Abstract
Background. Periprocedural myocardial injury remains the most common complication associated with percutaneous coronary intervention (PCI). Previous studies have demonstrated that even a small elevation of cardiac enzymes is associated with higher risk of mortality during follow-up.
Objective. We performed a meta-analysis based on all currently available randomized controlled trials (RCT) to evaluate the beneficial effects of hydroxymethylglutaryl-CoA reductase inhibitors (statins) given before PCI on preventing periprocedural myocardial infarction (MI).
Methods. The published literature was scanned by formal searches of electronic databases (PubMed, EMBASE, and the Cochrane Central Register of Controlled Trials) and conference proceedings up through August 2009. RCTs were eligible for inclusion if they compared preprocedural statins versus placebo treatment in patients not taking statins previously but scheduled for PCI and had the data of periprocedural MI reported by the trial investigators.
Results. Prespecified criteria were met by 6 RCTs involving 2,088 patients. During the periprocedural period, 81 of 1,051 patients (7.7%) in the statins pretreatment group developed periprocedural MI, significantly less than 147 of 1,037 (14.2%) patients assigned to the control group (OR 0.51, 95% CI 0.38–0.67; P< 0.001). During 1-month follow-up, only 4 deaths, 7 non-periprocedural Q-wave MIs, and 4 revascularizations occurred in all 2,088 enrolled patients. The composite of death, MI, or target vessel revascularization at 1 month, essentially driven by periprocedural MI, was reported in 8.0% in the statins pretreatment group and 15.3% in the control group (OR 0.48, 95% CI 0.36–0.64; P< 0.001). Conclusions. This meta-analysis supports the effectiveness of statins pretreatment on reducing the rate of periprocedural MI in patients undergoing PCI.
Key messages
Periprocedural myocardial injury, assessed by elevation of cardiac biomarkers, is associated with higher risk of mortality during follow-up.
We performed a meta-analysis based on all currently available randomized controlled trials to confirm that statins may lower the risk of periprocedural myocardial injury.
| Abbreviations | ||
| CI | = | confidence interval |
| CK | = | creatine kinase |
| MI | = | myocardial infarction |
| PCI | = | percutaneous coronary intervention |
| RCT | = | randomized controlled trials |
| OR | = | odds ratios |
| TVR | = | target vessel revascularization |
| ULN | = | upper limit of normal |
Introduction
Periprocedural myocardial injury, assessed by elevation of cardiac biomarkers, remains the most common complication associated with percutaneous coronary intervention (PCI) (Citation1). It has been reported to occur in up to 69% of patients undergoing PCI (Citation1) and should be labeled as myocardial infarction (MI) according to the new criteria (Citation2). Although most patients remain asymptomatic with no changes in cardiac function, even a small elevation of creatine kinase (CK)-MB isoenzyme is associated with higher risk of mortality during follow-up (Citation3). Recently, the results of several randomized controlled trials (RCT) evaluating the beneficial effects of hydroxymethylglutaryl-CoA reductase inhibitors (statins) given before coronary intervention on preventing myocardial injury have been reported (Citation4–11). Meta-analyses of randomized trials have the potential to increase the power and improve the precision of treatment effects and safety (Citation12). Therefore, we performed a meta-analysis based on all currently available RCTs to confirm the hypothesis that statins may lower the risk of periprocedural myocardial injury.
Methods
Data sources and selection criteria
To identify relevant trials, the electronic databases (PubMed, EMBASE, and the Cochrane Central Register of Controlled Trials) were searched using the following key words: randomized trial, statins, hydroxymethylglutaryl-CoA reductase inhibitor, pravastatin, atorvastatin, fluvastatin, simvastatin, lovastatin, cerivastatin, rosuvastatin, percutaneous coronary intervention, coronary artery disease. In addition, we scanned conference proceedings from the American College of Cardiology, American Heart Association, and European Society of Cardiology. The search was restricted to articles indexed as a clinical trial involving human subjects. Relevant reviews and editorials from major medical journals published within the last year were identified and assessed for possible information on trials of interest. Internet-based sources of information on the results of clinical trials in cardiology were also searched. The last search was performed in August 2009.
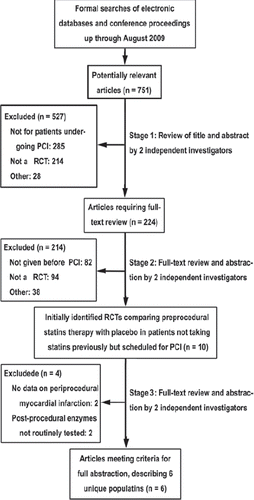
To be selected for this meta-analysis, studies comparing preprocedural statins versus placebo treatment in patients undergoing PCI had to be randomized and have their results of periprocedural MI reported by the trial investigators. All studies meeting the requirements, regardless of the language or form of publication, were considered to be eligible for this meta-analysis. When there were multiple reports from the same trial, we used the most complete and/ or recently reported data. Of the 751 potentially relevant articles initially screened, a total of 10 trials that compared preprocedural statins therapy with placebo in patients not taking statins previously but scheduled for PCI were initially identified (Citation4–11,Citation13,Citation14). Two studies (Citation10,Citation11) were then excluded because the MI event was not defined and no data were available on periprocedural MI. In another two trials (Citation13,Citation14), the cardiac enzymes were not routinely examined after the procedure. As the PCI-associated myocardial injury was mainly diagnosed by the postprocedural elevation of cardiac biomarkers, no exact data on periprocedural MI were available in these two trials. Thus, these two studies were also excluded. Finally, a total of six trials were included in this meta-analysis (Citation4–9). A flow diagram depicting the overall search strategy is demonstrated in .
Study outcomes and data abstraction
The primary end-point or outcome was periprocedural MI. Different cardiac markers and values were used by individual studies to define periprocedural MI: a postprocedural increase of CK-MB >2 times above the upper limit of normal (ULN) (Citation4), a CK-MB elevation >5 times ULN alone or associated with chest pain or ST-segment or T-wave abnormalities (Citation5), a postprocedural increase of CK-MB >2 times above the ULN in patients with normal base-line levels of CK-MB or a subsequent increase of more than 2-fold in CK-MB from baseline value in patients with elevated baseline levels of CK-MB (Citation6,Citation7), and a postprocedural CK-MB elevation >3 times ULN alone or associated with chest pain or ST-segment or T-wave abnormalities (Citation8,Citation9). We accepted these individual protocol definitions of periprocedural MI and did not attempt to retrospectively recategorize them. Other clinical outcomes of interest were: 1-month mortality, non-periprocedural MI, target vessel revascularization (TVR), by-pass surgery or repeat PCI of the target vessel, and the composite of death, MI, or TVR.
Two investigators (Z.F., D.L.) independently performed data abstraction. In addition to pertinent data on the outcomes of interest, we gathered information on trial names, first author, year of publication, and number of patients enrolled. Disagreements were resolved by consensus. The main characteristics of these trials are displayed in .
Table I. Characteristics of randomized controlled trials included in the meta-analysis.
Statistical analysis
All analyses were performed based on the intention-to-treat principle. Odds ratios (OR) with 95% confidence intervals (CI) were computed as summary statistics. The pooled OR was calculated with the Mantel-Haenszel method for fixed effects and the DerSimonian and Laird method for random effects (Citation15,Citation16). To assess heterogeneity across trials, we used Cochran's test and means of I2 statistic (Citation17). A funnel plot as well as the adjusted rank correlation test, according to the method of Begg and Mazumdar (Citation18), was used to assess publication bias with respect to the primary outcome of interest, periprocedural MI. A sensitivity analysis was performed by comparing the treatment effects obtained with each trial removed consecutively from the analysis with the overall treatment effects. Results were considered statistically significant at two-sided P< 0.05. Statistical analyses were performed with the Revman 5 freeware package program (The Cochrane Collaboration, Oxford, England) and the Stata version 9 statistical package (Stata Corp., College Station, Texas, USA).
Results
A total of 6 RCTs were finally included in this meta-analysis, involving 2,088 patients (1,051 in the statins pretreatment group and 1,037 in the control group) (Citation4–9). The study drug was atorvastatin with different dose and period before intervention (40 mg/d starting 7 days before the planned intervention (Citation4), 80 mg loading dose given a mean of 12 h before coronary angiography with a further 40 mg dose approximately 2 h before the procedure (Citation6), 80 mg/ day for 2 days prior to the index procedure (Citation8), or a single 80 mg loading dose within 24 hours (Citation9)) in four studies. In another trial (Citation7), a single rosuvastatin 40 mg loading was performed for 16 ± 5 hours (range 7–25) prior to the index procedure. And in the remaining trial (Citation5), the study drug was a multitude of different statins (atorvastatin, pravastatin, simvastatin, and fluvastatin) at variable doses, given for variable times before the procedure (between 3 and 31 days) according to the physician's discretion. In all studies, patients without contraindications were pretreated with aspirin (100 mg/d) and clopidogrel (a loading dose of 300 to 600 mg) or ticlopidine (250 mg twice a day at least 3 days) before the procedure. All patients continued ticlopidine 250 mg twice a day or clopidogrel 75 mg/d for at least 1 month (6 months for patients treated with drug-eluting stents). The use of platelet IIb/IIIa antagonist was comparable between patients assigned to either statins pretreatment group or control group (19% versus 22%, OR 0.81, 95% CI 0.64–1.03, P= 0.11; P= 0.34 for heterogeneity). In all trials included, the blood samples were taken before and at 6–24 hours after the procedure to assay CK-MB; additional determinations were performed if any patient developed postprocedural symptoms suggestive of myocardial ischemia.
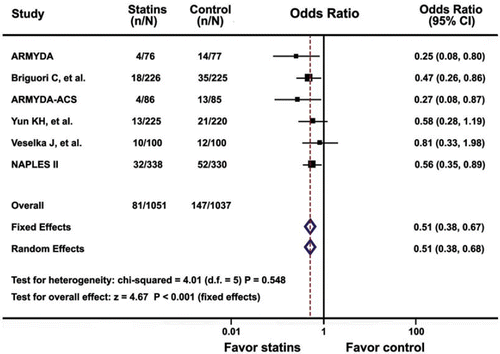
During the periprocedural period, 81 of 1,051 patients (7.7%) in the statins pretreatment group developed periprocedural MI, significantly fewer than 147 of 1,037 (14.2%) patients assigned to the control group (OR 0.51, 95% CI 0.38–0.67; P< 0.001) by the fixed-effect model (). There was no significant heterogeneity between trials (P= 0.55). No evidence of publication bias with respect to periprocedural MI was found using the Begg funnel plot and rank correlation test (P= 0.45). Omission of individual trials from the analysis did not have any relevant influence on the overall results.
Figure 2. Odds ratios of periprocedural myocardial infarction associated with statins pretreatment versus placebo in patients undergoing percutaneous coronary intervention. The size of the data marker is proportional to the weight of the individual studies, measured as the inverse of the variance in the study by the Mantel-Haenszel procedure.
In contrast, patients pretreated with statins and placebo did not differ significantly with respect to other outcomes of interest. Only 4 deaths (1 in the statins pretreatment group and 3 in the control), 7 non-periprocedural Q-wave MIs (2 in the statins pretreatment group and 5 in the control), and 4 TVRs (all in the control) occurred in all 2,088 enrolled patients at 30-day follow-up. Thus, the composite of death, MI, or TVR at 1 month, essentially driven by periprocedural MI, was reported in 84 patients (8.0%) in the statins pretreatment group and 159 (15.3%) in the control group (OR 0.48; 95% CI 0.36–0.64; P< 0.001; P= 0.40 for heterogeneity).
Discussion
The present meta-analysis of RCTs demonstrates the myocardioprotective effects of preprocedural statins therapy in patients undergoing PCI. Patients who received statins pretreatment before intervention had a 49% reduction in the odds of postprocedural MI compared with placebo.
A number of studies have indicated a correlation between postprocedural cardiac enzyme elevation and future major adverse cardiac events (MACE). Although periprocedural myocardial injury remains clinically silent in the majority of patients, a comparative analysis has demonstrated that the relative increase in 6-month mortality with each increase in postprocedural peak CK-MB level is similar for spontaneous and PCI-related myonecrosis (Citation19). Likewise, a comprehensive meta-analysis of seven studies, pooling data from 23,230 patients, showed a 1.5, 1.8, and 3.1 times higher risk of long-term mortality for patients with postprocedural CK-MB elevation of 1–3 times ULN, 3–5 times ULN, and >5 times ULN, respectively (Citation3). As any increase of CK-MB above normal limits is associated with increased risk of long-term mortality, therapies that decrease the incidence of periprocedural myocardial injury should beneficially affect clinical outcomes in these patients.
Several observational studies have shown that statins administration prior to PCI may be associated with a significant decrease in periprocedural MI and a trend toward a reduction in MACE for up to 12 months (Citation20–26). However, the conclusions of these studies were weakened by the limitations of non-randomized study designs. Likewise, a previous meta-analysis was mainly based on non-randomized studies and failed to include all currently available randomized trials (Citation27). In contrast, our meta-analysis of all available RCTs, which has the potential to increase the power and improve the precision of treatment effects and safety, demonstrated that pretreatment with statins decreases the incidence of myocardial injury during coronary intervention compared with placebo. Indeed, statins significantly reduced release of all markers of myocardial damage after coronary intervention, including myoglobin, troponin I, and CK-MB in those enrolled studies (Citation4–11).
The mechanism underlying the beneficial effects of statins pretreatment in reducing myonecrosis in patients undergoing PCI is not well elucidated, but unlikely attributable to cholesterol-lowering effects which require a longer duration of treatment (Citation28). In in-vivo and in-vitro studies, statins have demonstrated various lipid-independent pleiotropic effects, such as improvement of endothelial function, vasodilation of coronary microvessels, and direct antithrombotic effect (Citation29–31). These effects may start acutely after drug administration and before their lipid-lowering effects. In addition, the anti-inflammatory effect of statins may play an important role in reducing myocardial necrosis, as the benefit seems to be more pronounced in patients with a high C-reactive protein level at base-line (Citation7,Citation23). This is further supported by experimental evidence showing protective activity of statins on a model of ischemia/reperfusion via effects on microcirculation and cell adhesion and platelet function (Citation32,Citation33), and by clinical data indicating that statins could significantly decrease levels of CRP and enhance the decline in inflammation (Citation34–37).
A limitation of our meta-analysis is that the enrolled individual trials varied considerably in study design, with different durations, doses, and types of statins used. However, the purpose of our study was to assess whether statins pretreatment is useful in preventing periprocedural myocardial injury, but not to test a predefined dosage of a specific statin, or a specific delay between statins administration and the procedure. Additionally, different values of cardiac enzymes were used to define periprocedural MI in individual studies because all of these studies were designed before the publication of the new universal definition of myocardial infarction (Citation2). We accepted these individual protocol definitions of periprocedural MI and did not attempt to retrospectively recategorize them. The study follow-up was of relatively short duration and not sufficient to assess the long-term benefits of statins pretreatment, especially with respect to the reduction of mortality, because no data of more than 1 month's follow-up were available in all trials included in the present meta-analysis. By pooling three non-randomized trials, a previous meta-analysis has suggested that statins pretreatment before PCI was associated with a trend toward a reduction in MACE for up to 12 months (Citation27). Further RCTs with long-term follow-up data will certainly provide important additional information. Another limitation of the present meta-analysis is that it is not based on individual patient data and the time-to-event analyses could not be performed. Finally, this meta-analysis is based only on six trials, and therefore the findings should be interpreted with some caution.
In conclusion, this meta-analysis of RCTs supports the effectiveness of statins pretreatment on reducing the rate of periprocedural MI in patients undergoing PCI. Further studies are needed to identify the optimal statin type, dose, and time of onset before percutaneous revascularization.
Acknowledgements
This work was supported by the Young Scientific ‘Phosphor’ Foundation from the Shanghai Science and Technology Development (No. 08QA14019).
Declaration of interest: The authors report no conflicts of interest.
References
- Herrmann J. Periprocedural myocardial injury: 2005 update. Eur Heart J. 2005;26:2493–519.
- Thygesen K, Alpert JS, White HD; Joint ESC/ACCF/AHA/ WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. Eur Heart J. 2007;28:2525–38.
- Ioannidis JP, Karvouni E, Katritsis DG. Mortality risk conferred by small elevations of creatine kinase-MB isoenzyme after percutaneous coronary intervention. J Am Coll Cardiol. 2003;42:1406–11.
- Pasceri V, Patti G, Nusca A, Pristipino C, Richichi G, Di Sciascio G, . Randomized trial of atorvastatin for reduction of myocardial damage during coronary intervention: results from the ARMYDA (Atorvastatin for Reduction of MYocardial Damage during Angioplasty) study. Circulation. 2004;110:674–8.
- Briguori C, Colombo A, Airoldi F, Violante A, Focaccio A, Balestrieri P, . Statin administration before percutaneous coronary intervention: impact on periprocedural myocardial infarction. Eur Heart J. 2004;25:1822–8.
- Patti G, Pasceri V, Colonna G, Miglionico M, Fischetti D, Sardella G, . Atorvastatin pretreatment improves outcomes in patients with acute coronary syndromes undergoing early percutaneous coronary intervention: results of the ARMYDA-ACS randomized trial. J Am Coll Cardiol. 2007;49:1272–8.
- Yun KH, Jeong MH, Oh SK, Rhee SJ, Park EM, Lee EM, . The beneficial effect of high loading dose of rosuvastatin before percutaneous coronary intervention in patients with acute coronary syndrome. Int J Cardiol. 2008 Aug 13 (Epub ahead of print).
- Veselka J, Zemánek D, Hájek P, Malý M, Adlová R, Martinkovicová L, . Effect of two-day atorvastatin pre-treatment on the incidence of periprocedural myocardial infarction following elective percutaneous coronary intervention: a single-center, prospective, and randomized study. Am J Cardiol. 2009;104:630–3.
- Briguori C. NAPLES (Novel Approaches for Preventing or Limiting Event Study) II Trial: Impact of a single high loading dose of atorvastatin on periprocedural myocardial infarction. Presented at 58th annual scientific session of American College of Cardiology 2009 in Orlando, USA on 30 March 2009. Available at: http://assets.cardiosource.com/NAPLESII_Briguori.ppt. accessed on April 30th 2009.
- Bozbas H, Yildirir A, Mermer S, Konas D, Atar I, Aydinalp A, . Does pravastatin therapy affect cardiac enzyme levels after percutaneous coronary intervention? Adv Ther. 2007;24:493–504.
- Hara H, Nakamura M, Yokouchi I, Kimura K, Nemoto N, Ito S, . Aggressive statin therapy in multicenter and effectiveness for the reduction of intra-myocardial damage caused by non-ST elevation acute coronary syndrome: AMERICA study. Ther Adv Cardiovasc Dis. 2009;3:357–65.
- Egger M, Ebrahim S, Smith GD. Where now for meta-analysis? Int J Epidemiol. 2002;31:1–5.
- Weintraub WS, Boccuzzi SJ, Klein JL, Kosinski AS, King SB 3rd, Ivanhoe R, . Lack of effect of lovastatin on restenosis after coronary angioplasty. N Engl J Med. 1994;331:1331–7.
- Serruys PW, Foley DP, Jackson G, Bonnier H, Macaya C, Vrolix M, . A randomized placebo-controlled trial of fluvastatin for prevention of restenosis after successful coronary balloon angioplasty; final results of the fluvastatin angiographic restenosis (FLARE) trial. Eur Heart J. 1999; 20:58–69.
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101.
- Akkerhuis KM, Alexander JH, Tardiff BE, Boersma E, Harrington RA, Lincoff AM, . Minor myocardial damage and prognosis: are spontaneous and percutaneous coronary intervention-related events different? Circulation. 2002;105:554–6.
- Mulukutla SR, Marroquin OC, Smith C, Varghese R, Anderson WD, Lee JS, . Effect of statin therapy prior to elective percutaneous coronary intervention of frequency of periprocedural myocardial injury. Am J Cardiol. 2004;94:1363–8.
- Herrmann J, Lerman A, Baumgart D, Volbracht L, Schulz R, von Birgelen C, . Preprocedural statin medication reduces the extent of periprocedural non-Q-wave myocardial infarction. Circulation. 2002;106:2180–3.
- Chang SM, Yazbek N, Lakkis NM. Use of statins prior to percutaneous coronary intervention reduces myonecrosis and improves clinical outcomes. Catheter Cardiovasc Interv. 2004;62:193–7.
- Chan AW, Bhatt DL, Chew DP, Reginelli J, Schneider JP, Topol EJ, . Relation of inflammation and benefit of statins after percuatneous coronary inteventions. Circulation. 2003;107:1750–6.
- Auguadro C, Manfredi M, Scalise F, Mortara A, Specchia G. Protective role of chronic statin therapy in reducing myocardial damage during percutaneous coronary intervention. J Cardiovasc Med (Hagerstown). 2006;7:416–21.
- Gurm HS, Breitbart Y, Vivekanathan D, Yen MH, Fathi R, Ziada KM, . Preprocedural statin use is associated with a reduced hazard of postprocedural myonecrosis in patients undergoing rotational atherectomy—a propensity-adjusted analysis. Am Heart J. 2006;151 suppl:e1031–6.
- Veselka J, Prochazkova S, Duchonova R, Homolova I, Tesar D, Bybee KA. Preprocedural statin therapy reduces the risk and extent of cardiac biomarker release following percutaneous coronary intervention. Heart Vessels. 2006;21:146–51.
- Ebrahimi R, Saleh J, Toggart E, Shah AP, Azmoon S, Babaei H, . Effect of preprocedural statin use on procedural myocardial infarction and major cardiac adverse events in percutaneous coronary intervention: a meta-analysis. J Invasive Cardiol. 2008;20:292–5.
- Ray KK, Cannon CP. Early time to benefit with intensive statin treatment: could it be the pleiotropic effects? Am J Cardiol. 2005;96:54F–60F.
- Wassmann S, Faul A, Hennen B, Sheller B, Bohm M, Nickenig G. Rapid effect of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibition on coronary endothelial function. Circ Res. 2003;93:e98–103.
- Hinoi T, Matsuo S, Tadehara F, Tsujiyama S, Yamakido M. Acute effect of atorvastatin on coronary circulation measured by transthoracic Doppler echocardiography in patients without coronary artery disease by angiography. Am J Cardiol. 2005;96:89–91.
- Sanguigni V, Pignatelli P, Lenti L, Ferro D, Bellia A, Carnevale R, . Short-term treatment with atorvastatin reduces platelet CD40 ligand and thrombin generation in hypercholesterolemic patients. Circulation. 2005;111:412–9.
- Jones SP, Lefer DJ. Cardioprotective action of acute HMG-CoA reductase inhibition in the setting of myocardial infarction. Acta Physiol Scand. 2001;173:139–43.
- Notarbartolo A, Davì G, Averna M, Barbagallo CM, Ganci A, Giammarresi C, . Inhibition of thromboxane biosynthesis and platelet function by simvastatin in type IIa hyper-cholesterolemia. Arterioscler Thromb Vasc Biol. 1995;15:247–51.
- Albert MA, Danielson E, Rifai N, Ridker PM; PRINCE Investigators. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA. 2001;286:64–70.
- Correia LC, Spósito AC, Lima JC, Magalhães LP, Passos LC, Rocha MS, . Anti-inflammatory effect of atorvastatin (80 mg) in unstable angina pectoris and non-Q-wave acute myocardial infarction. Am J Cardiol. 2003;92:298–301.
- Macin SM, Perna ER, Farías EF, Franciosi V, Cialzeta JR, Brizuela M, . Atorvastatin has an important acute anti-inflammatory effect in patients with acute coronary syndrome: results of a randomized, double-blind, placebo-controlled study. Am Heart J. 2005;149:451–7.
- Kinlay S, Schwartz GG, Olsson AG, Rifai N, Leslie SJ, Sasiela WJ, . High-dose atorvastatin enhances the decline in inflammatory markers in patients with acute coronary syndromes in the MIRACL study. Circulation. 2003;108:1560–6.