
Table II. Treatment modalities of the survivin-positive (n = 391) and survivin-negative (n = 260) patients. The table shows DMARD treatment over time in survivin+ and survivin- patients. Survivin+ patients have consistently been treated more frequently with DMARDs than survivin-patients.
At base-line, the proportion of patients started on prednisolone was similar in the survivin-positive and survivin-negative groups (35% versus 32%), but prednisolone use was significantly more frequent in survivin-positive patients at 1, 2, and 5 years of follow-up (39% versus 31%, P = 0.05, 37% versus 27%, P = 0.009, and 24% versus 16%, P = 0.023, respectively).
Relations between survivin, aCCP, and RF
Survivin, aCCP, and RF correlated considerably with each other (r = 0.49 for survivin with aCCP, r = 0.56 for survivin with RF, and r = 0.69 for aCCP with RF, all P = 0.001). Thus, of positive tests for survivin 76% were also aCCP-positive, kappa 0.5, and 82% were also RF-positive, kappa 0.6. Of aCCP-positive tests, 90% were also RF-positive, kappa 0.7.
Correlations of survivin levels with radiological changes and clinical variables at base-line and over time
At base-line, the levels of survivin correlated with total vdH-Sharp score (r = 0.18, P = 0.001). At follow-up after 1, 2, and 5 years the correlation between base-line survivin levels and vdH-Sharp scores became more prominent (r = 0.25, r = 0.28, and r = 0.34, respectively). At these time points no correlation between survivin levels and DAS28, CRP, and HAQ was found. This prompted us to evaluate the predictive role of survivin for clinical and radiological outcome.
The total vdH-Sharp score at base-line was significantly higher in the survivin-positive group than in the survivin-negative group (mean 4.5 versus 3.0, P = 0.018). This difference became more apparent after 1, 2, and 5 years of follow-up (). Accordingly, the mean changes in total vdH-Sharp score from base-line to 1, 2, and 5 years were significantly larger in the survivin-positive compared with the survivin-negative group (5.0 versus 2.7, 9.2 versus 5.3, and 19. 0 versus 9.7, respectively, P = 0.001 for all comparisons).
Figure 1. Progression of radiological changes over time in survivin positive and survivin negative patients with early rheumatoid arthritis. (A) The total vdH-Sharp score was significantly higher in survivin posive as compared to survivin negative patients. This difference showed a gradual increase at each time point of follow-up. (B) Development of bone erosions occurred significantly faster in survivin positive patients. Thus, the proportion of patients without erosions was lower among survivin positive patients.
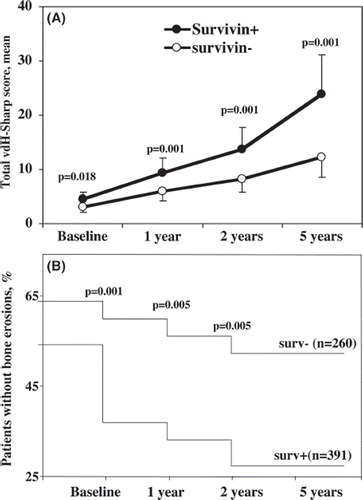
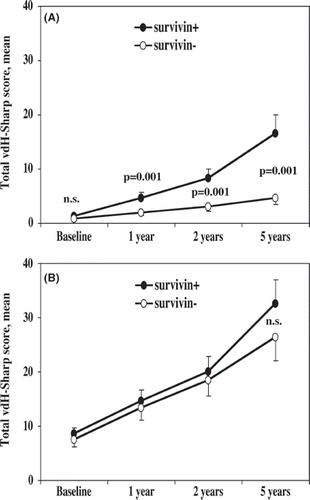
A total of 66% of survivin-positive patients and 34% of survivin-negative patients had erosive changes at the base-line X-ray examination (P = 0.017). Development of new erosions occurred significantly more often in the survivin-positive group (). The analysis of changes in the total vdH-Sharp score with respect to positive survivin test was performed separately in the group of patients with, as well as without, erosive disease at base-line (). In the group of patients who lacked erosions, the positive survivin test permitted a clear distinction in the progression of the total vdH-Sharp score already after 1 year of follow-up (). The propagation of the Sharp score was more pronounced in the survivin-positive group throughout the follow-up period of 5 years. In the group of patients with erosions at base-line X-ray examination, the propagation of the total vdH-Sharp score was similar in the survivin-positive and the survivin-negative groups. At the 5-year follow-up a trend to a more pronounced progression of the vdH-Sharp score in the survivin-positive group was revealed ().
Figure 2. The pattern of radiological changes differed between the patients with no erosions at baseline and in patients with one or more erosion. (A) In the patients with no erosions, the total vdH-Sharp score progressed significantly faster in survivin positive patients as compared to survivin negative. (B) In the patients with one or more erosion at baseline, the progression of the total vdH-Sharp score was similar in survivin positive and survivin negative patients.
Survivin as a strong predictor of radiological progression
A total of 55% of the patients had radiological progression from base-line to 5 years. In the univariate analyses of base-line variables, survivin, aCCP, RF, CRP, and total vdH-Sharp score were significantly associated with radiological progression (all variables P = 0.001), while this was not the case for age, disease duration, gender, DAS28, or HAQ.
To assess the predictive value of survivin, OR, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated. As shown in the crude (unadjusted) OR of a positive test for survivin for radiological progression was 3.8. The corresponding ORs for aCCP and RF were 5.9 and 3.9 (all, P = 0.001). The OR for radiological progression was 5.3 for patients with positive tests for both aCCP and survivin (survivin+aCCP).
Table III. Univariate analysis of predictors for radiological progression at 5 years.
Altogether 75% of the patients with radiological progression after 5 years had a positive test for survivin (sensitivity). aCCP, RF, and survivin+aCCP had corresponding sensitivities of 0.75, 0.73, and 0.65, respectively. The positive predictive value of survivin was 0.68, i.e. 68% of those with a positive test for survivin at base-line developed radiological progression 5 years later. In contrast, the probability of developing radiological progression in the survivin-negative group was 35%. The corresponding PPVs were 0.73 for aCCP, 0.69 for RF, and 0.75 for survivin+aCCP. Thus, all these predictors increased markedly the pre-test probability of radiological progression, which was 0.55.
To determine whether a positive test for survivin was independently predictive of radiological progression, a multiple regression analysis with backwards elimination was performed adjusting for those base-line variables that were univariately associated with radiological progression (n = 398). Survivin proved to be an independent predictor of radiological progression with an OR (95% confidence interval (CI)) of 2.1 (1.2–3.7), P = 0.04. Other independent predictors were aCCP (OR (95% CI) 3.6 (2–6.7), P = 0.001), base-line CRP (OR (95% CI) 1.01 (1.00–1.02), P = 0.008) and total vdH-Sharp score (OR (95% CI) 1.08 (1.03-1.13), P = 0.001), while RF was excluded from the model because of its close association with aCCP
Prediction of radiological progression in patients with or without radiological erosions at base-line
Adequate prediction of future joint damage is particularly important for patients with no bone erosions when first seen by the rheumatologist. Therefore, the predictive value of survivin for radiological progression was studied separately in the subgroup of 355 patients (60%), who lacked bone erosions at base-line and the subgroup of 242 patients (40%) having erosions already at base-line. Altogether 42% of the patients without base-line erosions and 72% of the patients with erosions developed radiological progression after 5 years.
shows that, in the subgroup of patients lacking base-line erosions, the association of a positive test for survivin, aCCP, RF, and survivin+aCCP with radiological progression was more pronounced than that found in the whole cohort. Thus, the OR for having radiological progression after 5 years when a test for survivin was positive at base-line was 4.3, P= 0.001. For patients with a positive test for both aCCP and survivin, the OR for progression was 7.63 P = 0.001. The sensitivity, specificity, PPV3 and NPV of base-line survivin, aCCP, RF, and survivin+aCCP for predicting radiological progression are also shown in . The figures are comparable to those obtained from the whole patient material. Thus, the PPV was highest for patients with positive tests for both survivin and aCCP, 0.71, thus increasing the pre-test probability by almost 30%. shows the predictive value of a positive survivin test in comparison to aCCP and RF for the analysis of patients with one or more erosions already at base-line. The ORs were generally somewhat smaller than those for the group without base-line erosions. The PPV of survivin for radiological progression in this group was 0.8, being somewhat higher than the PPVs of a positive test for aCCP or RF. Analogously, a positive survivin test had somewhat higher sensitivity and specificity compared with aCCP and RF. In all, the post hoc increase in predictive ability of a positive survivin test amounted to about 10% in this group of patients.
Table IV. Univariate analysis of predictors of radiological progression in rheumatoid arthritis at 5 years. A: Patients without bone erosions at base-line. B: Patients with bone erosions at base-line.
Prediction of clinical remission
At base-line there were no significant differences in mean DAS28 or HAQ between survivin-positive and survivin-negative patients. The mean improvement in DAS28 from base-line to 2 and 5 years was significantly less pronounced in the survivin-positive than in the survivin-negative group (mean 1.9 versus 2.3, P = 0.004, and 1.9 versus 2.4, respectively, P = 0.001).
As to aCCP and RF the mean difference in improvement in DAS28 over time between patients with positive and negative tests was significant only at 5 years (P = 0.031 and 0.012, respectively). The mean improvement in HAQ from base-line to 2 and 5 years was not significantly different between patients with positive and negative tests for these markers. According to both sets of criteria, survivin-positive patients were significantly less often in remission compared with survivin-negative patients. This was true for both point remission and sustained remission. Thus, by the EULAR criteria, 34% of survivin-positive patients versus 47% of survivin-negative patients were in point remission after 5 years (P = 0.002) and 20% versus 32% by the Mäkinen criteria (P = 0.001). Sustained remission was as a rule less frequent, but the differences between the survivin-positive and survivin-negative groups were consistent. Thus3 by the EULAR criteria, 22% versus 34% were in sustained remission (P = 0.001)., respectively, while by the Mäkinen criteria these figures were even smaller, 8% versus 19% (P = 0.001). The PPV of a positive test for survivin for not being in remission according to EULAR5 years, EULAR2+5 years, Mäkinen5 years, and Mäkinen2+5 years were 0.66, 0.78, 0.80, and 0.92, respectively. Multiple logistic regression models with backwards elimination including both sets of criteria for remission as dependent variables showed that a positive test for survivin was a consistent and significant independent predictor of not being in remission (). The presence of aCCP and RF was not found to be independent predictors in these models.
Table V. A positive test for survivin was an independent predictor of not being in remission.
Discussion
In this prospective study we show that the presence of survivin in circulation at early onset RA predicts progressive joint destruction. Indeed, levels of survivin correlated to the vdH-Sharp score at inclusion and prospectively at each time point of the study up to 5 years of follow-up. The progression of joint destruction was significantly more pronounced in the survivin-positive patients compared to survivin-negative. Importantly, the presence of survivin at disease onset has predictive value independently of other established variables, namely aCCP and RF (Citation5,Citation6).
Since the presence of erosions is an ultimate negative prognostic factor in RA leading to immuno-suppressive treatment of these patients, we addressed the question if survivin identifies patients at risk for development of erosions. Indeed, our results show that survivin-positive patients develop erosions significantly more frequently compared to survivin-negative patients. The use of DMARDs is known to play a major role delaying development of bone erosions (Citation1,Citation2,Citation4). The comparison of treatment modalities used by survivin-positive and survivin-negative patients showed that survivin-positive patients were treated significantly more often with DMARDs as monotherapy and in combination as compared to survivin-negative patients. Analogously, survivin-positive patients were significantly more often users of oral glucocorticoids. Thus, progressive development of erosions in survivin-positive patients was obvious despite more intensive treatment.
A positive survivin test was significantly associated with radiological progression (OR 3.8). The PPV, 0.68, indicates an increase in the pre-test probability of radiological progression of 0.55. The aCCP had a somewhat better prediction profile while that of RF was similar. In addition, testing for both survivin and aCCP gave some further increase in PPV above that seen with the single markers.
The evaluation of radiological progression showed that the predictive value of survivin increased predict-ability to 0.58 (the pre-test probability was 0.42) in patients lacking bone erosions at inclusion and also in the patients having erosions, 0.8 (the pre-test probability was 0.72). Thus, in patients who lacked bone erosions at base-line, survivin was found to be almost as predictive for radiographic progression as aCCP, which was the best single predictor. The evaluation of PPV in the group of patients having erosions at base-line showed somewhat higher predictability of survivin as compared to aCCP and RF. A positive test for both survivin and aCCP further increased PPV suggesting that survivin and aCCP may give partly different predictive information probably due to different mechanisms in the development of joint damage.
Furthermore, in the multivariate analyses, a positive test for survivin was an independent predictor of not being in remission, neither in point nor in sustained remission, irrespective of whether strict or less strict criteria for remission were applied. The aCCP and RF tests were not identified as predictors for this outcome, which is consistent with a recent study by Gossec et al. (Citation24). A cautious interpretation of these findings would be that a positive test for survivin might be regarded as a warning sign for persistent disease activity and failure to achieve remission. There were no consistent associations between survivin or aCCP and function, assessed by HAQ. This is in accordance with the study by Kastbom et al. (Citation25) and may reflect the fact that HAQ is only partly dependent on disease activity.
Some features distinguish survivin from the previously known predictive markers. Despite the high prevalence of positive survivin tests among the RA patients having autoantibody production (aCCP and RF), survivin is not a product of B-cells. A positive test for survivin may identify patients at risk of radiological progression even when negative for aCCP. The nature of survivin is distinct from that of an acute phase reactant in being resistant to change by sporadic treatment with corticosteroids. Survivin had strong predictability for radiological progression both in the group of patients with and without erosions at base-line. This suggests a value of survivin as a marker of insufficient anti-rheumatic treatment, identifying patients requiring change of treatment. The predictive role of survivin in established RA and for the response to treatment with conventional DMARDs and biologics must be confirmed in further studies on other patient cohorts and in clinical practice.
In a cross-sectional study of RA patients attending a specialized rheumatology ward we showed that the presence of survivin in circulation and synovial fluid was associated with an unfavourable and erosive course of joint disease (Citation17). The results of the present prospective study indicate survivin to be an important and early prognostic factor identifying patients at risk for development of joint destructions in RA. Recent experimental data favour the role of survivin as an essential mediator of arthritogenic properties in synovial fibroblasts (Citation26) and in regulation of integrin expression (Citation27). These findings give an argument for measuring survivin as a prognostic predictor of radiological progression and of clinical remission in patients with early RA.
To conclude, the present study shows that a test for survivin may help to identify patients with early RA at risk of developing joint damage and persistent disease activity. Furthermore, survivin gave predictive information additional to that obtained by aCCP, the most established predictive marker. Survivin may therefore play a role in the prediction of outcome in early RA, although further studies are needed to define the clinical value of this predictor in the group of patients with established RA.
Acknowledgements
The work has been supported by Göteborg Medical Society, Swedish Association against Rheumatism, King Gustaf V’s 80 year Foundation, Swedish Medical Research Council (521-2007 to AT and 2199-2008 to MB), Nanna Svartz’ Foundation, Börje Dahlin's Foundation, National Inflammation Network, Lundberg Foundation, Ugglas Foundation, Stig and Ragna Gorthon Foundation in Helsingborg, Stiftelsen för Rörelsehindrade i Skåne, the Regional agreement on medical training and clinical research (ALF) between Stockholm county council and the Karolinska Institute and between the Western Götaland county council and the University of Göteborg (LUA/ALF).
The patients were recruited and data collected by the BARFOT study group: Monica Ahlmén MD PhD, Johan Bratt MD PhD, Christina Dackhammar MD, Kristina Forslind MD PhD, Ingiäld Hafström MD PhD, Catharina Keller MD, Ido Leden MD, Bengt Lindell MD, Ingemar Petersson MD PhD, Björn Svensson MD PhD, Annika Teleman MD, and Jan Theander MD.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Wolfe F, Hawley DJ. The longterm outcomes of rheumatoid arthritis: Work disability: a prospective 18 year study of 823 patients. J Rheumatol. 1998;25:2108–17.
- Pugner KM, Scott DI, Holmes JW, Hieke K. The costs of rheumatoid arthritis: an international long-term view. Semin Arthritis Rheum. 2000;29:305–20.
- Harrison BJ, Symmons DP, Brennan P, Barrett EM, Silman AJ. Natural remission in inflammatory polyarthritis: issues of definition and prediction. Br J Rheumatol. 1996;35:1096–100.
- Van Aken J, van Dongen H, le Cessie S, Allaart CF, Breedveld FC, Huizinga TW. Comparison of long term outcome of patients with rheumatoid arthritis presenting with undifferentiated arthritis or with rheumatoid arthritis: an observational cohort study. Ann Rheum Dis. 2006;65:20–5.
- Gorman JD, Lum RF, Chen JJ, Suarez-Almazor ME, Thomson G, Criswell LA. Impact of shared epitope genotype and ethnicity on erosive disease: a meta-analysis of 3,240 rheumatoid arthritis patients. Arthritis Rheum. 2004;50:400–12.
- Forslind K, Ahlmén M, Eberhardt K, Hafström I, Svensson B. Prediction of radiological outcome in early rheumatoid arthritis in clinical practice: role of antibodies to citrullinated peptides (anti-CCP). Ann Rheum Dis. 2004;63:1090–5.
- Meyer O, Labarre C, Dougados M, Goupille P, Cantagrel A, Dubois A, . Anti-citrullinated protein/peptide antibody assays in early rheumatoid arthritis for predicting five year radiographic damage. Ann Rheum Dis. 2003;62:120–6.
- Bukhari M, Thomson W, Naseem H, Bunn D, Silman A, Symmons D, . The performance of anti-cyclic citrullinated peptide antibodies in predicting the severity of radiologic damage in inflammatory polyarthritis: results from the Norfolk Arthritis Register. Arthritis Rheum. 2007;56:2929–35.
- Nishimura K, Sugiyama D, Kogata Y, Tsuji G, Nakazawa T, Kawano S, . Meta-analysis: diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis. Ann Intern Med. 2007;146:797–808.
- Duffy MJ, O’Donovan N, Brennan DJ, Gallagher WM, Ryan BM. Survivin: A promising tumor biomarker. Cancer Lett. 2007;249:49–60.
- Altieri DC. Validating survivin as a cancer therapeutic target. Nat Rev Cancer. 2003;3:46–54.
- Pennati M, Folini M, Zaffaroni N. Targeting survivin in cancer therapy: fulfilled promises and open questions. Carcino-genesis. 2007;28:1133–9.
- Firestein GS, Echeverri F, Yeo M, Zvaifler NJ, Green DR. Somatic mutations in the p53 tumor suppressor gene in rheumatoid arthritis synovium. Proc Natl Acad Sci U S A. 1997;94:10895–900.
- Pap T, Franz JK, Hummel KM, Jeisy E, Gay R, Gay S. Activation of synovial fibroblasts in rheumatoid arthritis: lack of expression of the tumour suppressor PTEN at sites of invasive growth and destruction. Arthritis Res. 2000;2:59–64.
- Pap T, Nawrath M, Heinrich J, Bosse M, Baier A, Hummel KM, . Cooperation of Ras- and c-Myc-dependent pathways in regulating the growth and invasiveness of synovial fibro blasts in rheumatoid arthritis. Arthritis Rheum. 2004;50:2794–802.
- Hashiramoto A, Sano H, Maekawa T, Kawahito Y, Kimura S, Kusaka Y, . C-myc antisense oligodeoxynucleotides can induce apoptosis and down-regulate Fas expression in rheumatoid synoviocytes. Arthritis Rheum. 1999;42:954–62.
- Bokarewa M, Lindblad S, Bokarew D, Tarkowski A. Balance between survivin, a key member of the apoptosis inhibitor family, and its specific antibodies determines erosivity in rheumatoid arthritis. Arthritis Res Ther. 2005;7:R349–58.
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, . The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24.
- Prevoo ML, van’t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–8.
- Prevoo MLL, van Gestel AM, van’t Hof MA, van Rijswijk MH, van de Putte LB, van Riel PL. Remission in a prospective study of patients with rheumatoid arthritis. American Rheumatism Association preliminary remission criteria in relation to the disease activity score. Br J Rheumatol. 1996;35:1101–5.
- Mäkinen H, Kautiainen H, Hannonen P, Sokka T. Is DAS28 an appropriate tool to assess remission in rheumatoid arthritis? Ann Rheum Dis. 2005;64:1410–3.
- Ekdahl C, Eberhardt K, Andersson SI, Svensson B. Assessing disability in patients with rheumatoid arthritis. Use of a Swedish version of the Stanford Health Assessment Questionnaire. Scand J Rheumatol. 1988;17:263–71.
- van der Heijde DM, van Leeuwen MA, van Riel PL, Koster AM, van’t Hof MA, van Rijswijk MH, . Biannual radiographic assessments of hands and feet in a three-year prospective followup of patients with early rheumatoid arthritis. Arthritis Rheum. 1992;35:26–34.
- Gossec L, Dougados M, Goupille P, Cantagrel A, Sibilia J, Meyer O, . Prognostic factors for remission in early rheumatoid arthritis: a multiparameter prospective study. Ann Rheum Dis. 2004;63:675–80.
- Kastbom A, Strandberg G, Lindroos A, Skogh T. Anti-CCP antibody test predicts the disease course during 3 years in early rheumatoid arthritis (the Swedish TIRA project). Ann Rheum Dis. 2004;63:1085–9.
- Baran M, Möllers LN, Andersson S, Jonsson IM, Ekwall AK, Bjersing J, . Survivin is an essential mediator of arthritis interacting with urokinase signalling. J Cell Mol Med. 2009 Feb 27 (Epub ahead of print).
- Mera S, Magnusson M, Tarkowski A, Bokarewa M. Extra-cellular survivin up-regulates adhesion molecules on the surface of leukocytes changing their reactivity pattern. J Leukoc Biol. 2008;83:149–55.